Abstract
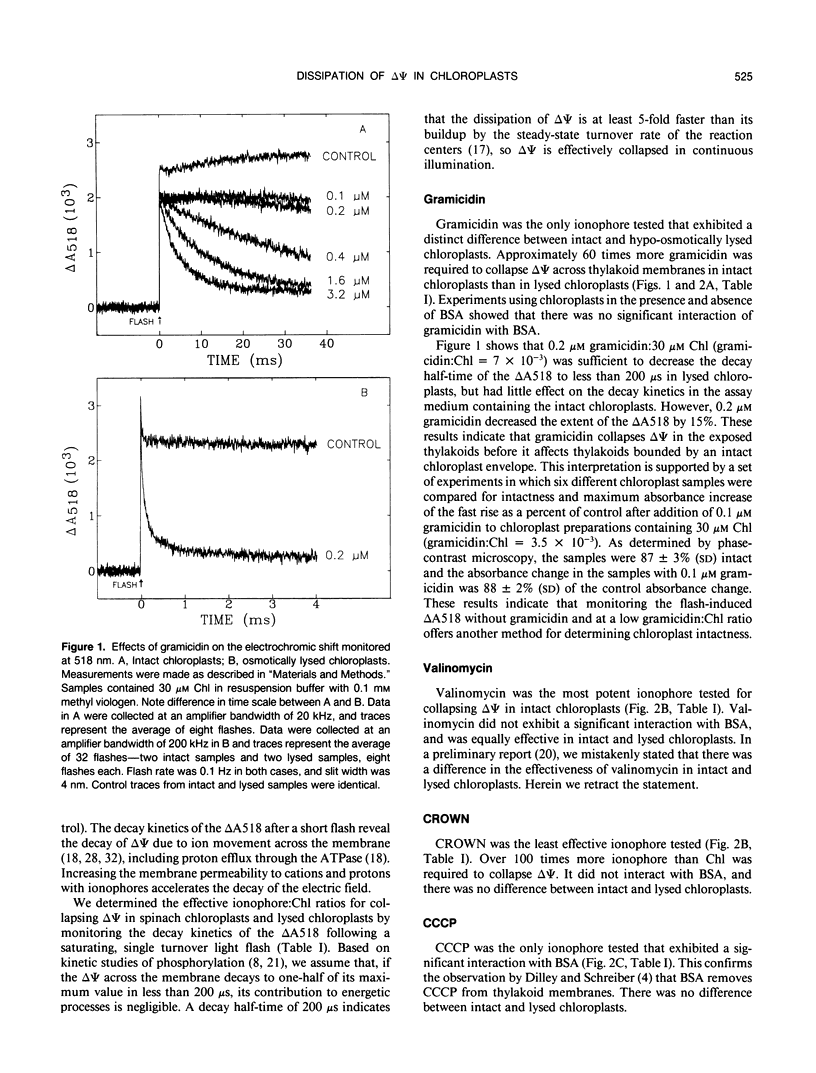
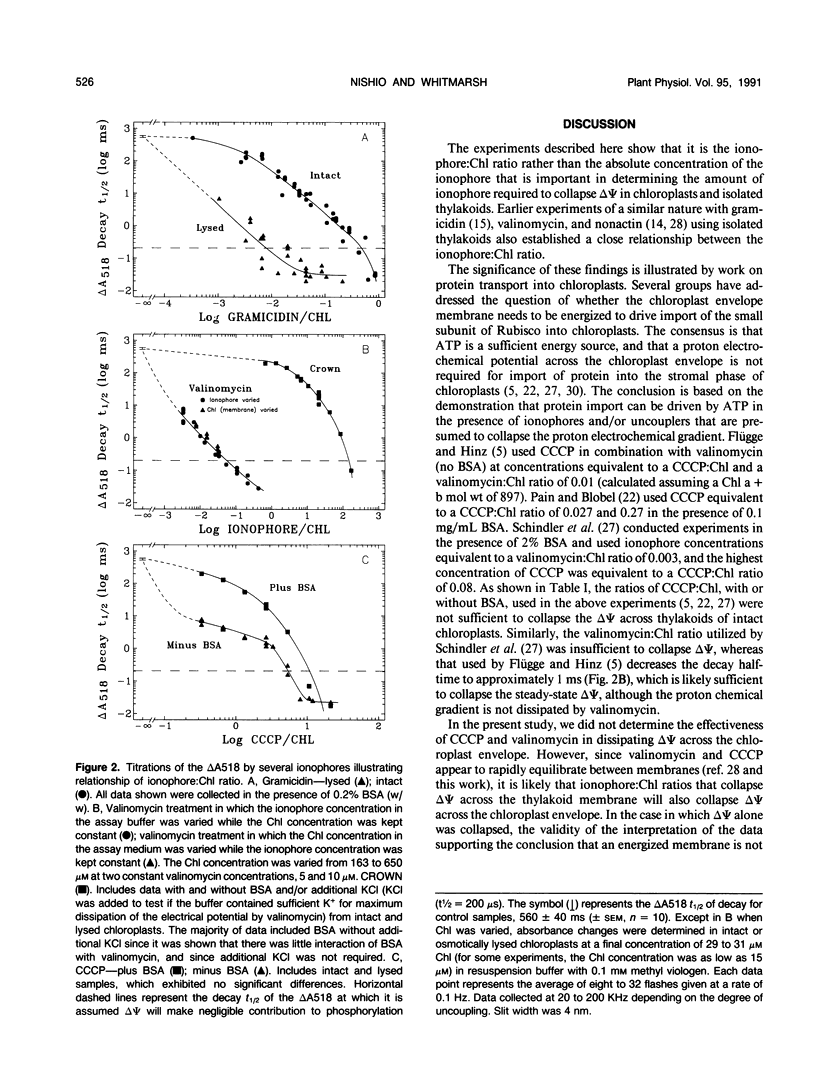
Effective ionophore:chlorophyll ratios were determined for various ionophores that decrease the electrical potential across thylakoid membranes in intact and hypo-osmotically lysed chloroplasts isolated from spinach (Spinacia oleracea). The efficacy of gramicidin D, valinomycin, carbonylcyanide m-chlorophenylhydrazone, and dicyclohexano-18-crown-6 in collapsing the electrical potential was determined spectrophotometrically by the decay half-time of the absorbance change at 518 nanometers induced by a saturating, single turnover flash. The results show that the effectiveness of the ionophores in collapsing the electrical potential in intact and lysed chloroplasts depends on the amount of ionophore-accessible membrane in the assay medium. Only gramicidin exhibited a significant difference in efficacy between intact and lysed chloroplasts. The ratio of gramicidin to chlorophyll required to collapse the electrical potential was more than 50 times higher in intact chloroplasts than in lysed chloroplasts. The efficacy of carbonylcyanide m-chlorophenylhydrazone was significantly reduced in the presence of bovine serum albumin. The other ionophores tested maintained their potency in the presence of bovine serum albumin. Valinomycin was the most effective ionophore tested for collapsing the electrical potential in intact chloroplasts, whereas gramicidin was the most potent ionophore in lysed chloroplasts. The significance of the ionophore:chlorophyll ratios required to collapse the electrical potential is discussed with regard to bioenergetic studies, especially those that examine the contribution of the transmembrane electrochemical potential to protein transport into chloroplasts.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- Cline K., Fulsom D. R., Viitanen P. V. An imported thylakoid protein accumulates in the stroma when insertion into thylakoids is inhibited. J Biol Chem. 1989 Aug 25;264(24):14225–14232. [PubMed] [Google Scholar]
- Dilley R. A., Schreiber U. Correlation between membrane-localized protons and flash-driven ATP formation in chloroplast thylakoids. J Bioenerg Biomembr. 1984 Jun;16(3):173–193. doi: 10.1007/BF00751048. [DOI] [PubMed] [Google Scholar]
- Flügge U. I., Hinz G. Energy dependence of protein translocation into chloroplasts. Eur J Biochem. 1986 Nov 3;160(3):563–570. doi: 10.1111/j.1432-1033.1986.tb10075.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W., Witt H. T. On the ion transport system of photosynthesis--investigations on a molecular level. Z Naturforsch B. 1968 Feb;23(2):244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Meadows J. W., Shackleton J. B., Musgrove J. E., Elderfield P. D., Mould R., Hay N. A., Robinson C. ATP-dependent import of a lumenal protein by isolated thylakoid vesicles. EMBO J. 1989 Aug;8(8):2251–2255. doi: 10.1002/j.1460-2075.1989.tb08349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. J., Whitmarsh J. Photosynthetic apparatus of pea thylakoid membranes : response to growth light intensity. Plant Physiol. 1989 Mar;89(3):932–940. doi: 10.1104/pp.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Dilley R. A. Photophosphorylation as a function of illumination time. I. Effects of permeant cations and permeant anions. Biochim Biophys Acta. 1976 Oct 13;449(1):95–107. doi: 10.1016/0005-2728(76)90010-4. [DOI] [PubMed] [Google Scholar]
- Pain D., Blobel G. Protein import into chloroplasts requires a chloroplast ATPase. Proc Natl Acad Sci U S A. 1987 May;84(10):3288–3292. doi: 10.1073/pnas.84.10.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of F1-ATPase subunit beta into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986 Dec 15;209(2):152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pick U., Weiss M., Rottenberg H. Anomalous uncoupling of photophosphorylation by palmitic acid and by gramicidin D. Biochemistry. 1987 Dec 15;26(25):8295–8302. doi: 10.1021/bi00399a041. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Ionophores. Methods Enzymol. 1979;55:435–454. doi: 10.1016/0076-6879(79)55058-7. [DOI] [PubMed] [Google Scholar]
- Roux B., Karplus M. The normal modes of the gramicidin-A dimer channel. Biophys J. 1988 Mar;53(3):297–309. doi: 10.1016/S0006-3495(88)83107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R., Junge W. Current-voltage studies on the thylakoid membrane in the presence of ionophores. Biochim Biophys Acta. 1975 Jun 11;394(1):76–92. doi: 10.1016/0005-2736(75)90206-0. [DOI] [PubMed] [Google Scholar]
- Theg S. M., Bauerle C., Olsen L. J., Selman B. R., Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989 Apr 25;264(12):6730–6736. [PubMed] [Google Scholar]
- Verner K., Schatz G. Import of an incompletely folded precursor protein into isolated mitochondria requires an energized inner membrane, but no added ATP. EMBO J. 1987 Aug;6(8):2449–2456. doi: 10.1002/j.1460-2075.1987.tb02524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


