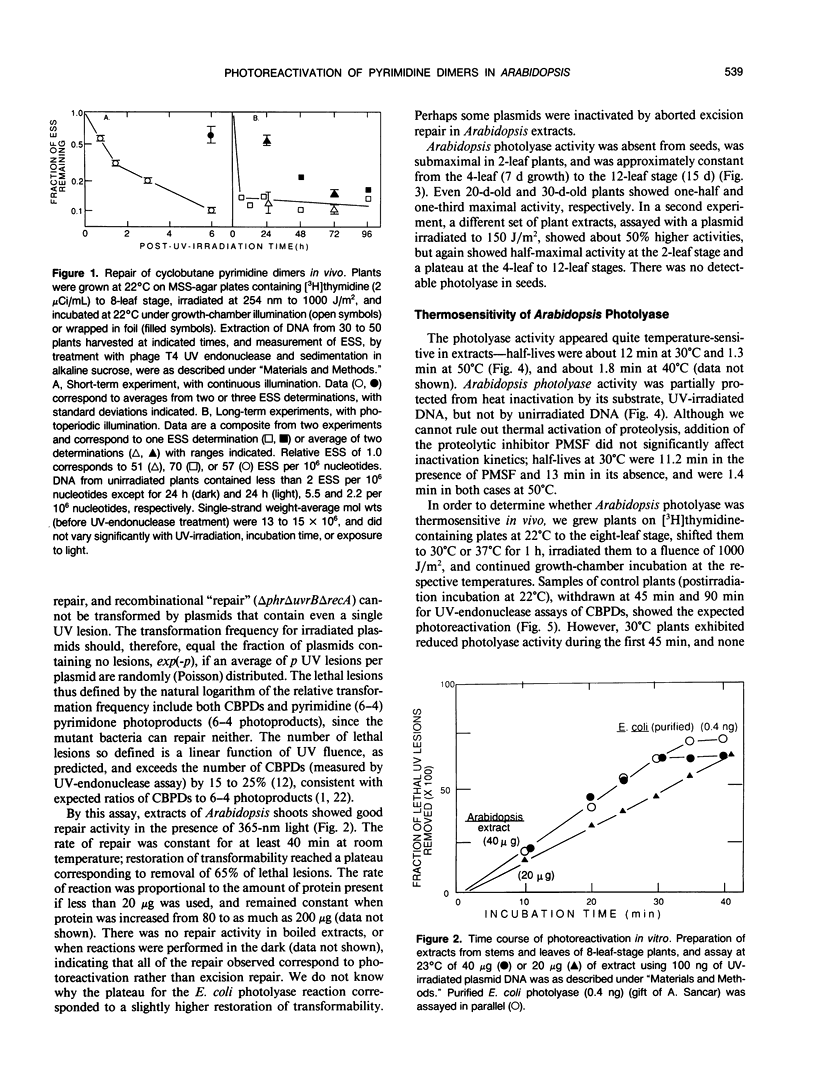
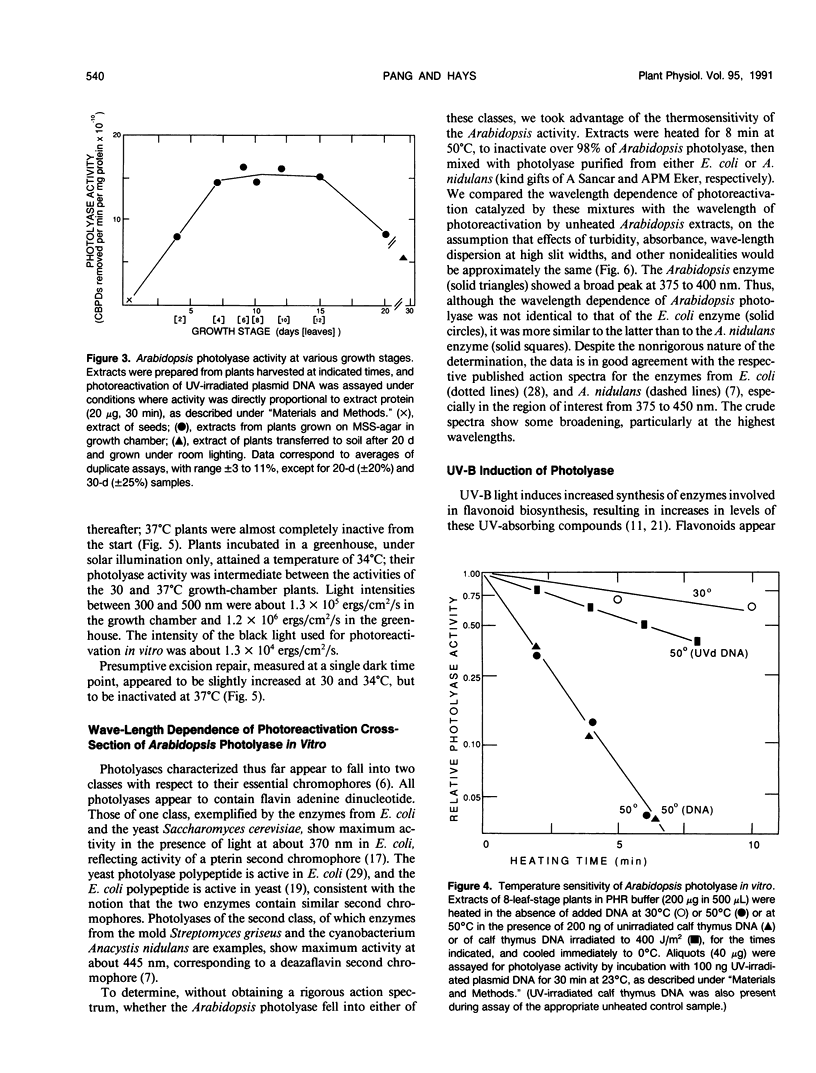
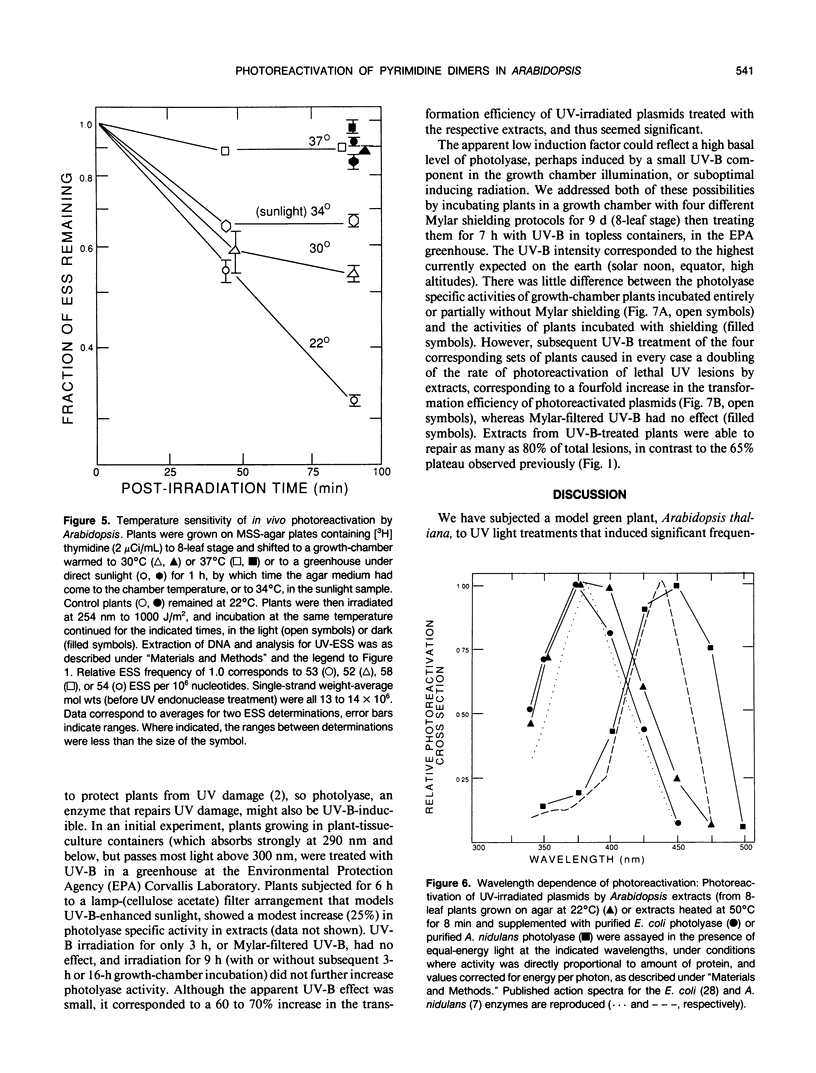
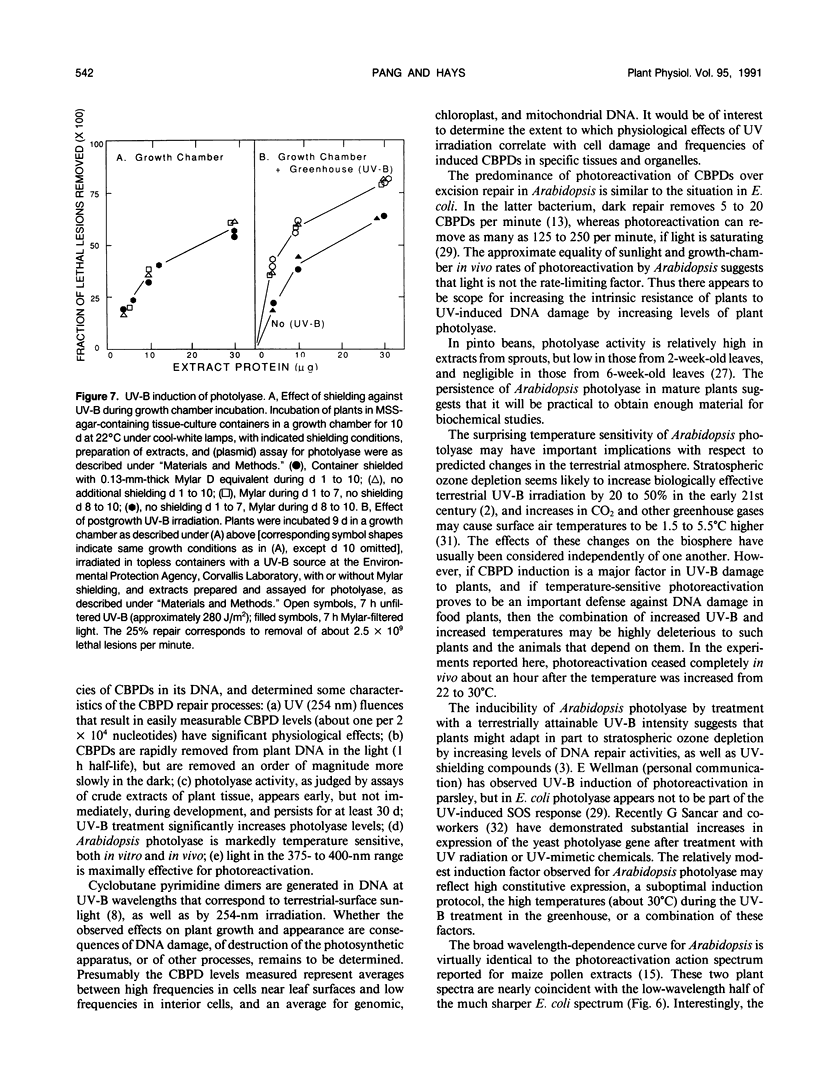
Abstract
Removal of cyclobutane pyrimidine dimers (CBPDs) in vivo from the DNA of UV-irradiated eight-leaf seedlings of Arabidopsis thaliana was rapid in the presence of visible light (half-life about 1 hour); removal of CBPDs in the dark, presumably via excision repair, was an order of magnitude slower. Extracts of plants contained significant photolyase in vitro, as assayed by restoration of transforming activity to UV-irradiated Escherichia coli plasmids; activity was maximal from four-leaf to 12-leaf stages. UV-B treatment of seedlings for 6 hours increased photolyase specific activity in extracts twofold. Arabidopsis photolyase was markedly temperature-sensitive, both in vitro (half-life at 30°C about 12 minutes) and in vivo (half-life at 30°C, 30 to 45 minutes). The wavelength dependency of the photoreactivation cross-section showed a broad peak at 375 to 400 nm, and is thus similar to that for maize pollen; it overlaps bacterial and yeast photolyase action spectra.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. L., Small G. D. Isolation of a photoreactivation-deficient mutant of Chlamydomonas. Mutat Res. 1985 Nov;146(3):249–255. doi: 10.1016/0167-8817(85)90065-3. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Kooiman P., Hessels J. K., Yasui A. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J Biol Chem. 1990 May 15;265(14):8009–8015. [PubMed] [Google Scholar]
- Ellison M. J., Childs J. D. Pyrimidine dimers induced in Escherichia coli DNA by ultraviolet radiation present in sunlight. Photochem Photobiol. 1981 Oct;34(4):465–469. [PubMed] [Google Scholar]
- Hahlbrock K., Knobloch K. H., Kreuzaler F., Potts J. R., Wellmann E. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1976 Jan 2;61(1):199–206. doi: 10.1111/j.1432-1033.1976.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Ackerman E. J., Pang Q. S. Rapid and apparently error-prone excision repair of nonreplicating UV-irradiated plasmids in Xenopus laevis oocytes. Mol Cell Biol. 1990 Jul;10(7):3505–3511. doi: 10.1128/mcb.10.7.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J. B., Martin S. J., Bhatia K. Repair of nonreplicating UV-irradiated DNA: cooperative dark repair by Escherichia coli uvr and phr functions. J Bacteriol. 1985 Feb;161(2):602–608. doi: 10.1128/jb.161.2.602-608.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland G. P. Dark-repair of ultraviolet-induced pyrimidine dimers in the DNA of wild carrot protoplasts. Nature. 1975 Mar 13;254(5496):160–161. doi: 10.1038/254160a0. [DOI] [PubMed] [Google Scholar]
- Jorns M. S., Sancar G. B., Sancar A. Identification of a neutral flavin radical and characterization of a second chromophore in Escherichia coli DNA photolyase. Biochemistry. 1984 Jun 5;23(12):2673–2679. doi: 10.1021/bi00307a021. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., Yasui A., Eker A. P. Expression of an Escherichia coli phr gene in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1985;199(3):396–400. doi: 10.1007/BF00330748. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Rosen H., Rehn M. M., Johnson B. A. The effect of caffeine on repair in Chlamydomonas reinhardtii. I. Enhancement of recombination repair. Mutat Res. 1980 May;70(3):301–309. doi: 10.1016/0027-5107(80)90020-2. [DOI] [PubMed] [Google Scholar]
- Saito N., Werbin H. Evidence for a DNA-photoreactivating enzyme in higher plants. Photochem Photobiol. 1969 Apr;9(4):389–393. doi: 10.1111/j.1751-1097.1969.tb07304.x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sancar G. B., Jorns M. S., Payne G., Fluke D. J., Rupert C. S., Sancar A. Action mechanism of Escherichia coli DNA photolyase. III. Photolysis of the enzyme-substrate complex and the absolute action spectrum. J Biol Chem. 1987 Jan 5;262(1):492–498. [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W., Heelis P. F. Purification of the yeast PHR1 photolyase from an Escherichia coli overproducing strain and characterization of the intrinsic chromophores of the enzyme. J Biol Chem. 1987 Nov 15;262(32):15457–15465. [PubMed] [Google Scholar]
- Schneider S. H. The greenhouse effect: science and policy. Science. 1989 Feb 10;243(4892):771–781. doi: 10.1126/science.243.4892.771. [DOI] [PubMed] [Google Scholar]
- Sebastian J., Kraus B., Sancar G. B. Expression of the yeast PHR1 gene is induced by DNA-damaging agents. Mol Cell Biol. 1990 Sep;10(9):4630–4637. doi: 10.1128/mcb.10.9.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko J. E., Mansour V. H. Photoreactivation of ultraviolet light-induced pyrimidine dimers in Ginkgo cells grown in vitro. Mutat Res. 1969 Jan-Feb;7(1):120–121. doi: 10.1016/0027-5107(69)90056-6. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Mansour V. H. Response of tobacco and Haplopappus cells to ultraviolet irradiation after posttreatment with photoreactivating light. Radiat Res. 1968 Nov;36(2):333–343. [PubMed] [Google Scholar]


