Abstract
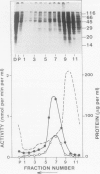
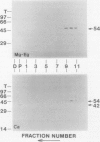
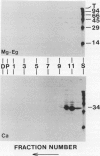
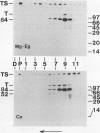
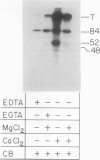
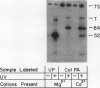
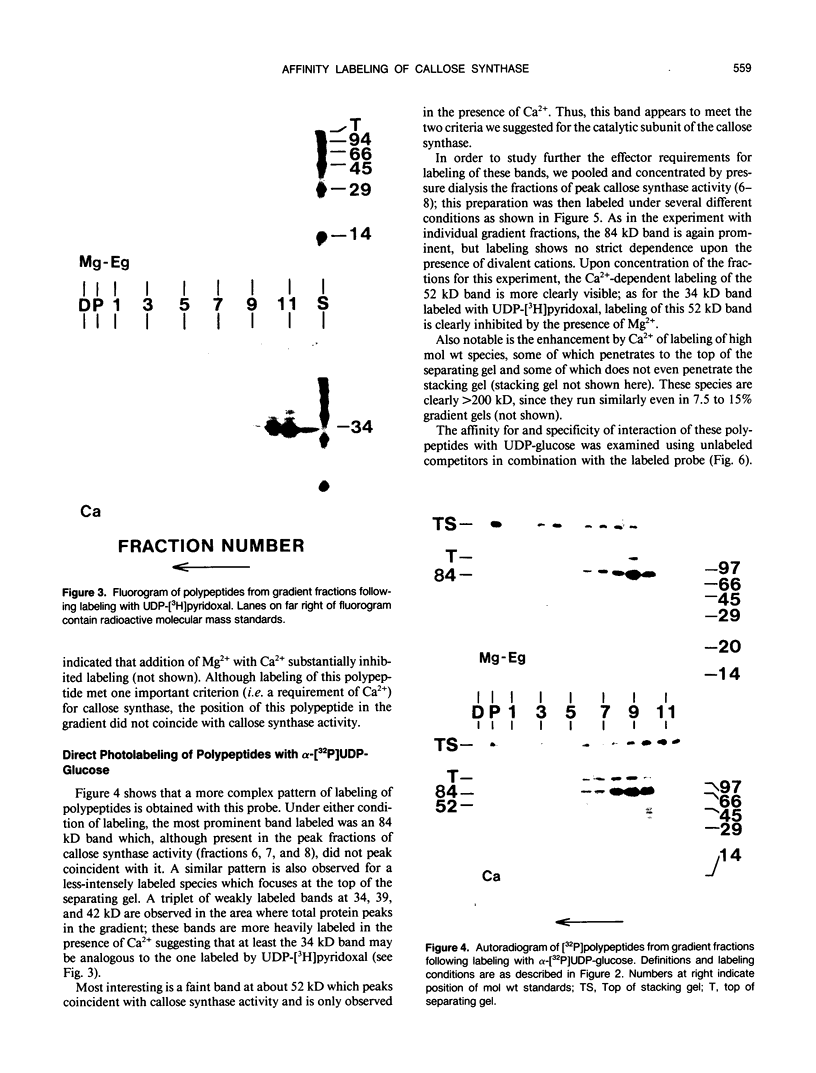
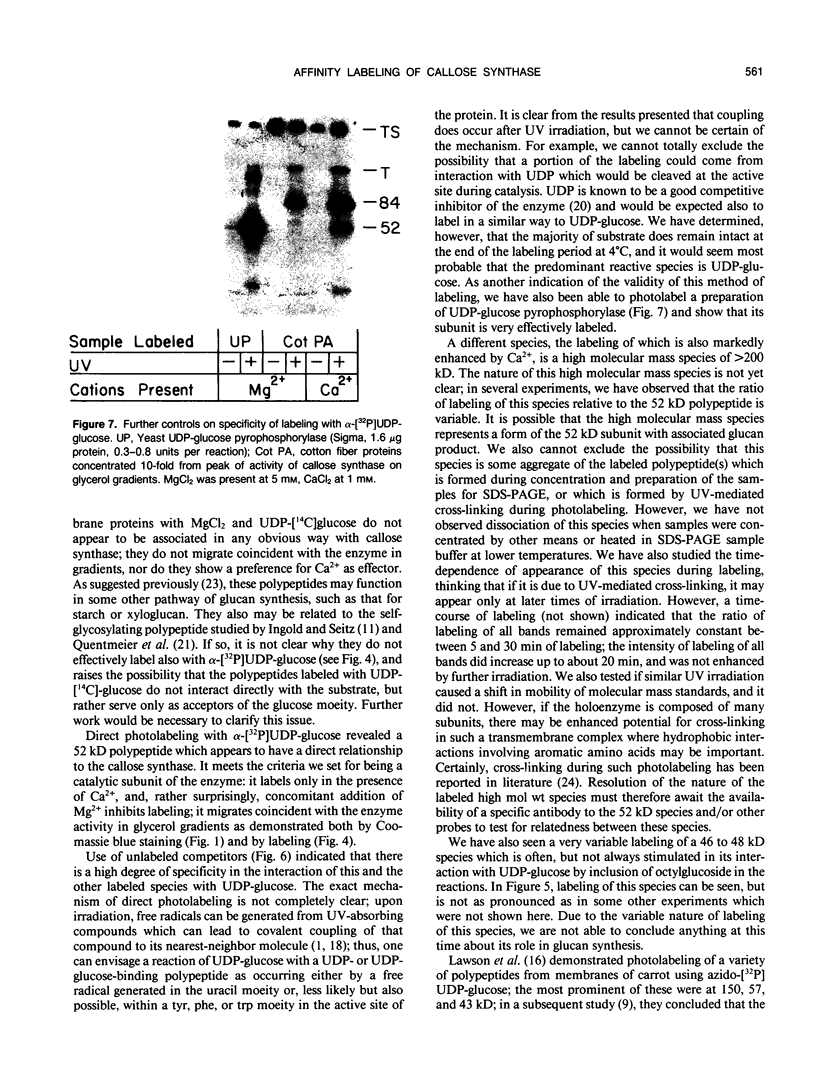
We have identified a 52 kilodalton polypeptide as being a likely candidate for the catalytic subunit of the UDP-glucose: (1→3)-β-glucan (callose) synthase of developing fibers of Gossypium hirsutum (cotton). Such a polypeptide migrates coincident with callose synthase during glycerol gradient centrifugation in the presence of EDTA, and can be directly photolabeled with the radioactive substrate, α-[32P]UDP-glucose. Interaction with the labeled probe requires Ca2+, a specific activator of callose synthase which is known to lower the Km of higher plant callose synthases for the substrate UDP-glucose. Using this probe and several other related ones, several other proteins which interact with UDP-glucose were also identified, but none satisfied all of the above criteria for being components of the callose synthase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonoff R. S., Obrig T., Ferguson J. J., Jr Direct photoaffinity labeling with cyclic nucleotides. Methods Enzymol. 1977;46:335–339. doi: 10.1016/s0076-6879(77)46038-5. [DOI] [PubMed] [Google Scholar]
- Callaghan T., Ross P., Weinberger-Ohana P., Benziman M. beta-Glucoside Activators of Mung Bean UDP-Glucose: beta-Glucan Synthase : II. Comparison of Effects of an Endogenous beta-Linked Glucolipid with Synthetic n-Alkyl beta-d-Monoglucopyranosides. Plant Physiol. 1988 Apr;86(4):1104–1107. doi: 10.1104/pp.86.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R. R., Jr, Evans R. K., Wolf M. J., Haley B. E. Synthesis and properties of 5-azido-UDP-glucose. Development of photoaffinity probes for nucleotide diphosphate sugar binding sites. J Biol Chem. 1989 Jul 15;264(20):11928–11933. [PubMed] [Google Scholar]
- Frost D. J., Read S. M., Drake R. R., Haley B. E., Wasserman B. P. Identification of the UDP-glucose-binding polypeptide of callose synthase from Beta vulgaris L. by photoaffinity labeling with 5-azido-UDP-glucose. J Biol Chem. 1990 Feb 5;265(4):2162–2167. [PubMed] [Google Scholar]
- Hayashi T., Read S. M., Bussell J., Thelen M., Lin F. C., Brown R. M., Delmer D. P. UDP-Glucose: (1-->3)-beta-Glucan Synthases from Mung Bean and Cotton: Differential Effects of Ca and Mg on Enzyme Properties and on Macromolecular Structure of the Glucan Product. Plant Physiol. 1987 Apr;83(4):1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. R., Northcote D. H. In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in determining the type of linkage formed. J Cell Sci Suppl. 1985;2:1–11. doi: 10.1242/jcs.1985.supplement_2.1. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Elango N., Mattia E., Au-Young J., Robbins P. W., Cabib E. Isolation of chitin synthetase from Saccharomyces cerevisiae. Purification of an enzyme by entrapment in the reaction product. J Biol Chem. 1984 Dec 10;259(23):14966–14972. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson S. G., Mason T. L., Sabin R. D., Sloan M. E., Drake R. R., Haley B. E., Wasserman B. P. UDP-Glucose: (1,3)-beta-Glucan Synthase from Daucus carota L. : Characterization, Photoaffinity Labeling, and Solubilization. Plant Physiol. 1989 May;90(1):101–108. doi: 10.1104/pp.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. C., Brown R. M., Jr, Drake R. R., Jr, Haley B. E. Identification of the uridine 5'-diphosphoglucose (UDP-Glc) binding subunit of cellulose synthase in Acetobacter xylinum using the photoaffinity probe 5-azido-UDP-Glc. J Biol Chem. 1990 Mar 25;265(9):4782–4784. [PubMed] [Google Scholar]
- Martyr R. J., Benisek W. F. Affinity labeling of the active sites of delta 5 -ketosteroid isomerase using photoexcited natural ligands. Biochemistry. 1973 May 22;12(11):2172–2178. doi: 10.1021/bi00735a025. [DOI] [PubMed] [Google Scholar]
- Morrow D. L., Lucas W. J. (1-->3)-beta-d-Glucan Synthase from Sugar Beet : I. Isolation and Solubilization. Plant Physiol. 1986 May;81(1):171–176. doi: 10.1104/pp.81.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Delmer D. P. Inhibition of Mung Bean UDP-Glucose: (1-->3)-beta-Glucan Synthase by UDP-Pyridoxal: Evidence for an Active-Site Amino Group. Plant Physiol. 1987 Dec;85(4):1008–1015. doi: 10.1104/pp.85.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop S. D., Charbonneau H., Beavo J. A. Direct photolabeling of the cGMP-stimulated cyclic nucleotide phosphodiesterase. J Biol Chem. 1989 Aug 15;264(23):13718–13725. [PubMed] [Google Scholar]
- Tagaya M., Nakano K., Fukui T. A new affinity labeling reagent for the active site of glycogen synthase. Uridine diphosphopyridoxal. J Biol Chem. 1985 Jun 10;260(11):6670–6676. [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]