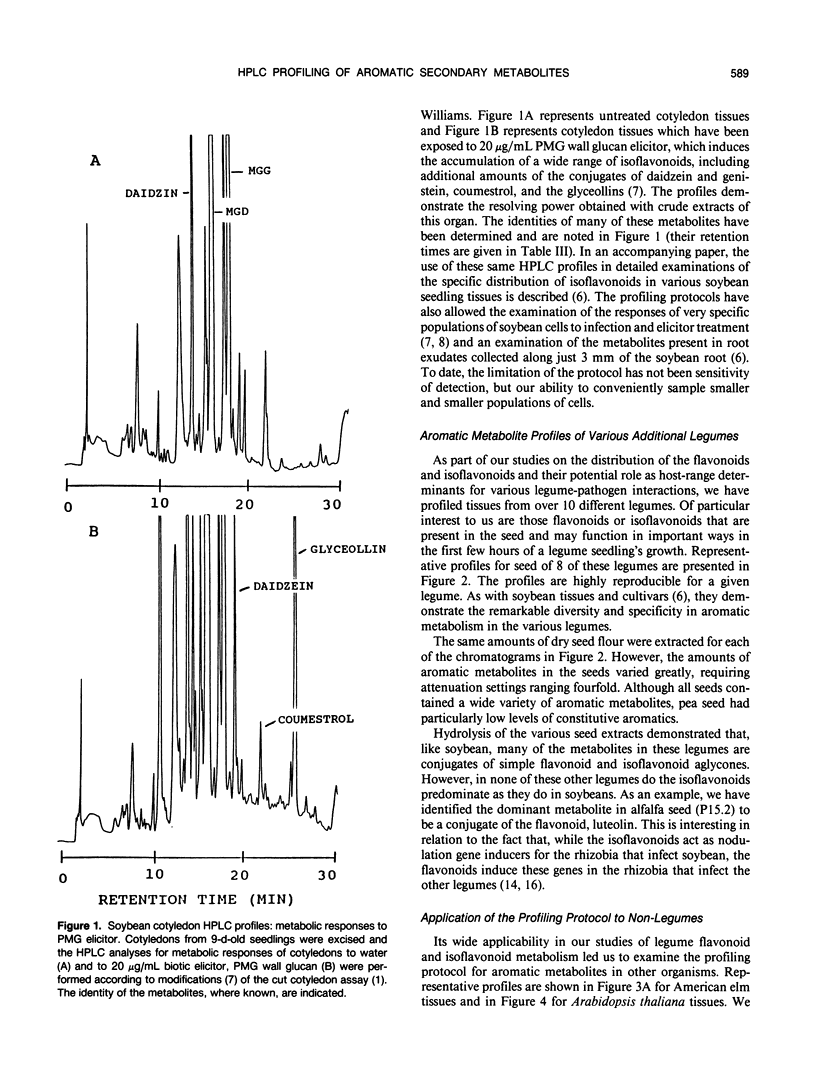
Abstract
High performance liquid chromatography protocols have been developed to allow the simultaneous analysis of a very wide range of soluble aromatic secondary metabolites in unfractionated biological extracts. The methods are simple, sensitive, and highly reproducible. They are applicable to a wide variety of natural product investigations in both plants and microorganisms. High resolution of metabolites is achieved in 25 minutes by chromatography on a reverse phase C18 column in a gradient of 0 to 55% acetonitrile in water at pH 3. For example, near-baseline resolution of over 20 phenylpropanoid metabolites and 18 naturally occurring metabolites of indole-3-acetic acid can be obtained. The methods can be applied directly to whole tissue extracts without prepurification or enrichment. Moreover, the simplicity and sensitivity of the protocols allow their application to a large number of very small tissue samples, such as those encountered in research on host-microbe interactions. Such profiles allow one to monitor simultaneously the various alternative metabolic fates of a complex array of molecules. Examination of the profiles over time thus provides one with a powerful tool to correlate many concurrent molecular events that may relate to a given biological phenomenon. The final protocol requires as little as 1 milligram of tissue, which is extracted directly in a microfuge tube in 80% ethanol. With a variable wavelength detector, as little as 100 femtomoles of a given metabolite can be analyzed. Examples of the application of the protocols to a number of plant and microbial secondary product investigations and to screening for flavonoid mutants of Arabidopsis thaliana (L.) Heynh. are given.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Osman S. F., Dunn M. F. Auxin production by plant-pathogenic pseudomonads and xanthomonads. Appl Environ Microbiol. 1987 Aug;53(8):1839–1845. doi: 10.1128/aem.53.8.1839-1845.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991 Feb;95(2):594–603. doi: 10.1104/pp.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Verma D. P. Phenolic compounds as regulators of gene expression in plant-microbe relations. Mol Plant Microbe Interact. 1990 Jan-Feb;3(1):4–8. doi: 10.1094/mpmi-3-004. [DOI] [PubMed] [Google Scholar]


