Abstract
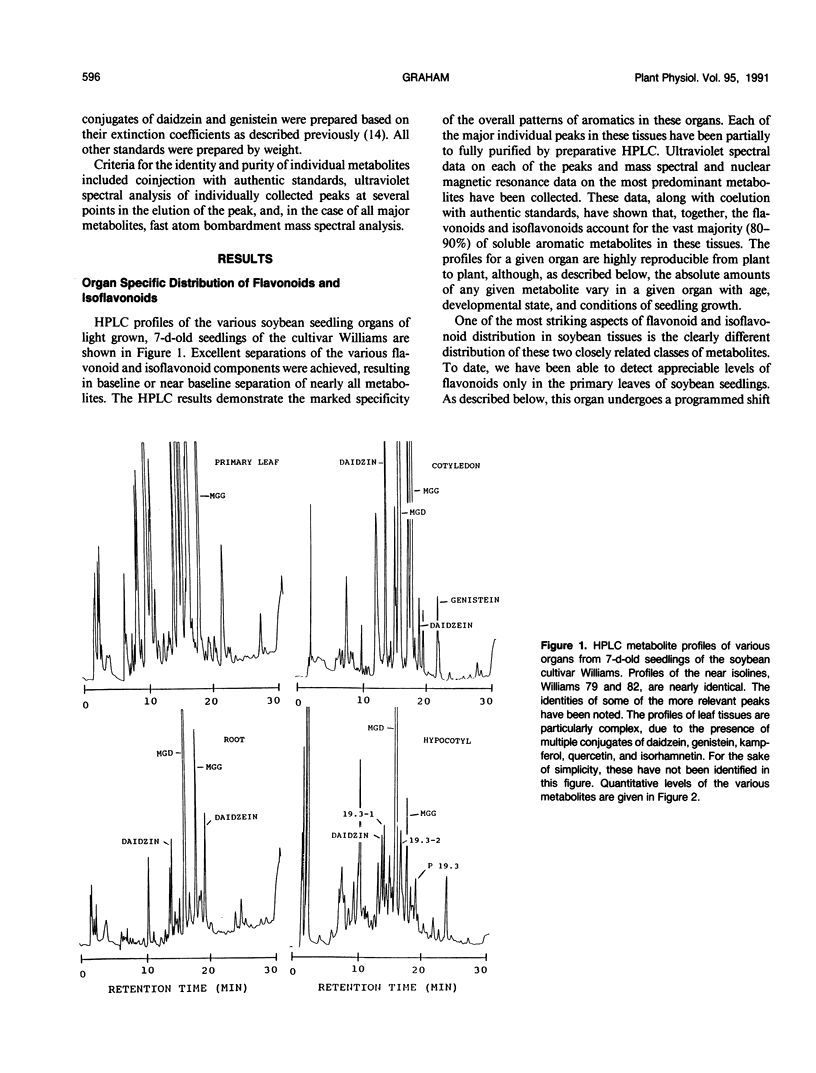
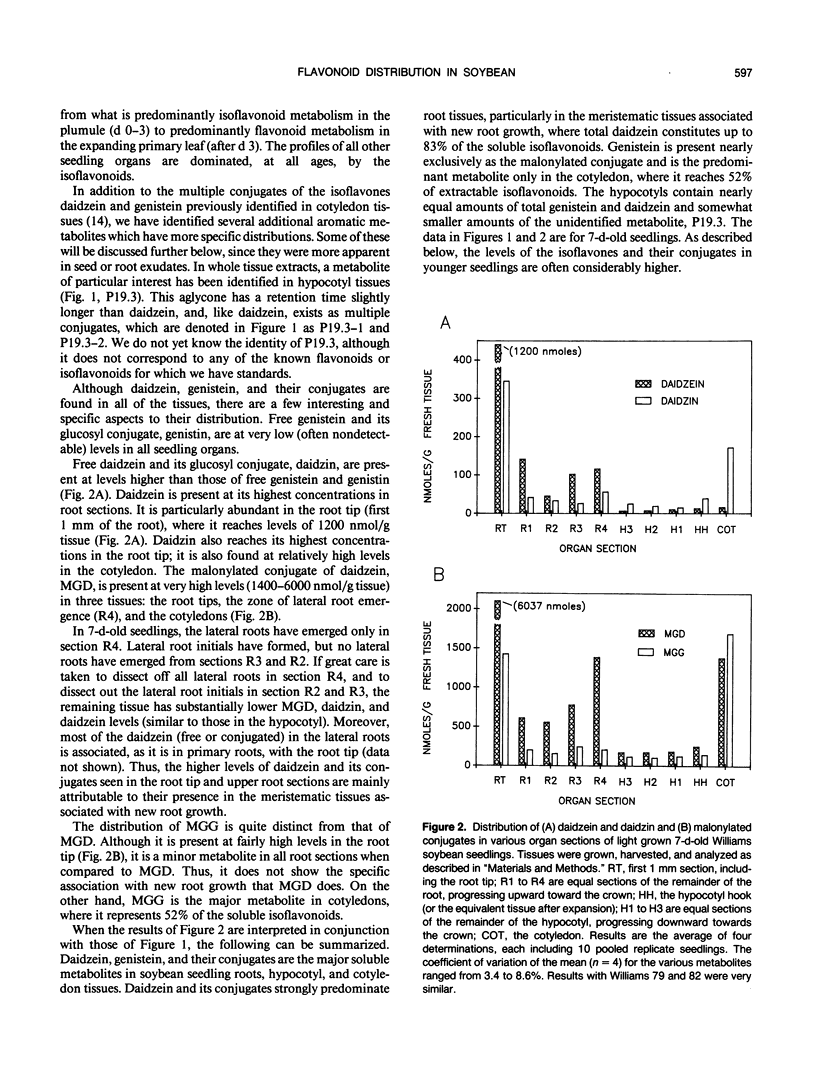
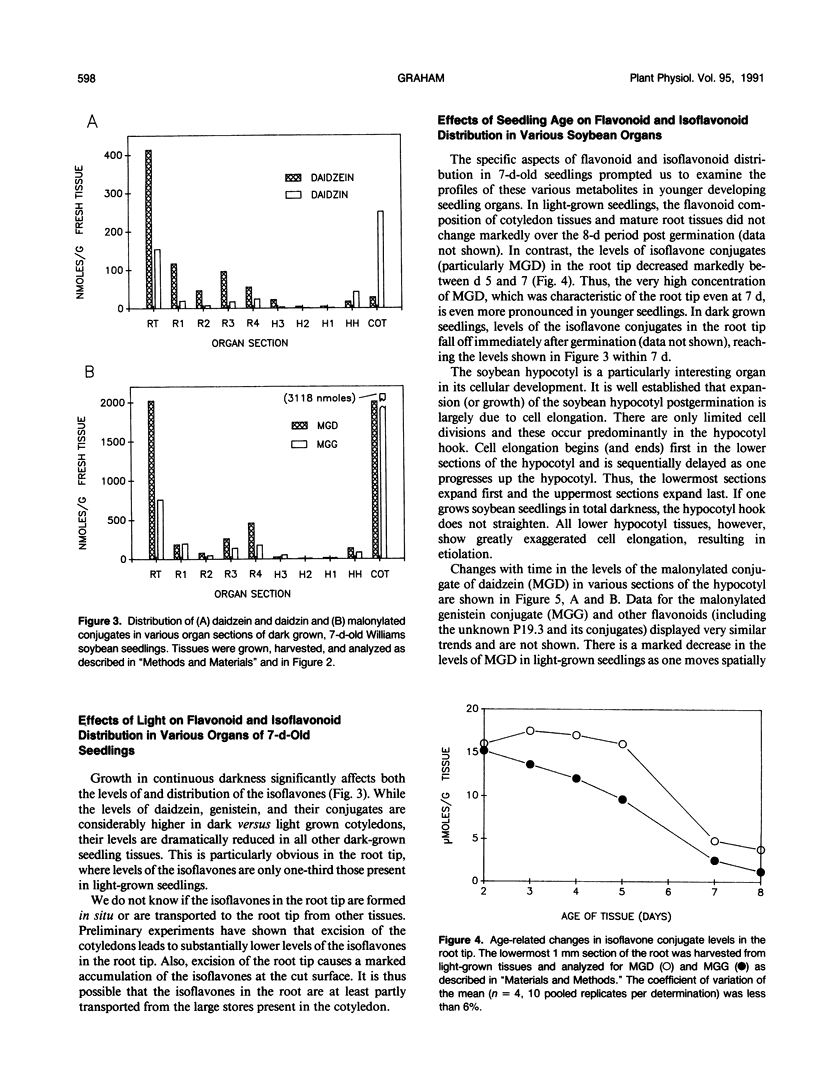
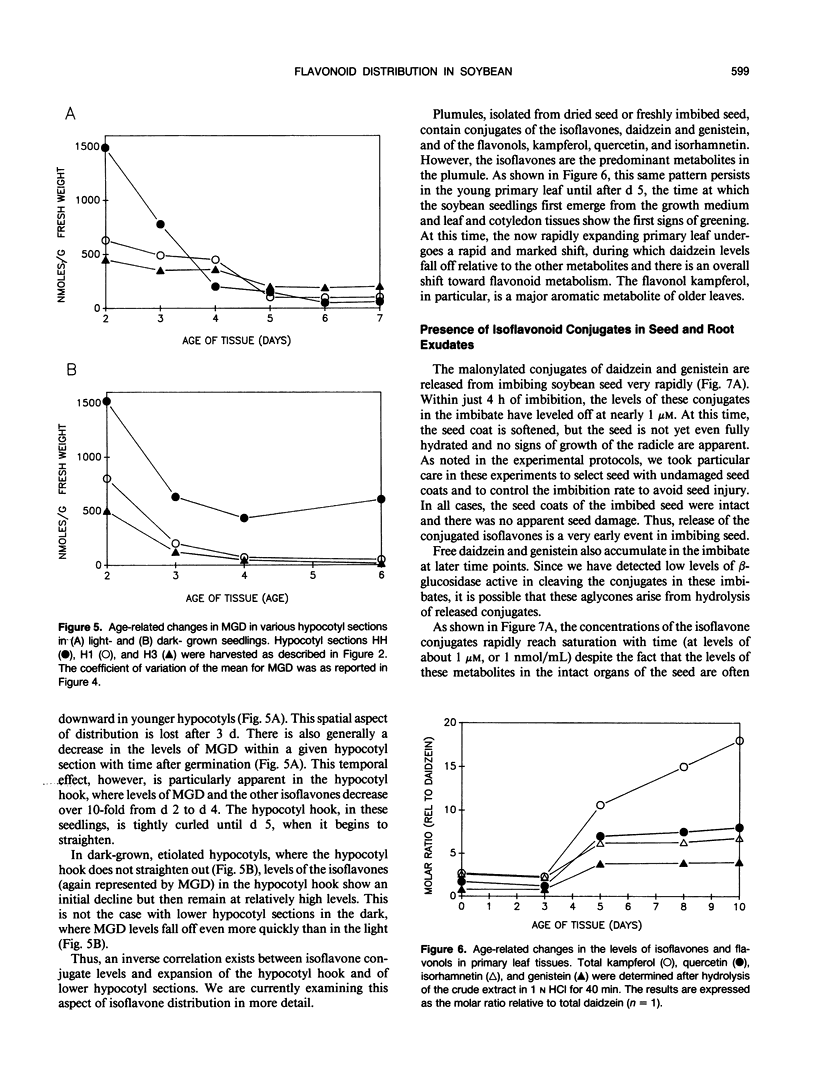
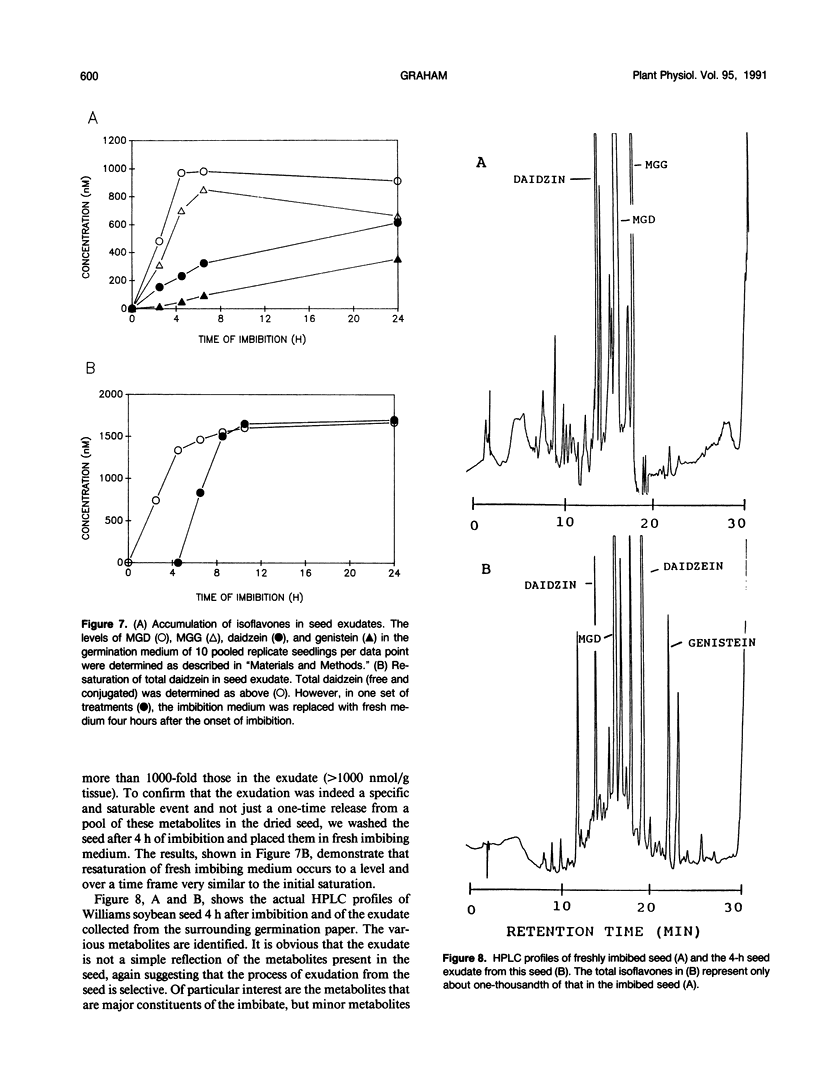
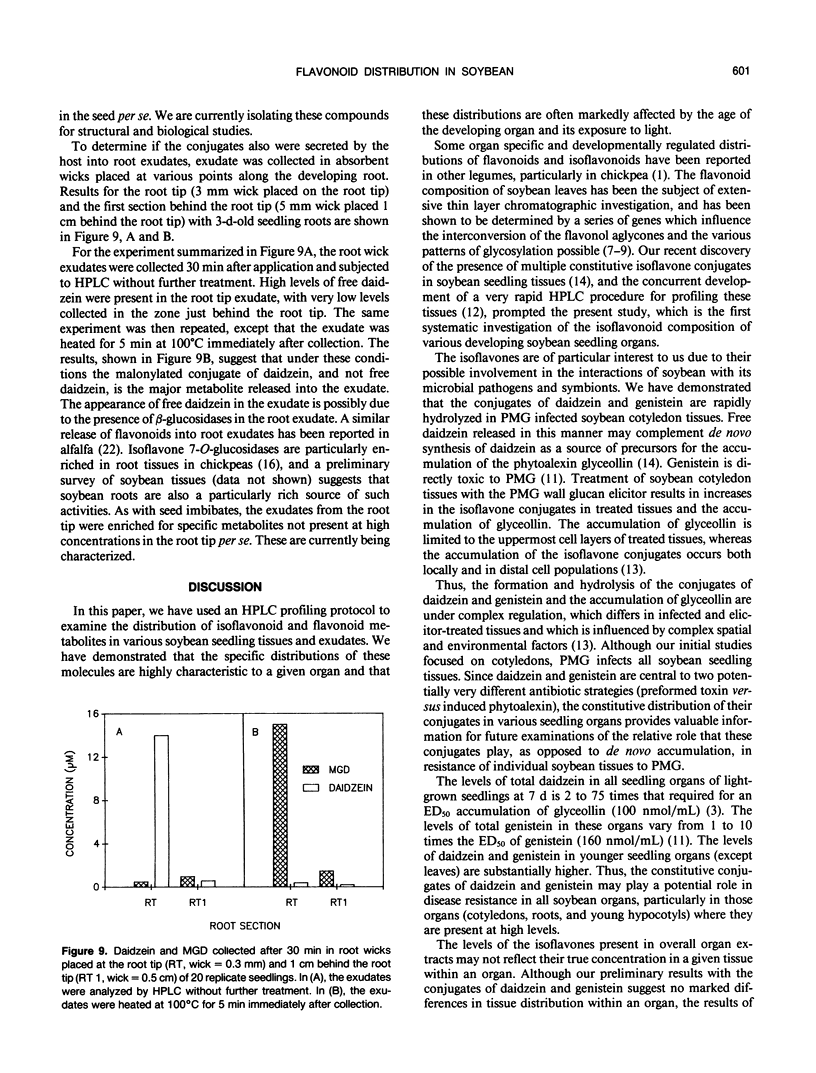
The distribution of flavonoids, isoflavonoids, and their conjugates in developing soybean (Glycine max L.) seedling organs and in root and seed exudates has been examined. Conjugates of the isoflavones daidzein and genistein are major metabolites in all embryonic organs within the dry seed and in seedling roots, hypocotyl, and cotyledon tissues at all times after germination. Primary leaf tissues undergo a programmed shift from isoflavonoid to flavonoid metabolism 3 days after germination and become largely predominated by glycosides of the flavonols kampferol, quercetin, and isorhamnetin by 5 days. Cotyledons contain relatively constant and very high levels of conjugates of both daidzein and genistein. Hypocotyl tissues contain a third unidentified compound, P19.3, also present in multiple conjugated forms. Conjugates of daidzein, genistein, and P19.3 are at their highest levels in the hypocotyl hook and fall off progressively down the hypocotyl. These isoflavones also undergo a programmed and dramatic decrease between 2 and 4 days in the hypocotyl hook. All root sections are predominated by daidzein and its conjugates, particularly in the root tip, where they reach the highest levels in the seedling. Light has a pronounced effect on the distribution of the isoflavones; in the dark, isoflavone levels in the root tips are greatly reduced, while those in the cotyledons are higher. Finally, the conjugates of daidzein and genistein and several unidentified aromatic metabolites are selectively excreted into root and seed exudates. Analysis of seed exudates suggests that this is a continuous, but saturable event.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell R. I., Buttery B. R. Inheritance of flavonol glycosides in soybeans. Can J Genet Cytol. 1973 Dec;15(4):865–867. doi: 10.1139/g73-101. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Crist-Estes D. K., Bauer W. D. Chemotaxis of Rhizobium meliloti to the plant flavone luteolin requires functional nodulation genes. J Bacteriol. 1988 Jul;170(7):3164–3169. doi: 10.1128/jb.170.7.3164-3169.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. L. A rapid, high resolution high performance liquid chromatography profiling procedure for plant and microbial aromatic secondary metabolites. Plant Physiol. 1991 Feb;95(2):584–593. doi: 10.1104/pp.95.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Bonhoff A., Grisebach H. Quantitative Localization of the Phytoalexin Glyceollin I in Relation to Fungal Hyphae in Soybean Roots Infected with Phytophthora megasperma f. sp. glycinea. Plant Physiol. 1985 Mar;77(3):591–601. doi: 10.1104/pp.77.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hósel W., Barz W. Beta-Glucosidases from Cicer arietinum L. Purification and Properties of isoflavone-7-O-glucoside-specific beta-glucosidases. Eur J Biochem. 1975 Sep 15;57(2):607–616. doi: 10.1111/j.1432-1033.1975.tb02336.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Verma D. P. Phenolic compounds as regulators of gene expression in plant-microbe relations. Mol Plant Microbe Interact. 1990 Jan-Feb;3(1):4–8. doi: 10.1094/mpmi-3-004. [DOI] [PubMed] [Google Scholar]


