Abstract
Endocrine-disrupting chemicals (EDCs) are a class of exogenous substances that mimic the effects of hormones in the body, inducing hormonal dysregulation and contributing to various disorders. Epigenome regulation has emerged as an important mechanism for maintaining organ function in health and disease. Dissecting epigenomic and resultant gene expression changes provides unprecedented insight into the chromatin state, which underlines disease development and shapes risk and phenotypic plasticity in response to the environment and internal cues. The cutting-edge, high throughput technologies provide new routes to understanding the etiology of disease and new footholds on the promising path to better treatment and disease prevention. We have recently revealed that myometrial stem cells (MMSCs), the cell origin of UFs, are the target of developmental EDC exposure. The EDC-induced epigenetic changes in MMSCs identified by multi-omics approaches include DNA methylation and histone modification modulated by DNA methyltransferases and MLL1, which characterized the molecular mechanism underlying EDC-related risk in hormone-dependent UFs. Future studies are needed to determine the link between real-life exposures to EDCs and their impact on the development of human diseases and transgenerational epigenetic inheritance, which can help explore strategies that may prevent adverse outcomes linked to EDC exposure.
Keywords: Leiomyoma, endocrine-disrupting chemicals, developmental reprogramming, myometrial stem cells, hormone-dependent tumor
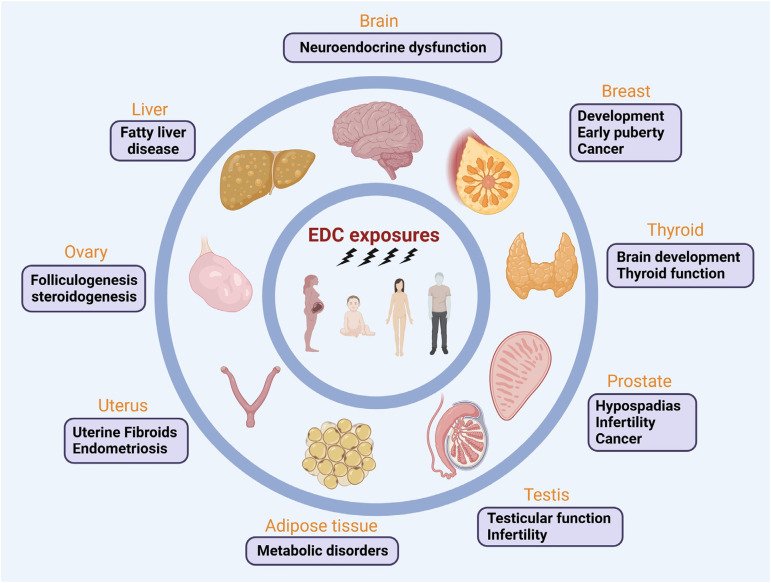
Endocrine-disrupting chemicals (EDCs) are a class of exogenous chemicals that mimic, block, or interfere with the hormones in the endocrine system, leading to various health issues. EDCs can intake by ingestion, inhalation, and absorption, and affect hormonal function in numerous ways, including targeting hormone receptors, acting on signal transduction in hormone-responsive cells, among others. 1 Humans are exposed to many EDCs daily. Epidemiological and experimental studies prove that EDC exposures adversely impact many tissues, leading to the onset of diverse diseases (Figure 1).
Figure 1.
EDCs: a promoter of diseases targeting multiple tissues. Growing evidence reveals that EDC exposures significantly impact on human health, targeting numerous tissues, including prostate, breast, liver, thyroid, adipose tissue, brain, ovary, testis, and uterus. Developmental exposures to EDCs have more negative impacts than adult exposure since organs during development are susceptible to adverse environmental exposures. EDCs can disrupt normal development patterns and alter disease susceptibility via various hormone axes and relevant pathways. Remarkably, developmental exposures to EDCs have been shown to have lifelong ramifications and even cause transgenerational effects on male and female reproduction and other diseases.
Uterine Fibroids (UFs) are the most common tumors of reproductive age women. The importance of impacting this disease cannot be overstated, as these tumors constitute a major health and financial burden: UF-related costs in the US alone are over $34 billion/year. UFs are also a public health and health disparity challenge, as they are more common in women of color. 2 An increasing body of evidence supports the hypothesis that these tumors arise from aberrant stem cells in the myometrial smooth muscle compartment of the uterus. 2 The myometrium undergoes dynamic changes beginning with its initial expansion during puberty, to fluctuations in proliferation and apoptosis during cyclical hormonal alterations, and ultimately to the robust expansion and terminal differentiation during pregnancy and involution during puerperium. To retain its capacity to meet the demands of regenerative cycles in monthly menstrual cycles and particularly for successive pregnancy and puerperia, the myometrium must maintain a reservoir of stem cells. Identifying and elucidating the biology of these stem cells have great significance for understanding normal myometrial function, as well as diseases such as UFs. 2 However, despite the remarkable advances in defining the cellular hierarchy in other hormonally regulated tissues, such as the breast 3 and prostate,4,5 little is known about the molecular mechanism underlying the cellular origin and initial steps in UF tumorigenesis.
Several studies demonstrated the existence of putative somatic stem cells in the mouse and human myometrium.6,7 The stem cells were also isolated from UFs participating in tumor formation (referred to as TICs = tumor-initiating cells). However, TICs may have undergone significant genomic and epigenomic changes relative to normal myometrial stem cells, making it difficult to rationalize using TICs to investigate factors influencing origin, predisposition, and risk for this disease. Additionally, human studies that address environmental exposures are limited in feasibility by ethical concerns for participant's safety. To overcome these limitations, we utilized Eker rats, which carry a germ-line mutation in the tuberous sclerosis complex 2 (Tsc2) tumor suppressor gene and are susceptible to developing UFs 8 to characterize the potential gene-environment interactions. It has been shown that early-life exposure to EDC (diethylstilbestrol) during the development of the uterus increased tumor-suppressor-gene penetrance from 65% to >90% and tumor multiplicity and size in Eker rats. 8
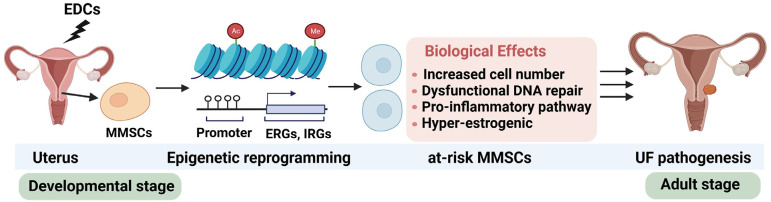
Although the role and relevance of epigenetics have been studied and reported in UFs, virtually nothing is known about epigenetic alterations in myometrial stem cells (MMSCs) that participate in the initiation of UF tumorigenesis or whether epigenetic signatures are associated with specific risk factors for this disease, such as environmental EDC exposures. Furthermore, few studies have utilized genome-wide epigenomic profiling methods to address fundamental questions about the etiology of common gynecologic disorders such as UFs. In a recently published paper, 1 we address the issue of how early-life EDC exposure increased the risk of UF development using the Eker rat model. We demonstrated that single EDC (diethylstilbestrol) exposure directly targeted MMSCs from the uterus and increased the risk of UF pathogenesis via reprogramming the MMSC epigenome toward the pro-fibroid landscape. The reprogrammed genes in MMSCs are known as estrogen-responsive genes (ERGs) and are activated by mixed lineage leukemia protein-1 (MLL1) and DNA hypo-methylation mechanisms. Additionally, we observed a marked elevation in the expression of ERGs in MMSCs from Eker rats exposed to natural steroids after developmental exposure to EDC, thereby augmenting estrogen activity. 1 Moreover, we investigated the paracrine effect of EDC-exposed MMSCs on myometrial cells. The secretome from the EDC-exposed MMSCs can enhance the myometrial cell proliferation compared to that of vehicle-exposed MMSCs, concurring with the activation of the β-catenin pathway. 1 These studies indicate that developmental exposure to EDC epigenetically targets MMSCs and leads to persistent changes in the expression of a subset of ERGs, imparting a hormonal imprint on the ERGs, resulting in a “hyper-estrogenic” phenotype, and increasing the hormone-dependent risk of UFs. Therefore, we incorporated the unique biology of reprogrammed MMSCs into our understanding of UF pathogenesis linked to EDC exposure. Notably, our previous and recent studies also demonstrated that single EDC exposure can increase the number of MMSCs, 9 decrease the DNA repair capacity,10–12 and activate the pro-inflammatory pathway, 13 indicating that developmental EDC exposure altered the MMSC characteristics in an orchestrated manner (Figure 2).
Figure 2.
Developmental exposure to EDCs can lead to an increased risk of UF development. MMSCs in the myometrium of the uterus are the cellular target for EDC. Epigenetic reprogramming can alter the MMSC characteristics. EDC-induced adverse effects on MMSCs include increased cell number, inflammation activation, DNA repair dysfunction, and hyper-estrogenic phenotype. Ac: acetylation modification on histones; EDCs: endocrine-disrupting chemicals; ERGs: estrogen-responsive genes; IRGs: inflammatory responsive genes; Me: methylation modification on histones; MMSCs: myometrial stem cells; UF: uterine fibroids.
Although the use of diethylstilbestrol was banned in the late 1970s, it was considered a representative example of EDC that causes adverse health outcomes by interfering with the hormone systems in the experimental setting. In UFs, several other EDCs, including phthalates, are associated with UF pathogenesis.14,15 Notably, the human population is exposed to EDC cocktails that co-exist in the environment, referring to the intricate reality of modern life, where individuals encounter a multitude of EDCs simultaneously. This simultaneous exposure results from a variety of sources, including pollutants in the air, food, water, personal care products, and industrial processes. These EDCs represent a mix of different chemicals with varying mechanisms of action and potency. The presence of multiple EDCs in human body tissues indicates that the adverse effects of EDC mixtures are not hypothetical but rather a pressing concern. When these various EDCs interact within the human body, they can lead to additive or even synergistic effects, compounding the disruption of the endocrine system. Understanding these complex interactions is crucial for evaluating the health risks associated with EDC exposure. Moreover, it's essential to consider various factors when studying EDCs. These factors include the timing of exposure, the duration of exposure, dosage levels, the route of exposure (e.g., oral, dermal, inhalation), and the selection of experimental models (e.g., animal models or in vitro systems). These considerations help researchers create more accurate and applicable models for studying the effects of EDCs, reflecting the complexity of real-world exposure scenarios.
Moreover, studies have shown that EDC exposure may cause transgenerational effects on reproduction in both males and females. This means that the adverse health effects of EDCs may extend beyond the exposed generation and affect the health of offspring. Understanding the molecular mechanisms underlying these transgenerational effects is crucial for identifying and mitigating the long-term health risks associated with EDC exposure. To unravel the intricate web of EDC exposure, cutting-edge technologies and omics tools (such as genomics, epigenetics, proteomics, and metabolomics) are essential. These advanced approaches enable the identification of molecular signatures and changes in various hormone axes and relevant pathways in response to EDC exposures. By combining these tools with traditional toxicological techniques, researchers can gain a comprehensive understanding of the multifaceted effects of EDCs on human health and reproduction, ultimately contributing to improved public health policies and regulatory decisions.
Acknowledgements
The figures were created using BioRender software.
Author biographies
Qiwei Yang is a research scientist in reproductive medicine. His area of research focuses on genetic, epigenetic, and biological pathways in uterine disorders.
Mohamed Ali is a staff scientist in reproductive disease. His area of research is pharmaceuticals and 3D models in uterine fibroids.
Maria Victoria Bariani is a postdoctoral fellow. Her area of research focuses on the risk factors for the development of uterine fibroids.
Somayeh Vafaei is a postdoctoral fellow. Her area of research focuses on the role of endocrine-disrupting chemicals in the pathogenesis of uterine fibroids.
Ayman Al-Hendy is a physician-scientist who delivers expert care for complex gynecologic conditions. He is also dedicated to using basic and translational research to better understand the cause of gynecologic conditions and investigate new treatments that offer improved outcomes and overall quality of life.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NIH, (grant number ES028615, HD106285).
ORCID iD: Qiwei Yang https://orcid.org/0000-0001-7131-8946
References
- 1.Yang Q, Ali M, Trevino LS, et al. Developmental reprogramming of myometrial stem cells by endocrine disruptor linking to risk of uterine fibroids. Cell Mol Life Sci 2023; 80: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, Ciebiera M, Bariani MV, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev 2022; 43: 678–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visvader JE, Lindeman GJ. The unmasking of novel unipotent stem cells in the mammary gland. EMBO J 2011; 30: 4858–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu WY, Hu DP, Xie L, et al. Keratin profiling by single-cell RNA-sequencing identifies human prostate stem cell lineage hierarchy and cancer stem-like cells. Int J Mol Sci 2021; 22. doi: 10.3390/ijms22158109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley L, Cambuli F, Aparicio L, et al. A single-cell atlas of the mouse and human prostate reveals heterogeneity and conservation of epithelial progenitors. Elife 2020; 9. doi: 10.7554/eLife.59465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mas A, Nair S, Laknaur A, et al. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril 2015; 104(1): 225–34 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono M, Qiang W, Serna VA, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One 2012; 7: e36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook JD, Davis BJ, Cai SL, et al. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci U S A 2005; 102: 8644–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mas A, Stone L, Connor O, et al. Developmental exposure to endocrine disruptors expands murine myometrial stem cell compartment as a prerequisite to leiomyoma tumorigenesis. Stem Cells 2017; 35: 666–678. [DOI] [PubMed] [Google Scholar]
- 10.Prusinski Fernung LE, Yang Q, Sakamuro D, et al. Endocrine disruptor exposure during development increases incidence of uterine fibroids by altering DNA repair in myometrial stem cells. Biol Reprod 2018; 99: 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkafas H, Ali M, Elmorsy E, et al. Vitamin D3 ameliorates DNA damage caused by developmental exposure to endocrine disruptors in the uterine myometrial stem cells of eker rats. Cells 2020; 9. doi: 10.3390/cells9061459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bariani MV, Cui YH, Ali M, et al. TGFbeta signaling links early life endocrine-disrupting chemicals exposure to suppression of nucleotide excision repair in rat myometrial stem cells. Cell Mol Life Sci 2023; 80: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q, Ali M, Trevino LS, et al. Epigenetic modulation of inflammatory pathways in myometrial stem cells and risk of uterine fibroids. Int J Mol Sci 2023; 24:11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bariani MV, Rangaswamy R, Siblini H, et al. The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Curr Opin Endocrinol Diabetes Obes 2020; 27: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka T, Yin P, Zuberi A, et al. Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation. Proc Natl Acad Sci U S A 2022; 119: e2208886119. [DOI] [PMC free article] [PubMed] [Google Scholar]