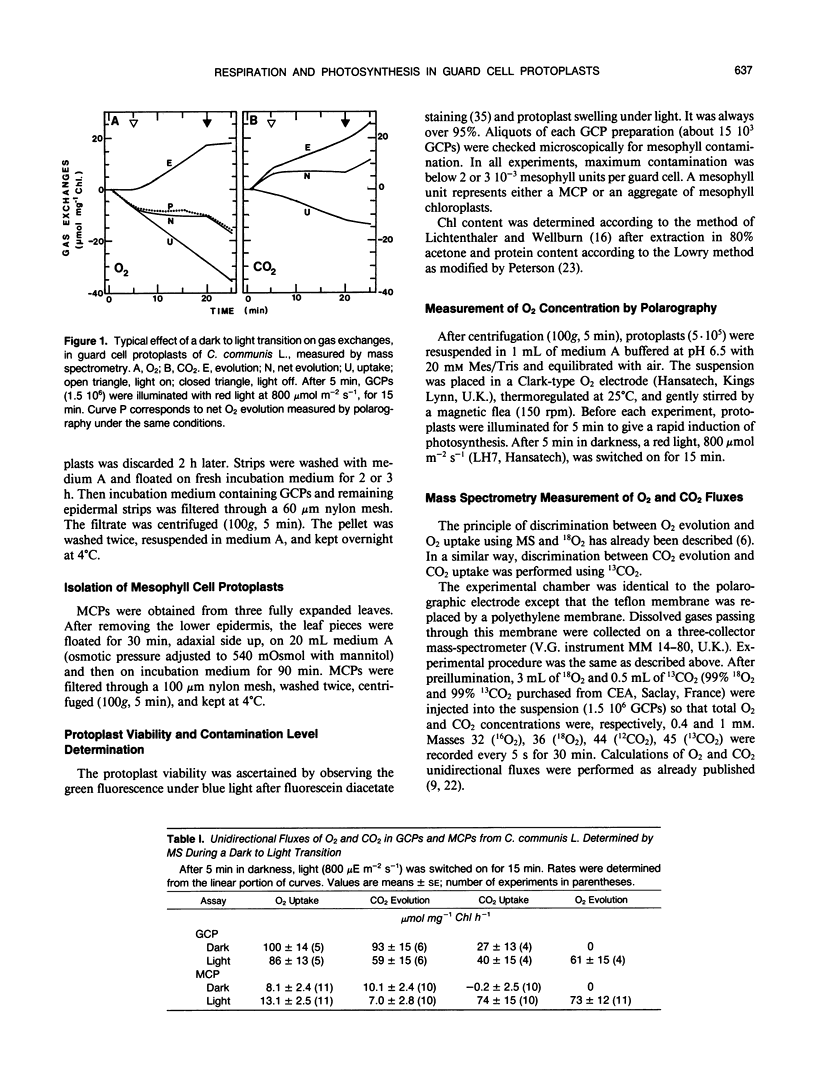
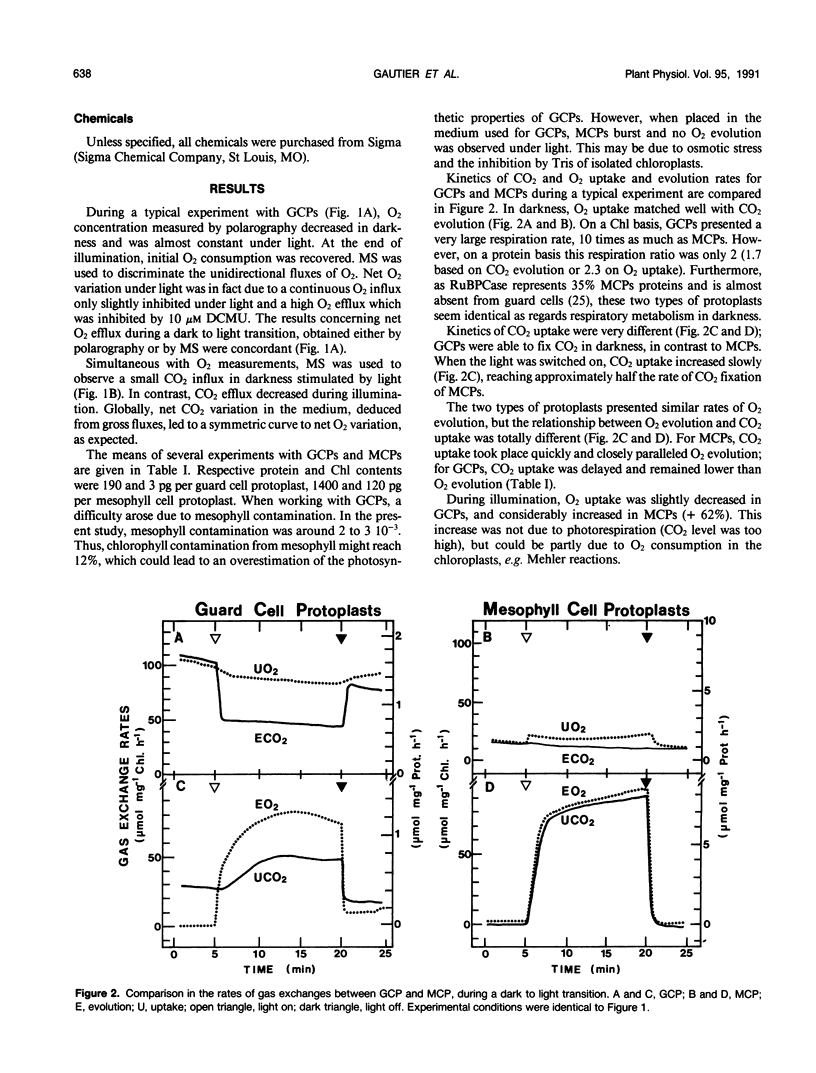
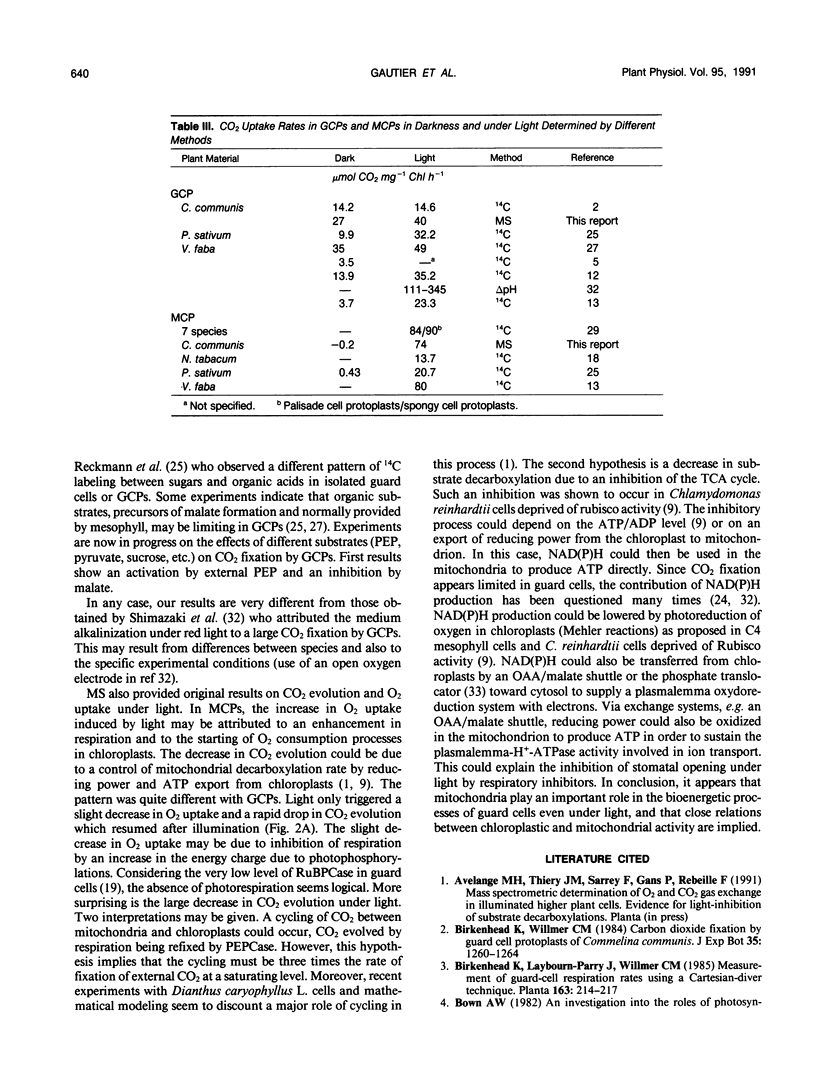
Abstract
A mass spectrometric method combining 16O/18O and 12C/13C isotopes was used to quantify the unidirectional fluxes of O2 and CO2 during a dark to light transition for guard cell protoplasts and mesophyll cell protoplasts of Commelina communis L. In darkness, O2 uptake and CO2 evolution were similar on a protein basis. Under light, guard cell protoplasts evolved O2 (61 micromoles of O2 per milligram of chlorophyll per hour) almost at the same rate as mesophyll cell protoplasts (73 micromoles of O2 per milligram of chlorophyll per hour). However, carbon assimilation was totally different. In contrast with mesophyll cell protoplasts, guard cell protoplasts were able to fix CO2 in darkness at a rate of 27 micromoles of CO2 per milligram of chlorophyll per hour, which was increased by 50% in light. At the onset of light, a delay observed for guard cell protoplasts between O2 evolution and CO2 fixation and a time lag before the rate of saturation suggested a carbon metabolism based on phosphoenolpyruvate carboxylase activity. Under light, CO2 evolution by guard cell protoplasts was sharply decreased (37%), while O2 uptake was slowly inhibited (14%). A control of mitochondrial activity by guard cell chloroplasts under light via redox equivalents and ATP transfer in the cytosol is discussed. From this study on protoplasts, we conclude that the energy produced at the chloroplast level under light is not totally used for CO2 assimilation and may be dissipated for other purposes such as ion uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bown A. W. An investigation into the roles of photosynthesis and respiration in h efflux from aerated suspensions of asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):803–810. doi: 10.1104/pp.70.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Outlaw W. H. Effect of Fusicoccin on Dark CO(2) Fixation by Vicia faba Guard Cell Protoplasts. Plant Physiol. 1982 Dec;70(6):1700–1703. doi: 10.1104/pp.70.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans P., Rebeille F. Light inhibition of mitochondrial respiration in a mutant of Chlamydomonas reinhardtii devoid of ribulose-1,5-bisphosphate carboxylase/oxygenase activity. Arch Biochem Biophys. 1988 Jan;260(1):109–117. doi: 10.1016/0003-9861(88)90430-4. [DOI] [PubMed] [Google Scholar]
- Gotow K., Sakaki T., Kondo N., Kobayashi K., Syōno K. Light-Induced Alkalinization of the Suspending Medium of Guard Cell Protoplasts from Vicia faba L. Plant Physiol. 1985 Nov;79(3):825–828. doi: 10.1104/pp.79.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow K., Taylor S., Zeiger E. Photosynthetic Carbon Fixation in Guard Cell Protoplasts of Vicia faba L. : Evidence from Radiolabel Experiments. Plant Physiol. 1988 Mar;86(3):700–705. doi: 10.1104/pp.86.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Role of o(2) and mitochondrial respiration in a photosynthetic stimulation of oat protoplast acidification of a surrounding medium. Plant Physiol. 1983 Jun;72(2):356–361. doi: 10.1104/pp.72.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Kiriyama T., Kato Y., Kogame Y., Kaneko K., Hishida H., Mizuno Y., Ejiri K., Kawai K., Takeuchi A. [Clinical reliability and limitation of 99m Tc-pyrophosphate myocardial scintigraphy for the assessment of acute myocardial infarction--with special reference to evaluation of the area affected by infarction]. Kaku Igaku. 1982 Jul;19(6):871–879. [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G., Thibault P. O(2) uptake in the light in chlamydomonas: evidence for persistent mitochondrial respiration. Plant Physiol. 1985 Sep;79(1):225–230. doi: 10.1104/pp.79.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Reckmann U., Scheibe R., Raschke K. Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 1990 Jan;92(1):246–253. doi: 10.1104/pp.92.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey P., Peltier G. Photorespiratory Properties of Mesophyll Protoplasts of Nicotiana plumbaginifolia. Plant Physiol. 1989 Mar;89(3):762–767. doi: 10.1104/pp.89.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Terada J., Tanaka K., Kondo N. Calvin-Benson Cycle Enzymes in Guard-Cell Protoplasts from Vicia faba L: Implications for the Greater Utilization of Phosphoglycerate/Dihydroxyacetone Phosphate Shuttle between Chloroplasts and the Cytosol. Plant Physiol. 1989 Jul;90(3):1057–1064. doi: 10.1104/pp.90.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Zeiger E. Red Light-Dependent CO(2) Uptake and Oxygen Evolution in Guard Cell Protoplasts of Vicia faba L.: Evidence for Photosynthetic CO(2) Fixation. Plant Physiol. 1987 May;84(1):7–9. doi: 10.1104/pp.84.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]


