Abstract
The gene organization and transcription of the Agrobacterium glg operon differ from those in other bacteria. Agrobacterium tumefaciens A348 contains a 9.1-kb gene cluster harboring genes for glycogen metabolism. The nucleotide sequence and gene organization of a region containing ADP-glucose pyrophosphorylase (glgC), glycogen synthetase (glgA), and phosphoglucomutase (pgm) genes have been previously described (A. Uttaro and R. A. Ugalde, Gene 150:117–122, 1994). In this work we report that the glycogen phosphorylase (glgP) and branching enzyme (glgB) genes are located immediately upstream of this region. The complete nucleotide sequences of the glgP and glgB genes were obtained, and mutants were constructed by targeted insertional mutagenesis with a kanamycin cassette. Enzymatic assays and reverse transcription PCR carried out with the wild type and with glgP and glgB mutants, as well as primer extension experiments and β-galactosidase fusions, revealed that this region containing five open reading frames (glgPBCA and pgm) is transcribed unidirectionally as a single operon under the control of a promoter located upstream of the glycogen phosphorylase gene (glgP). An alternative transcript was identified starting 168 bp upstream of an internal ATG start codon of the pgm gene, which is translated as a 71-amino-acid-shorter Pgm protein which complements in vivo a pgm mutant. This alternative transcript has a promoter with the motif TATCAAN5G, identified in octopine Ti plasmid as an autoinducible TraR promoter. This promoter is >200 times more efficient in A. tumefaciens than in Escherichia coli, as judged by the level of enzymatic activity of a lacZ-pgm fusion.
Glycogen is produced and accumulates in many bacteria. Although it is known that this polysaccharide is used as a stored source of energy, the precise role that it may play in bacteria is still not clear. Escherichia coli glycogen synthetase mutants have no relevant growth phenotype. However, some authors have suggested that the accumulation of glycogen may give advantages under starvation conditions, providing a stored source of energy (23).
The reactions that lead to the synthesis of glycogen in bacteria have been extensively studied (23, 24). ADP-glucose provides the donor sugar nucleotide, whose synthesis is catalyzed by the enzyme ADP-glucose pyrophosphorylase (EC 2.7.7.27). The glucosyl moiety of ADP-glucose is transferred, in a reaction catalyzed by a specific ADP-glucose-glycogen synthetase (EC. 2.4.1.21), to either a maltodextrin or a glycogen primer to form a new α-1,4-glucosidic bond. Subsequently, a branching enzyme (EC 2.4.1.18) catalyzes the formation of branched α-1,6-glucosidic linkages. All these reactions were observed to occur in extracts of more than 40 species of bacteria (25). The metabolic pathway that leads to the release of the energy stored in glycogen starts with the enzyme glycogen phosphorylase (EC 4.4.1.1), which releases glucose-1-phosphate from the nonreducing terminus of the α-1,4 chain (9).
The genetic organization of the glycogen operon (glg) was determined in E. coli (28, 38), Bacillus stearothermophilus (34), and Bacillus subtilis (18). The glg operon is located at approximately 75 min on the E. coli K-12 chromosome map (25). The arrangement and nucleotide sequence of the entire glg cluster revealed that a continuous DNA fragment of over 15 kb flanked by the genes asd (17) and glpD (1) contains the genes encoding the branching enzyme (glgB), ADP-glucose pyrophosphorylase (glgC), and glycogen synthetase (glgA) and two genes, glgX (homologous to genes encoding α-amylases) and glgP (homologous to the rabbit glycogen phosphorylase gene) (25, 28). None of the latter genes are required for glycogen synthesis, but they are needed for glycogen metabolism. Detailed inspection of the organization of the E. coli glg cluster suggests that glg genes may be transcribed as two operons, glgBX and glgCAP (25). The coding regions of the glgB and glgX open reading frames (ORFs) overlap by 1 bp. ORFs glgC and glgA are separated by 2 bp, and glgA and glgP are separated by 18 bp. This close proximity suggests translational coupling of the two operons glgBX and glgCAP (25).
The glgCAP(Y) operon is under the positive control of cyclic AMP (cAMP) and the cAMP receptor protein (CRP); both the cya gene, encoding adenylate cyclase (EC 4.6.1.1), and crp, encoding CRP, are required for optimal synthesis of glycogen (9). CRP binds to a site located upstream of the glgC gene, and consensus DNA sequences between the E. coli and Salmonella typhimurium glgC upstream regions were found (25). Glycogen synthesis in E. coli is also positively regulated by ppGpp, which stimulates the transcription of the glgCAP operon; neither ppGpp nor CRP affects the transcription of the glgB gene (25).
In E. coli, glycogen synthesis is also down-regulated at the level of transcription (25). Regulatory mutants designated glgR and glgQ mutants were identified (29).
The organization of the glg operons in B. stearothermophilus and B. subtilis is different from that in E. coli. In B. subtilis the operon is located downstream of trnB, which maps at 275 min on the chromosome. The operon glgBCDAP has extensive homologies to genes encoding enzymes involved in glycogen and starch metabolism in both prokaryotes and eukaryotes (18). glgD, not present in other bacteria, has high homology to glgC. This operon is presumably expressed under the control of a sporulation promoter. The same operon organization was described for B. stearothermophilus (34). Purification of ADP-glucose pyrophosphorylase (EC 2.7.7.27) demonstrated that it was heterotetrameric, formed by the GlgC and GlgD proteins (33). In this bacterium, as is the case in B. subtilis, the operon is preceded by a sporulation promoter (34).
The genes coding for ADP-glucose pyrophosphorylase, glycogen synthetase, and phosphoglucomutase in the plant pathogenic bacterium Agrobacterium tumefaciens were found to form a continuous cluster on the chromosome (35). Nucleotide sequence analysis of this cluster revealed that it is transcribed in the same orientation with no intergenic region, suggesting that it might be transcribed as a single operon (35).
In this work, we show that the genes encoding five enzymes (glycogen phosphorylase, branching enzyme, ADP-glucose pyrophosphorylase, glycogen synthetase, and phosphoglucomutase) are transcribed from a single operon in A. tumefaciens. However, an independent transcript for pgm was identified that is translated as a Pgm protein 71 amino acids shorter than the protein produced by the polycistronic messenger. The sequences of glycogen phosphorylase and branching-enzyme genes were obtained, completing the information for the whole glg operon in A. tumefaciens.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli K-12 | ||
| DH5α-F′IQ | F′ φ80d lacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1/F′ proAB+ lacIqZ ΔM15 zzf::Tn5[Kmr] | 37 |
| DH5α | F− φ80d lacZ ΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | 14 |
| XL1-Blue | Δ(mrcA)183 Δ(mcrCB-hsd SMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proAB lacIqZ ΔM15 Tn10(Tetr)] | 3 |
| MRF′ | ||
| A. tumefaciens | ||
| A348 | Wild type, pTiA6 Rifr Cmr | 12 |
| A1120 | glgB::Kmr | This study |
| A1121 | glgP::Kmr | This study |
| A5129 | pgm::Tn5 | 35 |
| Plasmids | ||
| pFC6251 | 30-kb fragment of genomic DNA of A348 in pVK102 that complements exoC mutant | 4 |
| pXH11 | 1.1-kb XhoI-HindIII fragment of pFC6251 containing 5′ end of glgB and 3′ end of glgP | 35 |
| pBH26 | 2.6-kb BamHI-HindIII fragment of pFC6251 containing glgB in pBluescript II KS (+) | This study |
| pBH26K | pBH26, glgB::Kmr | This study |
| pBB3 | 3-kb BglII-BamHI fragment of pFC6251 containing glgP in pBluescript II KS (+) | This study |
| pBB3K | pBB3K, glgP::Kmr | This study |
| pFus96 | lacZ in pBBR1MCS-2 | This study |
| pFus97 | Translational fusion pgm-lacZ in pBBR1MCS-2 | This study |
| pFus98 | Translational fusion glgP-lacZ in pBBR1MCS-2 | This study |
| pPGM1 | 756-bp fragment from bases 7768 to 8522 corresponding to 5′ end of pgm product in pGEX2 | This study |
| pCC15 | 1,642-bp fragment from bases 7733 to 9375 corresponding to pgm2 in pBBR1MCS-4 | This study |
| pBBR1MCS | Broad-host-range cloning vector (Kmr or Ampr) | 19 |
| pAB5002 | Vector for construction of translational fusions with β-galactosidase (Ampr Gmr) | 2a |
Cloning, DNA sequencing, and gene disruption.
Plasmid pFC6251 was digested with HindIII and BglII restriction enzymes, and a 2.6-kb fragment containing the glgB gene was recovered and ligated into pBluescript II KS (+) digested with BamHI and HindIII, in order to eliminate the BamHI site in the polylinker of the recombinant plasmid. In the resulting recombinant plasmid, pBH26, a kanamycin cassette (22) was ligated into a unique BamHI site. Plasmid pBH26::Km (pBH26K in Table 1) was recovered and electroporated into the A. tumefaciens A348 wild-type strain. Double recombination events (Kmr Cbs) were selected and confirmed by PCR with a set of primers that amplifies a 500-bp DNA fragment from the wild type and a 1.8-kb DNA fragment from the double recombinant. A mutant clone named A1120 was selected for further studies.
In order to clone and mutagenize the glgP gene, plasmid pFC6251 was digested with BglII and BamHI and a 3.0-kb DNA fragment that hybridized with a 0.6-kb probe containing the 3′ end of the glgP gene was isolated. This 3.0-kb DNA fragment was ligated into pBluescript II KS (+) digested with BamHI to obtain plasmid pBB3. In order to eliminate the PstI site of the pBluescript II KS (+) polylinker, pBB3 was digested with BamHI and EcoRV, filled in, and religated. The kanamycin cassette was introduced into a unique PstI site of the glgP gene (Fig. 1), the plasmid was transformed into E. coli and selected with kanamycin (50 μg/ml), and then pBB3::Km (pBB3K in Table 1) was recovered and electroporated into the A. tumefaciens A348 wild-type strain. Double recombination events were selected (Kmr Cbs) and confirmed by PCR with a set of primers that amplified a fragment of 400 bp from the wild-type gene and a 1.7-kb fragment from the kanamycin interrupted gene. A mutant clone named A1121 was selected for further studies. Both strands of the glgP and glgB genes and flanking DNA regions were sequenced by the dideoxy terminator method as described elsewhere (33).
FIG. 1.
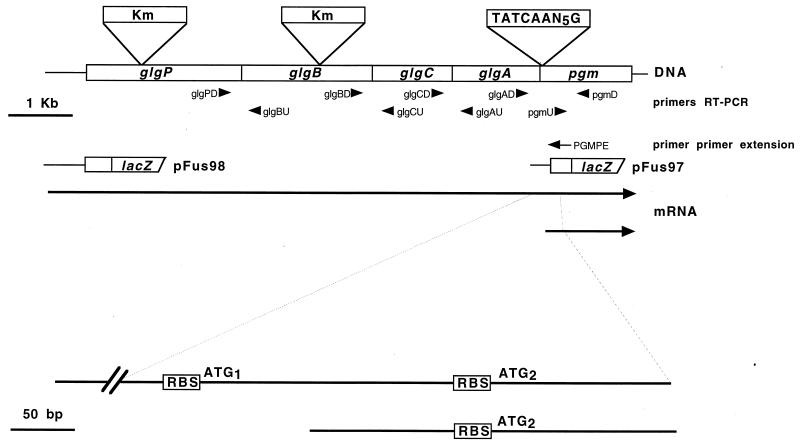
Scheme of A. tumefaciens glg operon organization and transcription. The genomic arrangement with the positions of the kanamycin cassette insertions and the putative alternative pgm promoter are shown in the upper part. Oligonucleotides used for RT-PCRs and the primer extension experiment are indicated by arrows. The DNA regions used for the construction of β-galactosidase fusion plasmids pFus98 and pFus97 are shown. The transcriptional organization of the operon, indicating the polycistronic mRNA and the alternative pgm2 mRNA (different scale), is shown in the lower part of the figure. RBS, Shine-Dalgarno conserved sequences; ATG1 and ATG2, initiation codons of Pgm1 and Pgm2, respectively.
Construction of pCC15 and complementation of A. tumefaciens A5129.
For the construction of the recombinant plasmid pCC15, a PCR was carried out with oligonucleotides 5′-CGGGATCCATGATCAAGACTATCAAGAC-3′ (positions 7733 to 7753) and 5′-AACTGCAGCGGGCGGACGTTATCAGGTA-3′ (positions 9355 to 9375), having BamHI and PstI sites (underlined) in their 5′ ends. This PCR amplified a DNA fragment of 1,642 bp, spanning from the ATG1 (Fig. 1) to the stop codon of the pgm gene. PCR product was digested with BamHI and PstI and ligated into pBBR1MCS-4 (19) digested with the same enzymes. This vector replicates in Agrobacterium and confers resistance to carbenicillin. It is worth noting that pgm was cloned in the opposite direction with respect to the transcription of the endogenous promoter of the multiple cloning site of pBBR1MCS-4. Recombinant plasmid pCC15 was introduced into A. tumefaciens A5129 by electroporation, and cells were plated on Luria-Bertani (LB) agar, containing carbenicillin (100 μg/ml), kanamycin (50 μg/ml), and Calcofluor (0.02%). Screening for complementation was carried out by searching for bright colonies under UV light.
RT-PCR experiments.
RNA was extracted from 10-ml stationary-phase cultures by a previously described protocol (2). RNA was treated with DNase, RNase free (Promega, Madison, Wis.), before use. Reverse transcription-PCRs (RT-PCRs) were carried out with primers glgPD (5′-CAGCGACTGGTTCATGGT-3′) (positions 2609 to 2626) and glgBU (5′-AATTTCCGTCCAGCGTCA-3′) (positions 2970 to 2987) for the glgP-glgB region, glgBD (5′-GCACGGCGCCTGGTGAAAAA-3′) (positions 4749 to 4768) and glgCU (5′-AATGTCGAAGCTTTCGTTAC-3′) (positions 5280 to 5299) for the glgB-glgC region, glgCD (5′-GCCGAGTGTGAAGATCGGGCG-3′) (positions 6100 to 6120) and glgAU (5′-CGCCGCAAAACGCTTCCAGT-3′) (positions 6605 to 6624) for the glgC-glgA region, glgAD (5′-AAATGCAGAAACTCGGAATG-3′) (positions 7631 to 7650) and pgmD (5′-GACGTCGTCATAAAGCTCCT-3′) (positions 8502 to 8521) for the glgA-pgm region, and pgmU (5′-AAAGATCACCGACGCGATCTA-3′) (positions 8140 to 8160) and pgmD for the amplification of an internal pgm gene region (Fig. 1).
Construction of lacZ fusions.
A pgm-lacZ fusion (pFus97) was constructed by amplifying by PCR with primers glgAD and pgmD (Fig. 1) a 872-bp DNA fragment, with cosmid pFC6251 as the template. The amplified fragment was ligated into pGEMT-easy (Promega Corporation) and transformed into E. coli. The recombinant plasmid was recovered, digested with NotI and SalI, and ligated into pAB5002 (Table 1). This construct leaves a DNA region of 315 bp upstream of the second ATG and the sequence for the first 104 amino acids encoded by pgm2 (Fig. 1) in frame with the promoterless lacZ of pAB5002. From this plasmid a 5.5-kb fragment, containing the pgm-lacZ fusion and acc1, was recovered by digesting with XbaI and XhoI and ligated into pBBR1MCS-2 (19). The resulting plasmid, named pFus97, was recovered from E. coli and electroporated into the A. tumefaciens A348 wild-type strain. The glgP-lacZ fusion (pFus98) consists of a 1.1-kb XbaI-SalI DNA fragment obtained from plasmid pBB3 (Table 1) cloned into pAB5002. This construct leaves a region of 650 bp upstream of the glgP ATG initiation codon with the sequence for the first 146 amino acids in frame with the promoterless lacZ of pAB5002. The subsequent steps for constructing pFus98 were the same as those used for pFus97. The control fusion plasmid pFus96 was constructed by cloning the XbaI/XhoI fragment obtained from pAB5002 in pBBR1MCS-2.
Primer extension.
The transcription start site was determined by primer extension analysis (32). Experiments were carried out with total RNA (20 μg) obtained from stationary-phase cultures of the A. tumefaciens A348 wild-type strain as described above. The synthetic oligonucleotide PGMPE (5′-ACTGGATGAAGTTCTCGG-3′) (positions 7830 to 7847 of the glycogen operon) was used as a primer (Fig. 1). The primer was labeled with [γ-32P]ATP catalyzed by T4 kinase (32). For primer-RNA annealing, the samples were heated at 90°C for 4 min, left standing at room temperature for 5 min, and then put in an ice bath. Synthesis of cDNA employing avian myeloblastosis virus reverse transcriptase (Promega Corporation) was carried out at 42°C for 1 h, and the reaction was stopped by heating at 99°C for 5 min. Products were subjected to polyacrylamide gel electrophoresis in parallel with the sequencing ladder.
Determination of enzymatic activities.
Cells from stationary-phase cultures were harvested by centrifugation at 10,000 × g for 20 min and resuspended (2 ml of buffer per g [wet weight] of cells) with 40 mM Tris-acetate buffer (pH 7.5)–5 mM EDTA–5 mM dithiothreitol (Sigma Chemical Co., St. Louis, Mo.). Lysozyme (2 μg/μl; Sigma Chemical Co.) was added. After incubation for 1 h at 4°C, the cells were disrupted by three compression-decompression cycles in a French press and 2 mM (final concentration) phenylmethylsulfonyl fluoride (Sigma Chemical Co.) was added. Extracts were centrifuged for 15 min at 11,000 × g, and the supernatants (crude extracts) were used for enzymatic assays.
(i) Branching enzyme (GlgB).
Assays for GlgB were carried out as described elsewhere (16), based on the ability of crude extracts to stimulate the formation of glycogen from glucose-1-phosphate (Glc-1-P) as the donor substrate by rabbit phosphorylase.
(ii) ADP-glucose pyrophosphorylase (GlgC).
Assays for GlgC were carried out following the synthesis of ATP as described previously (13).
(iii) Glycogen synthetase (GlgA).
Assays for GlgA were carried out following the synthesis of [14C]glycogen by using ADP-[14C]glucose as the substrate as described elsewhere (8).
(iv) Phosphoglucomutase (Pgm).
Assays for Pgm were carried out in a coupled reaction following the reduction of NADP at 340 nm as described previously (27).
(v) Glycogen phosphorylase (GlgP).
Assays for GlgP were carried out as described elsewhere (5), with some modifications. The reaction mixtures contained crude extract, in a final volume of 0.1 ml; 100 mM Na-citrate, pH 6; and glycogen (1.5 mg/ml). The reaction was started by the addition of 49.6 mM [14C]Glc-1-P (30 μCi/mmol), and the mixture was incubated for 45 min at 37°C. The reaction was stopped by heating at 100°C for 1 min, 1% glycogen was added as a carrier, and the mixture was precipitated with 75% methanol–1% KCl. The precipitates were washed three times with methanol-KCl and then resuspended in water, and the radioactivity incorporated into glycogen was counted in a liquid scintillator.
(vi) β-Galactosidase assays.
β-Galactosidase assays were carried out with whole cells as described elsewhere (32).
Preparation of phosphoglucomutase antibodies.
A 765-bp region spanning positions 7768 to 8522 of the glg operon was amplified by PCR and cloned into plasmid pGEX-2T (Pharmacia-Biotech, Uppsala, Sweden). The recombinant plasmid pPGM1 (Table 1) was introduced into E. coli DH5α and induced with isopropyl-β-d-thiogalactopyranoside, and inclusion bodies were subjected to polyacrylamide gel electrophoresis. Gels were stained with Coomassie brilliant blue R-250 (0.05% in water), and the recombinant protein was cut out of the gel and used to prepare rabbit antibodies by a standard protocol of immunization.
Western blot analysis.
Cultures (500 ml) of the A. tumefaciens wild-type strain A348, the pgm mutant A5129, and the glgB mutant A1120 were grown until stationary phase and harvested by centrifugation. Cell pellets were resuspended in 50 mM Tris-HCl buffer, pH 8.2, with 3 mM EDTA, 20% sucrose, 1 mM phenylmethylsulfonyl fluoride, and 200 μg of lysozyme per ml and incubated for 1 h at 4°C. Cells were recovered by centrifugation, resuspended in 50 mM Tris-HCl (pH 8.2)–20% sucrose–10 mM MgCl2–20 μg of DNase per ml and sonicated. Supernatants obtained after centrifugation for 30 min at 15,000 × g were salted out with (NH4)2SO4 at a final saturation of 30%, and the pellets were dissolved in 50 mM Tris-HCl (pH 8.2)–5% glycerol–3 mM β-mercaptoethanol and dialyzed overnight against the same buffer. Samples (30 μg of protein) were subjected to 8% polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and subjected to Western blot analysis using with anti-Pgm antibodies. Blots were developed afterwards with anti-rabbit immunoglobulin G-peroxidase (DAKO, Glostrup, Denmark).
Nucleotide sequence accession number.
The sequence comprising the genes for the A. tumefaciens glycogen phosphorylase (glgP) and branching enzyme (glgB) and the complete glg operon has been assigned GenBank accession no. AF033856.
RESULTS
Nucleotide sequences of the branching enzyme (glgB) and glycogen phosphorylase (glgP) genes.
Sequence analysis of the region located upstream from the A. tumefaciens A348 ADP-glucose pyrophosphorylase (glgC) gene (34) revealed the presence of two contiguous ORFs (ORF1 and ORF2) coding for two proteins, one 46.4% identical to the E. coli glycogen phosphorylase (EC 2.4.1.1) and the other 56% identical to the E. coli branching enzyme (EC 2.4.1.1.8). The complete organization of the A. tumefaciens glycogen operon, as deduced from the nucleotide sequence data, is shown in Fig. 1. The data shown in this figure were partially taken from our previous publication (35) and the results described below.
Comparison of the amino acid sequences deduced from ORF1 and ORF2 with GlgP and GlgB protein sequences.
A. tumefaciens ORF1 encodes a protein of 821 amino acids which is 46.4% identical to the E. coli glycogen phosphorylase protein, 43.8% identical to E. coli maltodextrin phosphorylase, and 43.2% identical to rat glycogen phosphorylase. Four of the eight glycogen storage sites described elsewhere (6) are conserved in the four proteins (residues Q394, N400, I424, and E426) (data not shown). Residue Y397 is not conserved in E. coli glycogen GlgP and E. coli maltodextrin GlgP, residues L404 and S422 are not conserved in E. coli maltodextrin GlgP, and residue S420 is not conserved in any of the phosphorylases. Seven regions of the protein were identified as belonging to the active site (6); five of them are conserved in A. tumefaciens GlgP. The region between residues Y282 and T287 contains two nonconservative changes in the E. coli and rat GlgP proteins. Sequences from A663 to G671 are conserved in rat GlgP but have one nonconservative change in the E. coli GlgP proteins (M665 replaced by T in glycogen phosphorylase and M665 replaced by K in maltodextrin phosphorylase).
A. tumefaciens ORF2 encodes a protein of 734 amino acid residues which is 56% identical to the E. coli glgB gene product. The four putative active-site residues H352, D417, H537, and D538 are all conserved (21). The regions with similarity among α-amylases, α-glucosidases, and other glucantransferases are also highly conserved. The percentages of identity between A. tumefaciens ORF2 and E. coli glgB are 80% for residues D307 to G316, 75% for residues T321 to G329, 90% for residues G343 to F353, 88% for residues W405 to M422, 90% for residues M466 to W475, and 100% for residues E529 to K544.
The homologous proteins were aligned by the program DNASTAR by using the algorithm developed by Lipman and Pearson (20).
The five glg genes form a single operon.
Insertional mutagenesis of glgP and glgB genes and enzymatic activity assays were carried out as described in Materials and Methods. A kanamycin cassette was introduced into a unique PstI site of the glgP gene and into a unique BamHI site of the glgB gene (Fig. 1). A. tumefaciens mutants A1120 (glgB::Kmr) and A1121 (glgP::Kmr) were obtained. Crude extracts were prepared from stationary-phase cultures of the wild-type strain A348 and mutant strains A1120, A1121, and A5129 (pgm mutant) (35). The activities of glycogen phosphorylase (EC 2.4.1.1), branching enzyme (EC 2.4.1.18), ADP-glucose pyrophosphorylase (EC 2.7.7.27), glycogen synthetase (EC 2.4.1.21), and phosphoglucomutase (EC 2.7.5.1) were determined as described in Materials and Methods. All strains were also scored for the accumulation of glycogen by the iodine assay (30). Table 2 shows that the mutant strain A1121 (glgP::Kmr) displayed a polar effect on the enzymatic activity of downstream mapping genes glgB, glgC, and glgA, with no detectable activity of branching enzyme, ADP-glucose pyrophosphorylase, or glycogen synthetase. This mutation, however, had only a partial effect on phosphoglucomutase activity (24% of the wild-type activity). Mutant A1120 (glgB::Kmr) had wild-type phosphorylase activity and a polar effect on the enzymatic activities of downstream mapping genes glgC and glgA. This mutant also displayed a partial polar effect on phosphoglucomutase (26% of wild-type activity). These results revealed that the region containing glgP, glgB, glgC, glgA, and pgm genes is organized as a single operon transcribed from glgP to pgm. The fact that mutant strains A1121 (glgP::Kmr) and A1120 (glgB::Kmr) had only a partial polar effect on pgm activity suggested that this downstream gene might be transcribed as part of this operon and also as a separate transcript. In order to further analyze the latter pos-sibility, RT-PCR, primer extension experiments, and β-galactosidase fusions were carried out as described below.
TABLE 2.
Enzymatic activities of A. tumefaciens wild-type and mutant strains
| Strain | Enzyme activity (%)a
|
Colony colorb | ||||
|---|---|---|---|---|---|---|
| GlgP | GlgB | GlgC | GlgA | Pgm | ||
| A348 | 100 | 100 | 100 | 100 | 100 | + |
| A1120 | 100 | 0 | 0 | 1.47 | 26 | −c |
| A1121 | 0.88 | 0 | 0 | 1.57 | 24 | − |
| A5129 | 100 | NDd | 74 | 100 | 0 | − |
Enzymatic activities were determined as described in Materials and Methods and expressed as percentages of the wild-type strain activity. Wild-type activities (in units per milligram of protein) were as follows: glycogen phosphorylase (GlpP), 0.0047; branching enzyme (GlgB), 0.021; ADP-glucose pyrophosphorylase (GlgC), 0.0156; glycogen synthase (GlgA), 0.091; and phosphoglucomutase (Pgm), 0.041. One unit of activity is defined as follows: for GlgB, the amount of enzyme that stimulated rabbit phosphorylase at 1 μmol/min; GlgC, the amount of enzyme that catalyzed the formation of 1 μmol of ATP per min; GlgA, the amount of enzyme that catalyzed the incorporation of glucose into insoluble glycogen at 1 μmol/min; Pgm, the amount of enzyme that catalyzed the formation of 1 μmol of NADPH per min, and GlgP, the amount of enzyme that catalyzed the incorporation of 1 μmol of Glc-1-P per min into insoluble glycogen.
Color of the colonies was detected after exposing a petri dish culture to iodine solution (30). Agrobacterium was grown at 28°C in AB medium (7) for 48 h, iodine solution was poured into the plate, and the color was scored after 2 to 5 min. +, brown color; −, no color.
A pale green color developed.
ND, not determined.
Two possible transcripts for the pgm gene.
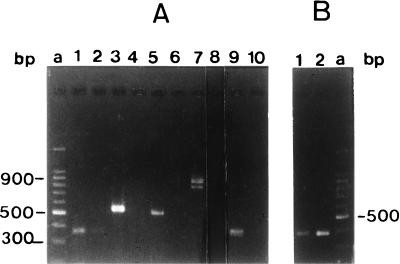
The nucleotide sequence of the entire A. tumefaciens glg region, as well as enzymatic assays of glg enzymes in two mutants obtained by gene disruption with a kanamycin cassette, suggested that in A. tumefaciens the glycogen genes glgP, glgB, glgC, glgA, and pgm form a single operon. In order to confirm these results, RT-PCR experiments were carried out as described in Materials and Methods. Fig. 2B shows that, when RNA extracted from mutant A1120 (glgB::Kmr) was used as the template, the expected product was amplified after RT-PCR with primers homologous to the 3′ and 5′ ends of the coding regions of glgP and glgB genes, respectively. On the other hand, no products were recovered after amplification with primers homologous to the 3′ and 5′ ends of the coding regions of glgB-glgC, glgC-glgA, or glgA-pgm genes (data not shown), thus indicating that the specific mRNA was absent in this mutant strain. However, when the amplification reaction was carried out with a set of primers internal to the pgm gene, an amplified product of the expected size (384 bp) was obtained (Fig. 2B). These results are consistent with the fact that a decreased but detectable level of Pgm activity was present in this mutant (Table 2), and with our previous observation that a plasmid (pH21) containing a DNA fragment expanding the glgA 5′ region and the pgm gene complemented a Tn5 pgm mutant (A5129 [35]) (Table 2). Control RT-PCRs carried out with wild-type RNA as the template produced the expected amplified products (Fig. 2A). These results suggested that the Agrobacterium glg operon is transcribed as a single mRNA containing the five genes glgPBCA and pgm, and that an alternative promoter might produce an mRNA leading to an active Pgm protein.
FIG. 2.
RT-PCRs carried out with total RNA obtained from wild-type A348 and A1120 (glgB) mutant strains. (A) Reactions carried out with total RNA of wild-type strain A348. Lane 1, glgP-glgB intergenic region; lane 3, glgB-glgC intergenic region; lane 5, glgC-glgA intergenic region; lane 7, glgA-pgm intergenic region; lane 9, internal region of pgm gene. Lanes 2, 4, 6, 8, and 10 represent control reactions carried out without the addition of reverse transcriptase. Lane a, molecular size markers. (B) Reactions carried out with total RNA of strain A1120 (glgB mutant). Lane 1, glgP-glgB intergenic region; lane 2, internal region of pgm gene. Lane a, molecular size marker. All the other intergenic regions gave negative results (data not shown).
Identification of two promoters in the glg operon.
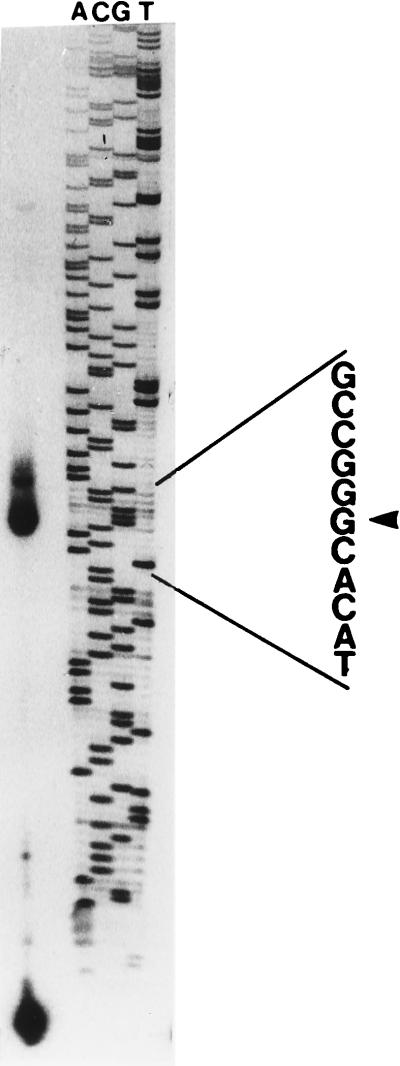
In order to study the activity of the regions identified by sequencing as putative promoters, β-galactosidase fusions were constructed as described in Materials and Methods. pFus97, a fusion of a 315-bp DNA region located upstream of the pgm2 ATG codon (Fig. 1), introduced in the A. tumefaciens wild-type background expressed β-galactosidase activity at 11.76 units of optical density at 420 nm (OD420) · mg of protein−1; on the other hand, in E. coli the β-galactosidase activity of this fusion was 0.05 OD420 unit · mg of protein−1. This indicates that the DNA region of 315 bp upstream of the putative ATG codon of pgm2 contains an active promoter that is more than 200 times stronger in A. tumefaciens than in E. coli. Fusion pFus98, containing a 650-bp DNA fragment located upstream of the glgP ATG start codon, introduced in the A. tumefaciens wild-type background expressed β-galactosidase activity at 37.07 OD420 units · mg of protein−1, which is 67 times higher than that observed in an E. coli background (0.55 OD420 unit · mg of protein−1). DNA sequence analysis of this 650-bp fragment revealed no homology to any gene in the database. The control fusion pFus96 expressed β-galactosidase activity neither in E. coli (0.02 OD420 unit · mg of protein−1) nor in A. tumefaciens (less than 0.01 OD420 unit · mg of protein−1), thus indicating that the β-galactosidase activity observed with pFus97 and pFus98 was indeed the result of the presence of active promoters upstream of the glgP and pgm2 genes. In order to define the transcription start site of the alternative promoter of pgm, a primer extension study was carried out. Total RNA was hybridized with an excess of single-stranded synthetic oligonucleotide PGMPE labeled with 32P at the 5′ terminus (see Materials and Methods and Fig. 1). As shown in Fig. 3, the transcription start site (the nucleotide shown by the arrow) of this alternative transcript is located 168 bp upstream of a second in-frame putative ATG codon, 8 bp upstream of which is a Shine-Dalgarno conserved sequence (Fig. 4). This transcript may produce a shorter Pgm protein, indicated as pgm2 in Fig. 4, that may account for the 24 and 26% remaining phosphoglucomutase activity observed in glgP and glgB polar mutants. This pgm promoter region contains 58% AT and has two copies of the sequence TATCAAN5G (Fig. 4), described to be present in the promoter regions of TraR autoinducible genes of octopine Ti plasmids (10, 11). It is noteworthy that the A5129 mutant completely lacks Pgm activity (Table 2). This is consistent with the fact that in A5129, the Tn5 insertion is located 430 bp downstream of the first ATG codon (35) and, consequently, downstream of both alternative promoters.
FIG. 3.
Mapping of the 5′ end of the pgm gene by primer extension analysis. The transcription start site is indicated by the arrow. Total RNA of the A. tumefaciens A348 wild-type strain was used to synthesize cDNA by employing avian myeloblastosis virus reverse transcriptase and the oligonucleotide PGMPE labeled with [γ-32P]ATP. The products were subjected to polyacrylamide gel electrophoresis in parallel with the sequencing ladder.
FIG. 4.
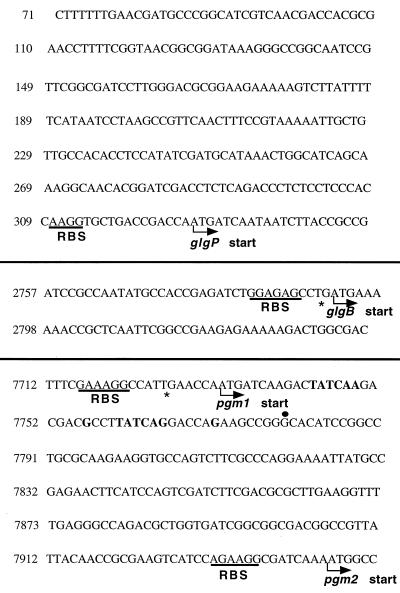
Nucleotide sequence of the glg gene cluster. Only regions that contained the putative promoters and transcriptional and translational start sites of the 9,100-bp DNA are shown. The sequence from positions 7716 to 7916 has been reported previously (35). Putative ribosome binding sites (RBS) are underlined, start codons are shown with an angled arrow, stop codons are shown with an asterisk, the transcriptional start site is shown with a dot, and putative promoters are in bold type. The complete sequence has been deposited in EMBL GenBank.
Detection of two phosphoglucomutase proteins by Western blot analysis and functional complementation.
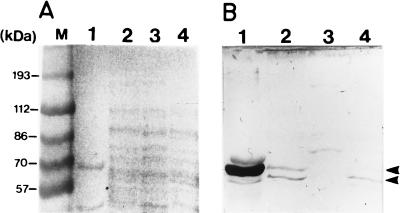
Primer extension and β-galactosidase fusion suggested that there is an alternative promoter located between two in-frame ATG codons of the pgm gene (Fig. 4) that might produce, if translated, a Pgm protein 71 amino acids shorter than the protein translated from the polycistronic glgPBCApgm mRNA (Fig. 1 and 4). It is shown in Fig. 5 that antibodies raised against a recombinant A. tumefaciens A348 Pgm recognized in extracts of the A. tumefaciens wild-type strain A348 two proteins with apparent molecular masses of 66 and 58 kDa (Fig. 5B, lane 2). Both proteins were absent in A. tumefaciens pgm mutant A5129 (Fig. 5B, lane 3), thus indicating that the two proteins represent two forms of Pgm with the molecular masses expected for Pgm1 and Pgm2 (Fig. 4). It can be observed that in cell extracts prepared from glgB mutant A1120, the amount of the smaller protein (58 kDa) was the same as that detected in wild-type extract; however, the amount of the protein with an apparent molecular mass of 66 kDa was severely reduced and barely detectable (Fig. 5B, lane 4). It can be observed that the antibody recognized a commercial rabbit Pgm (Fig. 5B, lane 1). These results indicate that in the glgB polar mutant A1120, Pgm protein was translated from the alternative transcript, which is expected to produce a protein 71 amino acids shorter than that translated from the polycistronic glg operon and could explain part of the remaining Pgm activity in the A1120 glgB mutant. The small amount of protein with a molecular mass of 66 kDa still detected in the A. tumefaciens A1120 mutant might be due to the presence of a cryptic promoter not detected by the primer extension experiment.
FIG. 5.
Western blot analysis. Cell extracts were subjected to polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes as described in Materials and Methods. (A) Protein stained with Ponceau S; (B) Western blot carried out with rabbit polyclonal antibody raised against recombinant A. tumefaciens Pgm developed with peroxidase-conjugated antibody against rabbit immunoglobulin G (Dako). Lanes 1, commercial rabbit Pgm; lanes 2, A. tumefaciens wild-type extract; lanes 3, A. tumefaciens pgm mutant A5129 extract; lanes 4, A. tumefaciens glgB mutant A1120 extract. M, prestained molecular mass standards. Arrows on the right indicate the positions of Pgm proteins.
In order to confirm that the short Pgm protein is active in vivo, a complementation experiment with the dark-phenotype A. tumefaciens pgm mutant A5129 (35) and plasmid pCC15 was carried out. The recombinant plasmid pCC15 (Table 1) was constructed by cloning in plasmid pBBR1MCS-4 (19) a DNA fragment containing the pgm gene from ATG1 (bp 7733 to bp 9377) as described in Materials and Methods. This construct does not contain the ribosome binding site upstream of ATG1 but contains the postulated promoter region of pgm2 upstream of ATG2 (Fig. 4). pCC15 was electroporated into A. tumefaciens pgm mutant A5129 and plated on LB medium containing 100 μg of carbenicillin per ml, 50 μg of kanamycin per ml, and 0.02% Calcofluor (Sigma Chemical Co.). All the transformants obtained were Cbr Kmr and bright under UV light, thus indicating that pgm function was restored and synthesis of exopolysaccharide had resumed. Since the DNA fragment was cloned in the opposite direction with respect to transcription of the endogenous promoter of the multiple cloning site of pBBR1MCS-4 (19), this result confirmed the presence of an alternative promoter between the two in-frame ATG codons (Fig. 4). Moreover, the experiment demonstrated that the short Pgm protein complements in trans the phenotype of the A5129 mutant. The possibility that recombination events were responsible for complementation was eliminated because 100% of the clones were Cbr Kmr and bright.
DISCUSSION
The glycogen (glg) operon of A. tumefaciens was studied, and its complete sequence was determined. The operon comprises five genes, glgP, glgB, glgC, glgA, and pgm, transcribed in the same direction as a single mRNA. A second promoter located downstream of glgA produces an alternative transcript of the pgm gene.
The overall organization of the operon in A. tumefaciens is different from that in E. coli, where it is formed by two operons, glgB and glgCAP (25); the pgm gene that is part of the Agrobacterium glg operon is not present in either of the two operons in E. coli.
The organization of the glg operon in B. stearothermophilus and B. subtilis (18, 34) is also different from that in E. coli or A. tumefaciens. In both bacilli, there is a single operon, glgBCDAP, in which glgD and glgC encode the subunits of a heterotetrameric ADP-glucose pyrophosphorylase. Neither the glgX nor the pgm homologous gene is part of the Bacillus operon.
RT-PCRs carried out with total RNA extracted from the wild type and the glgB mutant, assays of glg operon enzymes in the wild type and in glgP::Kmr and glgB::Kmr polar mutants, β-galactosidase fusions, and primer extension experiments confirmed the presence of a pgm mRNA transcribed from an alternative promoter. This alternative promoter is located within the coding region of the pgm gene and produces a Pgm protein 71 amino acids shorter than the Pgm protein translated from the polycistronic glgPBCApgm mRNA. Downstream of this alternative transcription start, a second in-frame ATG codon preceded by a Shine-Dalgarno sequence was identified. The predicted amino acid sequence of the N-terminal region of the protein translated from the polycistronic mRNA is 77% identical to the N-terminal region of rat Pgm (35); the active site and the potential phosphorylated serine are located downstream of this region (35). These results suggest that the enzymatic activity and pgm mRNA observed to be present in glgP::Kmr and glgB::Kmr polar mutants may be explained by transcription and translation of the pgm gene starting at this alternative pgm promoter. Moreover, a recombinant plasmid containing a pgm gene starting with the second ATG start codon complemented in trans a Tn5 A. tumefaciens pgm mutant, thus indicating that the 71-amino-acid-shorter protein is active in vivo. This alternative promoter may be turned on under specific metabolic or environmental conditions. A similar situation was described for the bovine β(1-4) galactosyltransferase, in which two promoters produce long and short mRNAs. The short mRNA starts in a region between two in-frame ATG codons (31). The promoter of the short mRNA was found to be a mammary gland-specific promoter, while the long mRNA functions as a housekeeping promoter (15).
In other bacteria the glg operon is regulated at the level of transcription, under metabolic conditions that lead to the accumulation of glycogen. Since in Agrobacterium the Leloir pathway is absent (36), Pgm is absolutely required for the biosynthesis of UDP-glucose, the sugar donor used for the synthesis of structural cell wall polysaccharides, exopolysaccharides, and cyclic β(1-2) glucans. This makes reasonable the hypothesis that an alternative promoter may be required to ensure, under certain conditions, the synthesis of Pgm independent of glg operon regulation.
The structure of the alternative pgm promoter contains a motif that resembles other Agrobacterium plant-inducible promoters (11), which might imply that pgm is induced when the bacteria reach the plant environment. It was previously described that cyclic β(1-2) glucans are required for virulence (26, 39). Cyclic glucans are synthesized by an inner membrane β(1-2) glucan transferase encoded by the chromosomal gene denominated chvB (7, 39). The enzyme is constitutively expressed and uses the sugar donor UDP-glucose (39). Thus, the supply of UDP-glucose must be guaranteed for the synthesis of cyclic glucans and for an effective infection. The presence of an alternative promoter that may be turned on when the bacterium reaches the plant environment might be required for the expression of pgm under nutrient-limiting conditions that may shut off the glg operon. Studies to investigate this possibility are in progress.
ACKNOWLEDGMENTS
This work was supported by grants from the Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET) (Argentina) and Universidad Nacional de General San Martin (Argentina). V.L., A.I., and R.U. are researchers of the CONICET.
We acknowledge Diego de Mendoza, University of Rosario, Rosario, Argentina, for kindly providing the kanamycin cassette; Anke Becker, Lehrstühl für Genetik, Universität Bielefeld, Bielefeld, Germany, for kindly providing the translation fusion vector; Fabio Fraga, University of General San Martín, Buenos Aires, Argentina, for preparing rabbit antibodies; and J. J. Cazzulo and A. C. Frasch, University of General San Martín, for critical reading of the manuscript and useful suggestions.
REFERENCES
- 1.Austin D, Larson T J. Nucleotide sequence of the glpD gene encoding aerobic sn-glycerol 3-phosphate dehydrogenase of Escherichia coli K-12. J Bacteriol. 1991;173:101–107. doi: 10.1128/jb.173.1.101-107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 1. New York, N.Y: Wiley Interscience; 1987. [Google Scholar]
- 2a.Becker, A. Unpublished data.
- 3.Bullock W O, Fernandez J M, Short J M. XL1-blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques. 1987;5:376–379. [Google Scholar]
- 4.Cangelosi G A, Hung L, Puvanesarajah V, Stacey G, Ozoa A D, Leigh J A, Nester E W. Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J Bacteriol. 1987;169:2086–2091. doi: 10.1128/jb.169.5.2086-2091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G S, Segel I H. Escherichia coli polyglucose phosphorylases. Arch Biochem Biophys. 1968;127:164–174. doi: 10.1016/0003-9861(68)90213-0. [DOI] [PubMed] [Google Scholar]
- 6.Choi Y L, Kawamukai M, Utsumi R, Sakai H, Komano T. Molecular cloning and sequencing of the glycogen phosphorylase gene from Escherichia coli. FEBS Lett. 1989;243:193–198. doi: 10.1016/0014-5793(89)80128-0. [DOI] [PubMed] [Google Scholar]
- 7.Douglas C J, Staneloni R J, Rubin R A, Nester E W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985;161:850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eidels L, Preiss J. Carbohydrate metabolism in Rhodopseudomonas capsulata: enzyme titers, glucose metabolism, and polyglucose polymer synthesis. Arch Biochem Biophys. 1970;140:75–89. doi: 10.1016/0003-9861(70)90011-1. [DOI] [PubMed] [Google Scholar]
- 9.Fletterick R J, Madsen N B. The structures and related functions of phosphorylase a. Annu Rev Biochem. 1980;49:31–61. doi: 10.1146/annurev.bi.49.070180.000335. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua C, Winans S C. Localization of OccR-activated and TraR-activated promoters that express two ABC-type permeases and traR gene of Ti plasmid pTiR10. Mol Microbiol. 1996;20:1199–1210. doi: 10.1111/j.1365-2958.1996.tb02640.x. [DOI] [PubMed] [Google Scholar]
- 12.Garfinkel D J, Simpson R B, Ream L W, White F F, Gordon M P, Nester E W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981;27:143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh H P, Preiss J. Adenosine diphosphate pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966;241:4491–4504. [PubMed] [Google Scholar]
- 14.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 15.Harduin-Lepers A, Shaper J H, Shaper N L. Characterization of two cis-regulatory regions in the murine beta 1,4,-galactosyltransferase gene. Evidence for a negative regulatory element that controls initiation at the proximal site. J Biol Chem. 1993;268:14348–14359. [PubMed] [Google Scholar]
- 16.Hawker J S, Ozbun J L, Ozaki H, Greenberg E, Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974;160:530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- 17.Haziza C, Stragier P, Patte J C. Nucleotide sequence of the asd gene of Escherichia coli: absence of a typical attenuation signal. EMBO J. 1982;1:379–384. doi: 10.1002/j.1460-2075.1982.tb01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiel J A, Boels J M, Beldman G, Venema G. Glycogen in Bacillus subtilis: molecular characterization of an operon encoding enzymes involved in glycogen biosynthesis and degradation. Mol Microbiol. 1994;11:203–218. doi: 10.1111/j.1365-2958.1994.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop II R M, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 20.Lipman D J, Pearson W R. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1440. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 21.MacGregor E A, Svensson B. A super-secondary structure predicted to be common to several α-1,4-d-glucan-cleaving enzymes. Biochem J. 1989;259:145–152. doi: 10.1042/bj2590145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka A, Sugisaki H, Takamani M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981;147:217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- 23.Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- 24.Preiss J, Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–238. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- 25.Preiss J, Romeo T. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1994;47:299–328. doi: 10.1016/s0079-6603(08)60255-x. [DOI] [PubMed] [Google Scholar]
- 26.Puvanesarajah V, Schell F M, Stacey G, Douglas C J, Nester E W. Role of 2-linked-beta-d-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985;164:102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian N, Stanley G A, Hahn-Hagerdal B, Radstrom P. Purification and characterization of two phosphoglucomutases from Lactococcus lactis subsp. lactis and their regulation in maltose- and glucose-utilizing cells. J Bacteriol. 1994;176:5304–5311. doi: 10.1128/jb.176.17.5304-5311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romeo T, Kumar A, Preiss J. Analysis of the Escherichia coli glycogen gene cluster suggests that catabolic enzymes are encoded among the biosynthetic genes. Gene. 1988;79:363–376. doi: 10.1016/0378-1119(88)90208-9. [DOI] [PubMed] [Google Scholar]
- 29.Romeo T, Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vitro effects of cyclic AMP and guanosine 5′-diphosphate 3′-diphosphate and analysis of in vitro transcripts. J Bacteriol. 1989;171:2773–2782. doi: 10.1128/jb.171.5.2773-2782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman-Denes L B, Cabib E. Two forms of yeast glycogen synthetase and their role in glycogen accumulation. Proc Natl Acad Sci USA. 1970;66:967–974. doi: 10.1073/pnas.66.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo R N, Shaper N L, Shaper J H. Bovine beta 1,4-galactosyltransferase: two sets of mRNA transcripts encode two forms of the protein with different amino-terminal domains. In vitro translation experiments demonstrate that both the short and the long form of the enzyme are type II membrane-bound glycoproteins. J Biol Chem. 1990;265:3324–3331. [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takata H, Takata T, Okada S, Takadi M, Imanaka T. Characterization of a gene cluster for glycogen biosynthesis and heterotetrameric ADP-glucose pyrophosphorylase from Bacillus stearothermophilus. J Bacteriol. 1997;179:4689–4698. doi: 10.1128/jb.179.15.4689-4698.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uttaro A, Ugalde R A. A chromosomal cluster of genes encoding ADP-glucose synthetase, glycogen synthetase and phosphoglucomutase in Agrobacterium tumefaciens. Gene. 1994;150:117–122. doi: 10.1016/0378-1119(94)90869-9. [DOI] [PubMed] [Google Scholar]
- 36.Uttaro A, Ielpi L, Ugalde R A. Galactose metabolism in Rhizobiaceae. Characterization of exoB mutants. J Gen Microbiol. 1993;139:1055–1062. [Google Scholar]
- 37.Woodcock D H, Crowther P J, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith S S, Michael M Z, Graham M W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Liu M Y, Romeo T. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the csrA gene product. J Bacteriol. 1996;178:1012–1017. doi: 10.1128/jb.178.4.1012-1017.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zorreguieta A, Ugalde R A. Formation in Rhizobium and Agrobacterium spp. of a 235-kilodalton protein intermediate in beta-d-(1-2) glucan synthesis. J Bacteriol. 1986;167:947–951. doi: 10.1128/jb.167.3.947-951.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]