Abstract
BACKGROUND
Pelvic radiation plus sensitizing chemotherapy with a fluoropyrimidine (chemoradiotherapy) before surgery is standard care for locally advanced rectal cancer in North America. Whether neoadjuvant chemotherapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) can be used in lieu of chemoradiotherapy is uncertain.
METHODS
We conducted a multicenter, unblinded, noninferiority, randomized trial of neoadjuvant FOLFOX (with chemoradiotherapy given only if the primary tumor decreased in size by <20% or if FOLFOX was discontinued because of side effects) as compared with chemoradiotherapy. Adults with rectal cancer that had been clinically staged as T2 node-positive, T3 node-negative, or T3 node-positive who were candidates for sphincter-sparing surgery were eligible to participate. The primary end point was disease-free survival. Noninferiority would be claimed if the upper limit of the two-sided 90.2% confidence interval of the hazard ratio for disease recurrence or death did not exceed 1.29. Secondary end points included overall survival, local recurrence (in a time-to-event analysis), complete pathological resection, complete response, and toxic effects.
RESULTS
From June 2012 through December 2018, a total of 1194 patients underwent randomization and 1128 started treatment; among those who started treatment, 585 were in the FOLFOX group and 543 in the chemoradiotherapy group. At a median follow-up of 58 months, FOLFOX was noninferior to chemoradiotherapy for disease-free survival (hazard ratio for disease recurrence or death, 0.92; 90.2% confidence interval [CI], 0.74 to 1.14; P = 0.005 for noninferiority). Five-year disease-free survival was 80.8% (95% CI, 77.9 to 83.7) in the FOLFOX group and 78.6% (95% CI, 75.4 to 81.8) in the chemoradiotherapy group. The groups were similar with respect to overall survival (hazard ratio for death, 1.04; 95% CI, 0.74 to 1.44) and local recurrence (hazard ratio, 1.18; 95% CI, 0.44 to 3.16). In the FOLFOX group, 53 patients (9.1%) received preoperative chemoradiotherapy and 8 (1.4%) received postoperative chemoradiotherapy.
CONCLUSIONS
In patients with locally advanced rectal cancer who were eligible for sphincter-sparing surgery, preoperative FOLFOX was noninferior to preoperative chemoradiotherapy with respect to disease-free survival. (Funded by the National Cancer Institute; PROSPECT ClinicalTrials.gov number, NCT01515787.)
Pelvic chemoradiotherapy for locally advanced rectal cancer reduces the risk of disease recurrence in the pelvis to less than 10% and has been standard care in North America since 1990.1–6 However, it is associated with short-term and long-term toxic effects7–9 that can adversely affect quality of life and physical function.8 In 2004, a randomized trial established the superiority of preoperative to postoperative pelvic chemoradiotherapy with fluorouracil sensitization.10 Also in 2004, postoperative (adjuvant) chemotherapy with the FOLFOX regimen, which combines fluorouracil, leucovorin, and oxaliplatin, was found to prolong disease-free survival as compared with fluorouracil alone among patients with stage III colon cancer.11 FOLFOX has also been shown to be associated with high response rates when administered before chemoradiotherapy.2,12
These findings motivated us to investigate whether neoadjuvant treatment with FOLFOX could allow the elimination of chemoradiotherapy without increasing the risk of recurrence. In a single-center pilot trial of neoadjuvant FOLFOX in which administration of chemoradiotherapy was reserved for patients whose tumors did not respond to chemotherapy, we found favorable outcomes, with few patients going on to receive radiation and none having local disease recurrence.13 On the basis of these results, we designed the PROSPECT trial (Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients with Locally Advanced Rectal Cancer Undergoing Surgery) to test the hypothesis that neoadjuvant FOLFOX, with chemoradiotherapy reserved for patients whose tumors responded poorly or in whom FOLFOX was discontinued because of side effects, would be noninferior to neoadjuvant chemoradiotherapy alone in patients with locally advanced rectal cancer that was amenable to sphincter-sparing surgery.
Methods
TRIAL DESIGN AND PATIENTS
We conducted a multicenter, unblinded, randomized, noninferiority trial with a seamless phase 2–3 design; the trial was sponsored by the Alliance for Clinical Trials in Oncology (Alliance, a cooperative research network funded by the National Cancer Institute) and was conducted in Canada, Switzerland, and the United States at 264 academic and community-based institutions (see the protocol, available with the full text of this article at NEJM.org). We recruited patients 18 years of age or older who had previously untreated, pathologically confirmed, locally advanced rectal cancer that had been clinically staged as T2 node-positive, T3 node-negative, or T3 node-positive (Table S1 in the Supplementary Appendix, available at NEJM.org) on the basis of the tumor–node–metastasis system in the Cancer Staging Manual of the American Joint Committee on Cancer, 7th edition. For a patient to be eligible, the patient’s primary surgeon had to consider neoadjuvant pelvic chemoradiotherapy followed by a sphincter-sparing operation as the appropriate treatment approach. Participants had to have an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0, 1, or 2 (scores range from 0 to 5, with higher scores indicating greater disability). Patients with T4 tumors, four or more pelvic lymph nodes with a short axis larger than 10 mm, or tumor visible within 3 mm of the radial margin seen on baseline pelvic imaging were ineligible. Among the other exclusion criteria were previous pelvic radiation therapy, chemotherapy within the previous 5 years, or abnormal laboratory measures.
TRIAL OVERSIGHT
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. The protocol was approved by the institutional review board at each participating institution or by the National Cancer Institute (NCI) central institutional review board. All the patients provided written informed consent before enrollment. The trial was monitored by the Alliance data and safety monitoring board twice per year. In April 2016, the data and safety monitoring board determined that the phase 2 data met the criteria for seamlessly proceeding to the phase 3 trial. Because fewer recurrences and deaths were noted than had been projected, the coprimary end points of disease-free survival and local recurrence (in a time-to-event analysis) were modified to disease-free survival alone. The statistical redesign was led by an independent statistician who was unaware of the trial data in collaboration with the NCI Cancer Therapy Evaluation Program and was approved by the data and safety monitoring board in May 2021. The amendments and statistical analysis plan are provided in the protocol. The first two authors developed the trial design, and the second author had access to the raw data after release and analyzed the data. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The NCI funded the trial and approved the design but had no role in the interpretation of the data.
RANDOMIZATION AND PROCEDURES
Pelvic magnetic resonance imaging (MRI) was recommended at baseline, but contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis plus endorectal ultrasonography was an acceptable alternative. After eligibility was confirmed, patients underwent randomization in a 1:1 ratio on the basis of a dynamic randomization scheme with stratification according to ECOG performance-status score (a score of 0 or 1 vs. 2).
Patients in the FOLFOX group received six cycles of modified FOLFOX614 administered intravenously every 2 weeks, followed by restaging with pelvic imaging and rectal endoscopy. Patients who were unable to complete at least five cycles of FOLFOX were given chemoradiotherapy (with the use of the procedures used in the chemoradiotherapy group; see below). Patients whose primary tumor had decreased in size by at least 20% as determined by the surgeon on the basis of restaging imaging, proctoscopy, and physical examination proceeded to surgery, and those whose primary tumor had decreased in size by less than 20% received chemoradiotherapy. Postoperative chemoradiotherapy was recommended for patients in the FOLFOX group whose resection was not pathologically complete (R0). Postoperative adjuvant chemotherapy with an additional six cycles of FOLFOX was suggested but not mandated.
Patients in the chemoradiotherapy group received pelvic radiotherapy with 50.4 Gy delivered in 28 fractions, together with sensitizing fluoropyrimidine chemotherapy delivered either as a continuous intravenous infusion of fluorouracil at a dose of 225 mg per square meter of body-surface area per day or as oral capecitabine at a dose of 825 mg per square meter twice daily, 5 days per week on days of radiation therapy, with the choice at the discretion of the patient and medical oncologist. Postoperative adjuvant chemotherapy with eight cycles of FOLFOX was suggested but not mandated. In both treatment groups, the choice between three-dimensional conformal radiotherapy or intensity-modulated radiotherapy was at the discretion of the radiation oncologist. The surgical approach (open resection vs. laparoscopic or robot-assisted) was chosen at the surgeon’s discretion.
END POINTS
The primary objective was to determine whether neoadjuvant FOLFOX with selective use of chemoradiotherapy would be noninferior to standard neoadjuvant chemoradiotherapy with respect to disease-free survival, defined as survival free from disease recurrence or death from any cause, assessed in a time-to-event analysis. Data from patients who did not die or have disease recurrence were censored at the date of the last disease evaluation.
Secondary end points included overall survival, local recurrence (in a time-to-event analysis), R0 resection, pathological complete response, and toxic effects. Overall survival was assessed in a time-to-event analysis as survival free from death from any cause. In the time-to-event analysis of local recurrence, isolated local recurrences were identified on the basis of their location in the pelvis, irrespective of the mode of detection or whether they could be surgically resected. R0 resection was identified on the basis of a pathology report showing no involvement of proximal, distal, or radial tumor within 1 mm of any surgical margin. Pathological complete response was confirmed if the surgical pathology report showed no evidence of tumor.
Adverse events were reported by clinicians using the NCI Common Toxicity Criteria and Adverse Events (CTCAE), version 4.15 Patients reported their symptoms and adverse events with the use of the patient-reported outcomes version of the CTCAE (PRO-CTCAE).16
STATISTICAL ANALYSIS
Noninferiority with respect to the primary end point required a hazard ratio for disease recurrence or death with a margin of less than 1.29, corresponding to 5-year disease-free survival that was 5 percentage points lower in the FOLFOX group than in the chemoradiotherapy group. In the original design, the prespecified acceptable maximum absolute between-group difference in 3-year disease-free survival was 5 percentage points. This noninferiority margin was considered to be clinically important and appropriate by more than 50 surgeons and radiation and medical oncologists, as well as by patient representatives attending the Alliance Gastrointestinal Oncology Meetings in November 2010 and May 2011 who participated in interactive polling to establish a noninferiority threshold. This margin was maintained with 5-year (instead of 3-year) disease-free survival in the revised statistical design. One interim analysis was performed before the major revision, and two interim analyses were removed to preserve power with a remaining one-sided alpha level of 0.049. The revised design required 210 instances of disease recurrence or death in order to have 85% power to reject the null hypothesis. Noninferiority of the intervention could be claimed if the upper limit of the two-sided 90.2% confidence interval of the hazard ratio for disease recurrence or death did not exceed the 1.29 noninferiority margin. Hazard ratios and their confidence intervals were estimated with the use of stratified Cox proportional-hazards models.
Because noninferiority testing was planned, the analysis of the primary end point was based on all the patients who received any dose of treatment (per-protocol population). Multivariate analyses were adjusted for age and baseline nodal status (node-positive vs. node-negative), given the prognostic importance of these features. Because the statistical analysis plan did not include a provision for correcting for multiplicity when conducting tests for secondary or other end points, results are reported as point estimates and 95% confidence intervals. The widths of the confidence intervals have not been adjusted for multiplicity, and therefore the intervals should not be used to infer definitive treatment effects for secondary end points. Chi-square and logrank tests were used to compare categorical and time-to-event secondary end points, respectively, between the treatment groups. Supplemental analyses were based on all the patients who underwent randomization, as well as on the per-protocol population that excluded patients who were found to be ineligible after randomization. Analyses were conducted with the use of SAS software, version 9.4 (SAS Institute).
Results
PATIENTS
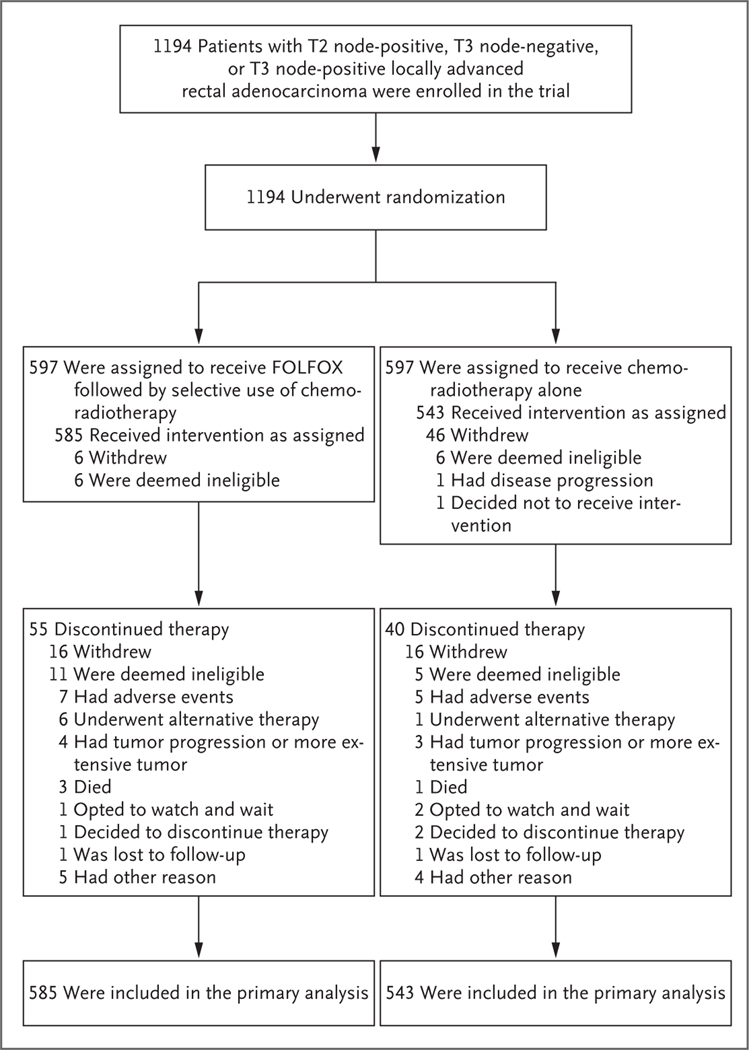
From June 2012 through December 2018, a total of 1194 patients with pathologically confirmed rectal adenocarcinoma that had been clinically staged as T2 node-positive, T3 node-negative, or T3 node-positive were randomly assigned to the FOLFOX group (597 patients) or the chemoradio therapy group (597 patients) at 263 participating institutions. After randomization, 66 patients (12 in the FOLFOX group and 54 in the chemoradiotherapy group) did not receive any protocol treatment. A total of 1128 patients (585 in the FOLFOX group and 543 in the chemoradiotherapy group) began treatment and were included in the primary per-protocol analysis (Fig. 1). The pretreatment demographic and clinical characteristics of these patients are shown in Table 1. The median follow-up for end points was 58 months. The trial database was locked on December 15, 2022, when 227 of the target 210 events in the analysis of disease-free survival had occurred.
Figure 1. Enrollment, Randomization, and Follow-up.
Sites were not required to provide screening logs during the recruitment phase, and therefore the number of patients assessed for eligibility is not available. FOLFOX consists of fluorouracil, leucovorin, and oxaliplatin; the chemoradiotherapy used in the trial consisted of pelvic radiation therapy plus sensitizing chemotherapy with a fluoropyrimidine. Patients in the FOLFOX group received six cycles of FOLFOX, with chemoradiotherapy given only if the primary tumor decreased in size by less than 20% or if FOLFOX was discontinued because of side effects; patients in the chemoradiotherapy group received chemoradiotherapy alone.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline (Per-Protocol Population).*
| Characteristic | FOLFOX Group (N = 585) | Chemoradiotherapy Group (N = 543) |
|---|---|---|
| Age — yr | ||
| Mean | 57.3±10.9 | 57.0±11.1 |
| Median (range) | 57 (19–91) | 57 (25–84) |
| Sex — no. (%) | ||
| Female | 216 (36.9) | 173 (31.9) |
| Male | 369 (63.1) | 370 (68.1) |
| Race — no. (%)† | ||
| White | 492 (84.1) | 467 (86.0) |
| Black | 32 (5.5) | 17 (3.1) |
| Asian | 31 (5.3) | 19 (3.5) |
| Other or not reported | 30 (5.1) | 40 (7.4) |
| Hispanic or Latino ethnic group† | ||
| Yes | 48 (8.2) | 48 (8.8) |
| No | 516 (88.2) | 475 (87.5) |
| Unknown or not reported | 21 (3.6) | 20 (3.7) |
| Country of residence — no. (%) | ||
| Canada | 51 (8.7) | 45 (8.3) |
| Switzerland | 10 (1.7) | 9 (1.7) |
| United States | 524 (89.6) | 489 (90.1) |
| Body-mass index‡ | ||
| Mean | 29.3±6.0 | 29.1±6.7 |
| Median (range) | 28.4 (14.5–65.4) | 28.1 (15.8–81.4) |
| Distribution — no. (%) | ||
| <18.5 | 4 (0.7) | 6 (1.1) |
| ≥18.5 to <25 | 127 (21.7) | 139 (25.6) |
| ≥25 to <30 | 225 (38.5) | 200 (36.8) |
| ≥30 | 229 (39.1) | 198 (36.5) |
| History of diabetes — no. (%) | ||
| Yes | 81 (13.8) | 83 (15.3) |
| No | 504 (86.2) | 460 (84.7) |
| History of cardiovascular disease — no. (%) | 106 (18.1) | 98 (18.0) |
| Starting neoadjuvant treatment — no. (%) | 479 (81.9) | 445 (82.0) |
| Highest education level — no./total no. (%) | ||
| Less than high school | 29/568 (5.1) | 29/531 (5.5) |
| High school diploma or GED certificate | 214/568 (37.7) | 201/531 (37.9) |
| Some college | 119/568 (21.0) | 102/531 (19.2) |
| College degree or higher | 206/568 (36.3) | 199/531 (37.5) |
| ECOG performance-status score — no. (%)§ | ||
| 0 or 1 | 582 (99.5) | 540 (99.4) |
| 2 | 3 (0.5) | 3 (0.6) |
| Primary rectal tumor on digital examination — no./total no. (%) | ||
| Rectal tumor not palpable | 290/580 (50.0) | 259/536 (48.3) |
| Rectal tumor palpable | 290/580 (50.0) | 277/536 (51.7) |
| Rectal tumor location — cm from anal verge | ||
| No. of patients with data | 585 | 542 |
| Mean | 8.6±2.9 | 8.5±2.8 |
| Median (range) | 8 (2–25) | 8 (2–18) |
| Rectal tumor location — no. (%) | ||
| ≤5 cm from anal verge | 83 (14.2) | 90 (16.6) |
| >5 to ≤10 cm from anal verge | 375 (64.1) | 344 (63.4) |
| >10 cm from anal verge | 127 (21.7) | 109 (20.1) |
| Clinical stage — no./total no. (%) | ||
| T2 node positive | 63/584 (10.8) | 38/543 (7.0) |
| T3 node negative | 232/584 (39.7) | 198/543 (36.5) |
| T3 node positive | 289/584 (49.5) | 307/543 (56.5) |
| Staging performed with MRI — no. (%) | ||
| Yes | 494 (84.4) | 458 (84.3) |
| No | 91 (15.6) | 85 (15.7) |
Plus–minus values are means ±SD. The per-protocol population included all the patients who received any dose of treatment. FOLFOX consists of fluorouracil, leucovorin, and oxaliplatin; the chemoradiotherapy used in the trial consisted of pelvic radiation therapy plus sensitizing chemotherapy with a fluoropyrimidine. Patients in the FOLFOX group received six cycles of FOLFOX, with chemoradiotherapy given only if the primary tumor decreased in size by less than 20% or if FOLFOX was discontinued because of side effects; patients in the chemoradiotherapy group received chemoradiotherapy alone. Percentages may not total 100 because of rounding. GED denotes General Educational Development.
Race and ethnic group were reported by the patients.
The body-mass index is the weight in kilograms divided by the square of the height in meters. Three patients had large values (>65), two as a result of an unusually short height (≤108 cm) and one because of an unusually high weight (205 kg). The sites confirmed that these data were correct.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
DISEASE-FREE SURVIVAL
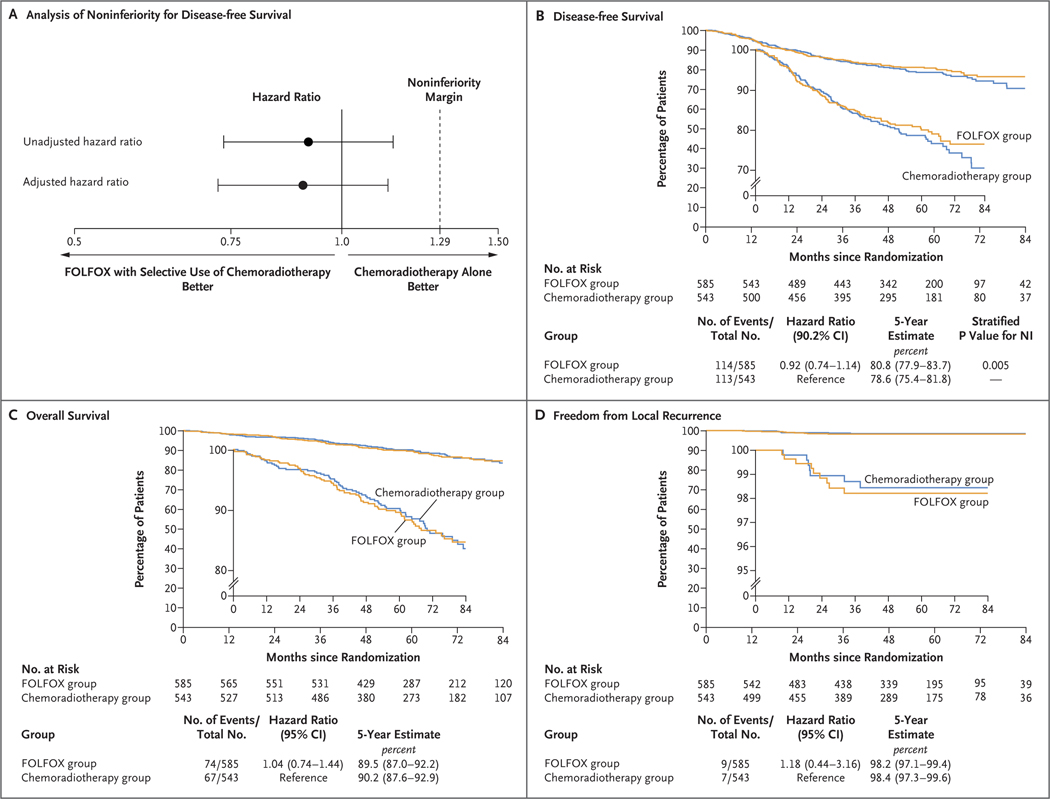
FOLFOX with selective use of chemoradiotherapy was found to be noninferior to chemoradiotherapy with respect to disease-free survival (hazard ratio for disease recurrence or death, 0.92; two-sided 90.2% confidence interval [CI], 0.74 to 1.14; P = 0.005 for noninferiority), and an analysis with adjustment for age and clinical nodal status yielded consistent results (Fig. 2A and 2B). Five-year disease-free survival was 80.8% (95% CI, 77.9 to 83.7) in the FOLFOX group and 78.6% (95% CI, 75.4 to 81.8) in the chemoradiotherapy group. Figure S3 shows a comparison of disease-free survival in prespecified subgroups of interest. In a supplementary analysis of disease-free survival involving all 1194 patients who underwent randomization, the hazard ratio for disease recurrence or death was 0.91 (90.2% CI, 0.73 to 1.13; P = 0.004 for noninferiority); results were similar for patients in the per-protocol population who were later found to be ineligible. The proportional-hazards assumption was not violated (P = 0.30 by the Schoenfeld residuals method).
Figure 2. Noninferiority Margin and Kaplan–Meier Curves for Disease-free Survival, Overall Survival, and Freedom from Local Recurrence.
In Panel A, the dashed line at a hazard ratio of 1.29 indicates the noninferiority margin. Values to the left of 1.29 are those for which FOLFOX with selective use of chemoradiotherapy would be considered noninferior to chemoradiotherapy alone with respect to disease-free survival. The adjusted hazard ratio was estimated with a multivariable Cox model with adjustment for age and nodal status (node-positive vs. node-negative). The widths of the confidence intervals in Panels C and D have not been adjusted for multiplicity, and therefore these intervals should not be used to infer definitive treatment effects. NI denotes noninferiority.
OVERALL SURVIVAL AND LOCAL RECURRENCE
Five-year overall survival was 89.5% in the FOLFOX group and 90.2% in the chemoradiotherapy group (hazard ratio for death, 1.04; 95% CI, 0.74 to 1.44) (Fig. 2C). Local recurrence occurred in nine patients in the FOLFOX group and seven patients in the chemoradiotherapy group; the incidence of local recurrence at 5 years was 1.8% and 1.6%, respectively (hazard ratio, 1.18; 95% CI, 0.44 to 3.16) (Fig. 2D).
PATHOLOGICAL AND SURGICAL SECONDARY END POINTS
Surgical and pathological end points are shown in Table 2. In the per-protocol population, resection was pathologically complete (R0) in 90.4% of the patients in the FOLFOX group and in 91.2% of those in the chemoradiotherapy group. Among the patients in the per-protocol population who underwent surgery, the corresponding percentages were 98.9% and 97.1%, respectively (Table 2). Among the patients in the per-protocol population who underwent surgery, 117 of 535 patients (21.9%) in the FOLFOX group and 124 of 510 (24.3%) in the chemoradiotherapy group had a complete pathological response.
Table 2.
Surgical and Pathological Secondary and Exploratory End Points in Patients in the Per-Protocol Population Who Underwent Surgery.
| End Point | FOLFOX Group (N = 535) | Chemoradiotherapy Group (N = 510) |
|---|---|---|
| Secondary end points | ||
| Completeness of rectal resection — no. (%)* | ||
| R0 | 529 (98.9) | 495 (97.1) |
| R1 | 6 (1.1) | 14 (2.7) |
| R2 | 0 | 1 (02) |
| Pathological complete response — no. (%)† | ||
| Yes | 117 (21.9) | 124 (24.3) |
| No | 418 (78.1) | 386 (75.7) |
| Other surgical and pathological end points | ||
| Median time from randomization to surgery (interquartile range) — wk | 19.0 (17.1–21.1) | 15.6 (14.6–17.0) |
| Median time from end of preoperative therapy to surgery (interquartile range) — wk‡ | 4.6 (3.1–6.3) | 7.7 (6.9–9.0) |
| Type of surgery — no. (%) | ||
| Abdominal perineal resection | 13 (2.4) | 10 (2.0) |
| Low anterior resection | 522 (97.6) | 500 (98.0) |
| Histologic grade — no./total no. (%)§ | ||
| G1 or G2 | 396/535 (74.0) | 344/504 (68.3) |
| G3 or G4 | 22/535 (4.1) | 27/504 (5.4) |
| GX | 117/535 (21.9) | 133/504 (26.4) |
| Radial margin category — no./total no. (%)¶ | ||
| ≤1 mm | 6/509 (1.2) | 7/469 (1.5) |
| >1 mm but ≤3 mm | 26/509 (5.1) | 31/469 (6.6) |
| >3 mm | 477/509 (93.7) | 431/469 (91.9) |
| Pathological tumor stage after neoadjuvant therapy — no./total no. (%) | ||
| ypT0 | 121/534 (22.7) | 125/506 (24.7) |
| ypT1 | 56/534 (10.5) | 50/506 (9.9) |
| ypT2 | 183/534 (34.3) | 156/506 (30.8) |
| ypT3 | 169/534 (31.6) | 173/506 (34.2) |
| ypT4 | 5/534 (0.9) | 2/506 (0.4) |
| Pathological node status after neoadjuvant therapy — no. (%) | ||
| ypN0 | 400 (74.8) | 390 (76.5) |
| ypN1 | 108 (20.2) | 104 (20.4) |
| ypN2 | 27 (5.0) | 16 (3.1) |
| Pathological metastatic status — no./total no. (%) | ||
| M0 | 520/521 (99.8) | 494/499 (99.0) |
| M1a | 1/521 (0.2) | 5/499 (1.0) |
| Tumor regression grade — no./total no. (%)‖ | ||
| Pathological complete response or grade 0 | 123/533 (23.1) | 127/510 (24.9) |
| Grade 1 | 161/533 (30.2) | 200/510 (39.2) |
| Grade 2 | 146/533 (27.4) | 151/510 (29.6) |
| Grade 3 | 103/533 (19.3) | 32/510 (6.3) |
An R0 (complete) resection was defined as a surgical specimen with no tumor identified within 1 mm of any surgical margin and no macroscopic evidence of residual tumor.
Pathological complete response was confirmed if the surgical pathology report showed no evidence of tumor.
The end of neoadjuvant therapy was defined as the start date of the last cycle of FOLFOX plus 2 weeks for patients who received neoadjuvant FOLFOX only and as the end date of preoperative radiation treatment for patients in either group who received neoadjuvant chemoradiotherapy.
A histologic grade of G1 indicates well differentiated, G2 moderately differentiated, G3 poorly differentiated, G4 undifferentiated or anaplastic, and GX not assessable.
A margin of 1 mm or less was considered positive in accordance with the Cancer Staging Manual of the American Joint Committee on Cancer, 7th edition; greater than 1 mm but no greater than 3 mm is considered negative (but close to positive), and greater than 3 mm is considered negative.
Tumor regression grades range from 0 to 3, with higher grades indicating greater degrees of pathological response.
NEOADJUVANT TREATMENT DURATION AND ADHERENCE
The median time from randomization to surgery was 19.0 weeks (interquartile range, 17.1 to 21.1) in the FOLFOX group and 15.6 weeks (interquartile range, 14.6 to 17.0) in the chemoradiotherapy group (Table 2). In the FOLFOX group, 555 of 585 patients (94.9%) received at least five cycles of neoadjuvant therapy (Table S3A) and 53 of 585 (9.1%) received neoadjuvant chemoradiotherapy. Eight patients (1.4%) in the FOLFOX group received fewer than six cycles of FOLFOX, 38 patients (6.5%) did not meet the clinical response threshold of a 20% decrease in primary tumor size, and in 7 cases (1.2% of the patients) the treating physician or patient opted for chemoradiotherapy after randomization for other reasons. Among the patients who were randomly assigned to the FOLFOX group who subsequently received chemoradiotherapy, 52 of 53 (98%) received the full dose of 50.4 Gy. In the chemoradiotherapy group, 515 of 543 patients (94.8%) completed the full dose of 50.4 Gy (Table S3B).
POSTOPERATIVE TREATMENT
In the FOLFOX group, 438 of 585 patients (74.9%) received adjuvant chemotherapy; 348 of these 438 patients (79.5%) received adjuvant FOLFOX for a median of six cycles. In the chemoradiotherapy group, 423 of 543 patients (77.9%) received adjuvant chemotherapy; 281 of these 423 patients (66.4%) received FOLFOX for a median of eight cycles, and an additional 60 patients (14.2%) received postoperative capecitabine and oxaliplatin (CAPOX). Ultimately, 61 of 585 patients (10.4%) in the FOLFOX group received chemoradiotherapy; 53 patients (9.1%) received it before surgery and 8 (1.4%) received it after surgery. The median total duration of treatment (from randomization to the last date of postoperative treatment) was 35.6 weeks (interquartile range, 32.9 to 39.3) in the FOLFOX group and 37.0 weeks (interquartile range, 34.0 to 40.4) in the chemoradiotherapy group (Table S4). Among the patients who underwent resection, 438 of 535 (81.9%) in the FOLFOX group and 423 of 510 (82.9%) in the chemoradiotherapy group received any postoperative therapy.
SAFETY
Details of clinician-reported toxic effects during neoadjuvant therapy (Table S2A) showed a higher incidence of severe (grade ≥3) adverse events in the FOLFOX group than in the chemoradiotherapy group (41.0% vs. 22.8%). However, the treatment period was twice as long in the FOLFOX group (minimum of 12 weeks, vs. 5.5 weeks in the chemoradiotherapy group). Neuropathy was more frequent and severe in the FOLFOX group than in the chemoradiotherapy group, and diarrhea was more frequent and severe in the chemoradiotherapy group than in the FOLFOX group. In the FOLFOX group, the most frequent grade 3 or higher toxic effects of neoadjuvant therapy were neutropenia, pain, and hypertension, reported by clinicians for 20.3%, 3.1%, and 2.9% of the patients, respectively. In the chemoradiotherapy group, the most frequent grade 3 or higher toxic effects reported by clinicians were lymphopenia, diarrhea, and hypertension, in 8.3%, 6.4% and 1.7% of the patients, respectively.
Among the patients who received any adjuvant therapy (Table S2B), severe (grade ≥3) postoperative adverse events occurred in a lower percentage of patients in the FOLFOX group than in the chemoradiotherapy group (25.6% vs. 32.6%). The most commonly reported postoperative grade 3 or higher toxic effects were neutropenia (in 3.9% of the patients), diarrhea (in 2.7%), and hyponatremia (in 2.3%) in the FOLFOX group and diarrhea (in 5.2%), dehydration (in 4.3%), and lymphopenia (in 4.3%) in the chemoradiotherapy group. No unanticipated toxic effects of either FOLFOX or chemoradiotherapy were observed.
Discussion
In patients with rectal cancer that had been clinically staged as T2 node-positive, T3 node-negative, or T3 node-positive who were candidates for sphincter-sparing surgery, neoadjuvant FOLFOX and selective use of pelvic chemoradiotherapy was noninferior to the current North American standard of neoadjuvant pelvic chemoradiotherapy with respect to disease-free survival. Among the patients assigned to receive neoadjuvant FOLFOX, 89.6% were ultimately able to avoid receiving chemoradiotherapy. Overall survival was also similar with the two treatment strategies.
The percentage of patients free from local recurrence was also similar in the two groups and exceeded 98% at 5 years. The major benefit of pelvic radiation therapy that has been shown in previous clinical trials is a decrease in the risk of pelvic recurrence.5,6,17,18 The very low incidence of local recurrence seen in the FOLFOX group in our trial supports the premise underpinning the trial: modern therapies, including staging MRI, chemotherapy with oxaliplatin and a fluoropyrimidine, and total mesorectal excision, obviate the need for universal application of pelvic radiation. The incidence of local recurrence was even lower than that reported in previous trials, most likely because the previous trials, including the Neoadjuvant FOLFOX6 Chemotherapy with or without Radiation in Rectal Cancer (FOWARC) trial,19 included patients who could not undergo sphincter-sparing surgery or who had other high-risk features. In that trial of neoadjuvant therapy, 165 patients were randomly assigned to receive FOLFOX and 165 to receive chemoradiotherapy, and 3-year disease-free survival was 72.9% and 73.5%, respectively.19
Most trial participants (61.8%) had clinical evidence of enlarged lymph nodes that were likely to contain a tumor, and most tumors were in the mid-rectum, with a median distance of 8 cm from the anal verge. When the trial was designed in 2011, concern was expressed about compromising the low incidence of local recurrence that would be expected to occur with administration of neoadjuvant pelvic radiation. Therefore, patients who were thought to be at high risk for incomplete resection, such as those with T4 stage disease or low-lying tumors, were excluded. Patients with four or more pelvic lymph nodes with a short axis measuring more than 10 mm were also excluded, although it is plausible that such patients might have the most to gain from neoadjuvant FOLFOX therapy.
Because our trial was launched in 2012, alternative approaches to the treatment of rectal cancer have emerged. The Rectal Cancer and Pre-operative Induction Therapy Followed by Dedicated Operation (RAPIDO) trial20 evaluated short-course pelvic radiation with preoperative CAPOX as compared with chemoradiotherapy and discretionary use of postoperative CAPOX. Although treatment for the control groups was similar in the two trials, the RAPIDO trial enrolled patients with high-risk tumors (e.g., T4) who were excluded from our trial, and differences in eligibility account for the higher incidence of recurrence in the RAPIDO trial.20 That trial showed that preoperative short-course radiation and chemotherapy were associated with a lower incidence of recurrence than preoperative chemoradiotherapy.20 This finding has been attributed to the lower rates of chemotherapy use in the chemoradiotherapy group in that trial. The 5-year risk of local recurrence was higher with short-course radiation, and this may explain the limited adoption of this treatment strategy in North America.21
Although the eligibility criteria used in our trial may limit its generalizability to high-risk patients, an analysis of Surveillance Epidemiology and End Results data reveals that patients with cancer clinically staged as T2 node-positive, T3 node-negative, or T3 node-positive account for more than half the patients with a diagnosis of locally advanced rectal cancer in the United States. In our trial, the percentage of patients with a pathological complete response was similar in the two groups (21.9% in the FOLFOX group and 24.3% in the chemoradiotherapy group), but further research will be necessary to determine whether distinctive molecular features predict responsiveness to chemotherapy as compared with radiation.
The widespread treatment of average-risk rectal cancer with neoadjuvant chemoradiotherapy, which has predominated in North America since publication of the German trial in 2004,10 increases the potential for overtreatment. It is plausible that the low incidence of recurrence in our trial could have been achieved with up-front surgery and selective use of adjuvant chemotherapy or pelvic chemoradiotherapy based on surgical pathological assessment. Although our trial shows that it is safe for some patients with locally advanced rectal cancer to forgo pelvic radiation, substitution of preoperative chemoradiotherapy for preoperative FOLFOX does not reduce the fundamental risk of overtreatment.
Because survival and the incidence of recurrence were similar in the two groups, the short-term and long-term symptoms reported by patients enrolled in our trial will be especially important. The incidence of toxic effects was higher during the 12 weeks of preoperative treatment with FOLFOX than during the 6 weeks of preoperative treatment with chemoradiotherapy. However, during the postoperative period, this pattern reversed, with a higher incidence of toxic effects among patients in the chemoradiotherapy group who received more postoperative cycles of FOLFOX. Thus, the differences in the incidence of toxic effects reflect the timing of FOLFOX administration. The patients who were enrolled in our trial were invited to report their symptoms and quality of life, and these data (not reported in this article) may enable patients and physicians to tailor treatment to individual risk profiles and preferences.22 Pelvic radiation therapy can cause late complications, including pelvic fracture, second cancers,9 and myelosuppression, which may impair a patient’s ability to receive chemotherapy in the event of recurrence.23 Longer follow-up is required in order to evaluate the magnitude of late effects.
The increasing number of treatment options for locally advanced rectal cancer enables customized treatment that is based on tumor-specific features. For example, patients whose tumors have high microsatellite instability can have a durable complete response with immunotherapy alone.24 These newer approaches, including total neoadjuvant therapy25,26 with the potential for nonoperative management,27 have not yet been evaluated in phase 3 trials, but they tailor treatment intensity to recurrence risk and may minimize toxic effects and the risk of overtreatment. The findings in this trial have extended the therapeutic options by showing the noninferiority of neoadjuvant FOLFOX with selective use of chemoradiotherapy to the prevailing standard of neoadjuvant chemoradiotherapy. In this trial, neoadjuvant FOLFOX was an effective treatment option for patients with T2 node-positive, T3 node-negative, or T3 node-positive rectal cancer who were candidates for sphincter-sparing surgery.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by grants from the National Cancer Institute of the National Institutes of Health (U10CA180821, U10CA180882, and UG1CA189823, to the Alliance for Clinical Trials in Oncology; UG1CA232760; UG1CA233180; UG1CA233290; UG1CA233329; UG1CA233373; P30 CA008748; P30CA006516; U10CA180863; CCS#707213, to the Canadian Cancer Trials Group; U10CA180820, to the ECOG-ACRIN Cancer Research Group; U10CA180868, to NRG Oncology; and U10CA180888, to SWOG). The Swiss Group for Clinical Cancer Research provided services in support of the trial participants in Switzerland.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank Greg Yothers, Ph.D., for statistical support; Jennifer Wind, M.A., and Christine Cronin, B.A., from the Dana-Farber Cancer Institute for project management; Garth Nelson, M.S., Brian Colgrove, B.S., and Deb Papenfus, B.A., from the Alliance Statistics and Data Management Center (Mayo Clinic) for statistical and data-management contributions; the many physicians, advanced practitioners, nurses, study managers, and patient advocates who dedicated effort to the execution of the trial; and, most important, the patients who enrolled in the trial.
References
- 1.NIH consensus conference: adjuvant therapy for patients with colon and rectal cancer. JAMA 1990; 264:1 444–50. [PubMed] [Google Scholar]
- 2.Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006; 24:6 68–74. [DOI] [PubMed] [Google Scholar]
- 3.Douglass HO Jr, Moertel CG, Mayer RJ, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med 1986; 315:1 294–5. [DOI] [PubMed] [Google Scholar]
- 4.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324:7 09–15. [DOI] [PubMed] [Google Scholar]
- 5.Peeters KCMJ Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007; 246: 693–701. [DOI] [PubMed] [Google Scholar]
- 6.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005; 23: 5644–50. [DOI] [PubMed] [Google Scholar]
- 7.Wolff HA, Conradi L-C, Beissbarth T, et al. Gender affects acute organ toxicity during radiochemotherapy for rectal cancer: long-term results of the German CAO/ARO/AIO-94 phase III trial. Radiother Oncol 2013; 108:4 8–54. [DOI] [PubMed] [Google Scholar]
- 8.Downing A, Glaser AW, Finan PJ, et al. Functional outcomes and health-related quality of life after curative treatment for rectal cancer: a population-level study in England. Int J Radiat Oncol Biol Phys 2019; 103: 1132–42. [DOI] [PubMed] [Google Scholar]
- 9.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA 2005; 294: 2587–93. [DOI] [PubMed] [Google Scholar]
- 10.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40. [DOI] [PubMed] [Google Scholar]
- 11.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010; 28: 859–65. [DOI] [PubMed] [Google Scholar]
- 13.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014; 32: 513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;2 2: 23–30. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Therapy Evaluation Program (CTEP). Common terminology criteria for adverse events (CTCAE) v5.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50). [Google Scholar]
- 16.National Cancer Institute. What is the PRO-CTCAE measurement system? Overview (https://healthcaredelivery.cancer.gov/pro-ctcae/overview.html ). [Google Scholar]
- 17.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012; 30:1 926–33. [DOI] [PubMed] [Google Scholar]
- 18.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst 2015;1 07(11): djv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC Trial. J Clin Oncol 2019; 37:3 223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22:2 9–42. [DOI] [PubMed] [Google Scholar]
- 21.Dijkstra EA, Nilsson PJ, Hospers GAP, et al. Locoregional failure during and after short-course radiotherapy followed by chemotherapy and surgery compared to long-course chemoradiotherapy and surgery — a five-year follow-up of the RAPIDO Trial. Ann Surg 2023. January 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basch E, Dueck AC, Mitchell SA, et al. Patient-reported outcomes during and after treatment for locally advanced rectal cancer in the PROSPECT trial. J Clin Oncol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruheim K, Guren MG, Skovlund E, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010; 76: 1005–11. [DOI] [PubMed] [Google Scholar]
- 24.Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 2022; 386:2 363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018; 4(6): e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahma OE, Yothers G, Hong TS, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol 2021; 7:1 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol 2022; 40:2 546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.