Abstract
Nisin is a pore-forming antimicrobial peptide. The capacity of nisin to induce transmembrane movement of a fluorescent phospholipid in lipid vesicles was investigated. Unilamellar phospholipid vesicles that contained a fluorescent phospholipid (1-acyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]caproyl}-sn-glycero-3-phosphocholine) in the inner leaflet of the bilayer were used. Nisin-induced movement of the fluorescent phospholipid from the inner leaflet to the outer leaflet of the membrane reached stable levels, which were dependent on the concentration of nisin added. The rate constant k of this nisin-induced transmembrane movement increased with the nisin concentration but was not dependent on temperature within the range of 5 to 30°C. In contrast, the rate constant of movement of fluorescent phospholipid from vesicle to vesicle strongly depended on temperature. The data indicate that nisin transiently disturbs the phospholipid organization of the target membrane.
Asymmetric distribution of phospholipids over the inner and outer leaflets of biological membranes is essential for membrane function (28, 29). In eukaryotic membranes, the phospholipid distribution is maintained by proteins termed “flippases” and by interactions with cytoskeletal proteins (12). For instance, the ATP-dependent flippase in erythrocytes translocates amino phospholipids from the outer leaflet to the inner leaflet of the membrane (36). Also, MDR P-glycoprotein translocates phospholipid (43). In bacterial membranes, little is known about the distribution of phospholipids over the inner and outer leaflets. Rapid movement of fluorescent phospholipids across the inner membrane of Escherichia coli has been demonstrated and is probably a protein-mediated process (14). Movement of a fluorescent phospholipid from the outer leaflet to the inner leaflet was demonstrated in the membrane of Bacillus megaterium (13).
Channel-forming cytotoxins (from Staphylococcus aureus [alpha-toxin] and Pseudomonas aeruginosa) in addition to relatively small “pore-forming” antimicrobial peptides, such as melittin, the synthetic peptide GALA (10), and mastoparan X (24), are known to cause movement of membrane phospholipids from one leaflet to the other (35). In the cases mentioned above, the peptide-induced phospholipid movement might reflect an important mechanistic aspect of pore formation. For example, fusion of the inner and outer leaflets of the target membrane may play a role such as that proposed for magainin-induced “toroidal” pores (22).
Nisin is a cationic peptide antibiotic of 34 amino acids that contains five intramolecular rings. Its genetics (18), synthesis regulation (19), and structure (21, 39–42) are known, and several mutants have been generated (16–18, 20). Because of its antimicrobial activities, of which permeabilization of the membranes of the target cells is the most important (25), nisin is used as a food preservative. Nisin preferentially interacts with anionic phospholipid-containing (3, 8, 9) membranes, transiently enhancing the permeability for small ions and solutes (5, 11, 15, 27, 31–34). We previously proposed a “wedge-like” model for pore formation by nisin (9, 26). The positively charged carboxyl terminus of nisin, together with the bound lipids, enter into the membrane. Both the transmembrane electrical potential (ΔΨ) (9, 32, 33) and the ΔpH (cis acid) stimulate nisin-mediated membrane permeabilization (26). In this study, we demonstrate that nisin induces rapid movement of a fluorescent phospholipid from the inner leaflet to the outer leaflet of unilamellar phospholipid vesicles. This provides support for nisin-induced disturbance of the phospholipid organization in the membrane, as proposed previously in the wedge-like model for nisin-induced pore formation.
MATERIALS AND METHODS
Materials.
Nisin was obtained as a gift from Aplin & Barrett. Dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylglycerol (DOPG), l-α-phosphatidylethanolamine-N-(lissamine rhodamine B sulfonyl) (Rh-PE), 1-acyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]caproyl}-sn-glycero-3-phosphocholine (C6-NBD-PC), and 1-acyl-2{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]cap-royl}-sn-glycero-3-[phospho-rac-(1-glycerol)] (C6-NBD-PG) were obtained from Avanti Polar Lipids. The 1-acyl chains of C6-NBD-PC and C6-NBD-PG represent the fatty acid content of egg lysophosphatidylcholine.
Preparation of small unilamellar phospholipid vesicles.
Phospholipids dissolved in organic solvent were dried under nitrogen. Subsequently, a small volume of ethanol was added, and the lipids were dried again. Multilamellar vesicles were formed by vortexing in the presence of buffer and glass beads. Small unilamellar vesicles were obtained by sonication (tip diameter, 3 mm) (Soniprep 150; Beun de Ronde, Abcoude NL) of the multilamellar vesicles under the following conditions: 30 min, 10-μm amplitude, pulses of 30 s on and 30 s off, incubation on ice under a stream of nitrogen. Metal particles and large vesicles were removed by centrifugation for 15 min at 80,000 × g at 4°C. Vesicles composed of DOPC–Rh-PE (98:2 [wt/wt]) were prepared by ethanol injection (2), followed by dialysis to remove ethanol.
Preparation of large unilamellar phospholipid vesicles with symmetric transmembrane distribution of C6-NBD-phospholipids.
Phospholipid vesicles labelled with 2% (wt/wt) C6-NBD-phospholipid were prepared by reverse-phase evaporation (38), followed by extrusion performed 11 times through 400-nm-pore-size polycarbonate filters (Avestin) in either piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), or phosphate buffer (pH 7.0) containing 0.21 M sucrose and 50 mM K+ or 50 mM Na+. Extravesicular sucrose was removed by dialysis against the same buffer without sucrose.
Preparation of large unilamellar phospholipid vesicles with C6-NBD-lipids exclusively in the inner leaflet.
Vesicles prepared as in the preceding paragraph, which contain C6-NBD-phospholipid in both inner and outer membrane leaflets, were incubated four times at 20°C with sonicated small DOPG vesicles that do not contain C6-NBD-phospholipid, essentially as described previously (10). Each incubation was followed by centrifugation for 15 min at 80,000 × g and at 4°C, after which the supernatant with the sonicated small DOPG vesicles was discarded and the DOPG–C6-NBD-phospholipid vesicles were resuspended. The incubation with sonicated small DOPG vesicles was at a 5- to 10-fold (first incubation) or one- to twofold (subsequent three incubations) excess quantity over the amount of total phospholipid present in the DOPG–C6-NBD-phospholipid vesicles.
Preparation of large unilamellar phospholipid vesicles with C6-NBD-phospholipids exclusively in the outer leaflet.
DOPG vesicles without C6-NBD-phospholipids were prepared as described above by reverse-phase evaporation (38) followed by extrusion. These acceptor vesicles were incubated twice during 1 h at 20°C with twice the amount (on a phospholipid basis) small unilamellar donor vesicles, prepared by sonication as described above in “Preparation of small unilamellar phospholipid vesicles” but containing 6% (wt/wt) C6-NBD-phospholipid. Centrifugation for 15 min at 80,000 × g and at 4°C followed each incubation. The supernatant with small unilamellar vesicles was discarded, and the vesicles with C6-NBD-phospholipid in the outer leaflet were resuspended.
Transmembrane movement of C6-NBD-phospholipids.
Transmembrane movement of C6-NBD-phospholipids was measured in two ways. In both assays, nisin causes transmembrane movement of C6-NBD-phospholipids from the inner leaflet of the unilamellar DOPG (58 μM) vesicles to the outer leaflet, which initially does not contain C6-NBD-phospholipids.
The first method makes use of the decrease of measurable NBD emission due to energy transfer to Rh-PE. Nisin-induced transmembrane movement of C6-NBD-phospholipids to the outer leaflet of DOPG (58 μM) vesicles is followed by the diffusion of C6-NBD-phospholipid to DOPC–Rh-PE (98:2 [wt/wt]) (1 mM DOPC) vesicles, resulting in a decrease of the NBD emission, which was measured as a percentage at the point at which a stable level of fluorescence decrease was reached. The NBD fluorescence measured when vesicles composed of C6-NBD-phospholipid–DOPC–Rh-PE were prepared was taken as the 100% control of fluorescence decrease.
In the second assay, transmembrane movement of C6-NBD-phospholipids from the inner leaflet to the outer leaflet of DOPG vesicles is measured in the absence of quenching DOPC–Rh-PE vesicles. The NBD fluorescence increase due to shielding of NBD groups in the outer leaflet from the aqueous phase by membrane-bound nisin is measured (9). Analysis of the kinetics of transmembrane movement was performed by assuming second-order kinetics and by using the table curve program (Jandel). The NBD fluorescence increase was fitted according to the equation that gave the best fit: y = a + b(1 − e−kx) + d(1 − e−ex). The value of the second rate constant e was very small (generally between 10−2 and 10−5 times smaller than k) compared to the first rate constant k. In parallel experiments, the NBD fluorescence increase was measured for DOPG vesicles with symmetric transbilayer distribution of C6-NBD-phospholipid.
All fluorescence measurements were performed at 30°C, unless otherwise indicated, using a Perkin Elmer LS-50 spectrofluorimeter. For the NBD fluorescence, excitation and emission wavelengths were 475 and 530 nm, respectively. The slit widths were set at 4 nm.
Transfer of C6-NBD-phospholipids between phospholipid vesicles.
Interbilayer movement of lipids was measured as follows: DOPG–Rh-PE vesicles (98:2 [wt/wt]) containing either 2% (wt/wt) C6-NBD-PG or C6-NBD-PC were incubated with DOPG or DOPC vesicles without fluorescent probe. Rate constants of phospholipid transfer were derived from the NBD fluorescence increase.
Calcein leakage.
Liposomes were prepared by reverse-phase evaporation (38) in a solution containing 0.21 M sucrose, 8 mM calcein, and 50 mM K-PIPES, pH 7.0. After dialysis against 50 mM K-PIPES (pH 7.0), external calcein was removed by centrifugation for 15 min at 80,000 × g and 4°C, followed by two washes (each wash done by resuspension and then centrifugation). The integrity of the phospholipid vesicles was assayed by measuring the fluorescence increase after the addition of nisin and after subsequent addition of 0.2% Triton X-100 to calcein-loaded phospholipid vesicles. Excitation and emission wavelengths were 470 and 520 nm, respectively, and the slit widths were 2.5 nm.
Phosphate determinations.
Phosphate determinations were performed by the method of Rouser et al. (30).
RESULTS
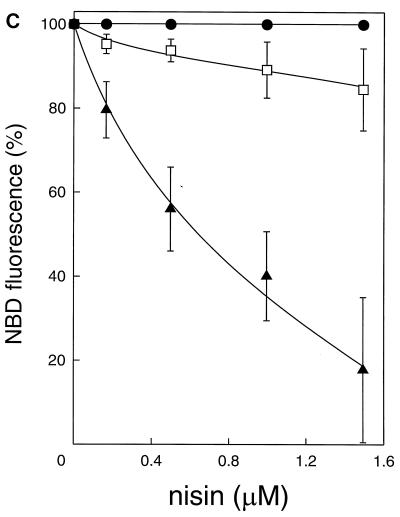
In the present study, we investigated the capacity of nisin to induce transmembrane movement of the C6-NBD-phospholipid. In a first assay, unilamellar DOPG vesicles which contained C6-NBD-phospholipids in the inner leaflet were incubated with an excess of DOPC–Rh-PE vesicles. Nisin is known to bind to negatively charged phospholipids, like DOPG, but not to zwitterionic DOPC phospholipids (9). After nisin-mediated movement to the outer leaflet of the membrane, the C6-NBD-phospholipids diffused to the Rh-PE-containing DOPC vesicles. Once inserted into these vesicles, the NBD emission was largely used for the excitation of rhodamine, which gave rise to a decrease in the emission of C6-NBD-PC (Fig. 1A and C) and C6-NBD-PG (Fig. 1B and C). In the absence of nisin or when DOPC vesicles (Fig. 1C) were used instead of DOPG vesicles, no decrease in the NBD fluorescence was observed. This implied that the acidic phospholipid DOPG was needed for the nisin-induced transmembrane movement of the C6-NBD-phospholipid. Interestingly, the decrease in NBD fluorescence was much greater for C6-NBD-PC than for C6-NBD-PG (Fig. 1C). Control experiments showed that nisin did not inhibit the transfer of either C6-NBD-PG or C6-NBD-PC from DOPG vesicles towards DOPC vesicles. These control experiments were performed by following the increase in NBD fluorescence due to transfer of C6-NBD-phospholipid from DOPG (58 μM)–C6-NBD-phospholipid (2% [wt/wt] in both leaflets)–Rh-PE (2% [wt/wt] in both leaflets) vesicles, in which the NBD fluorescence was quenched by the Rh-PE, towards DOPC (1 mM) vesicles (data not shown).
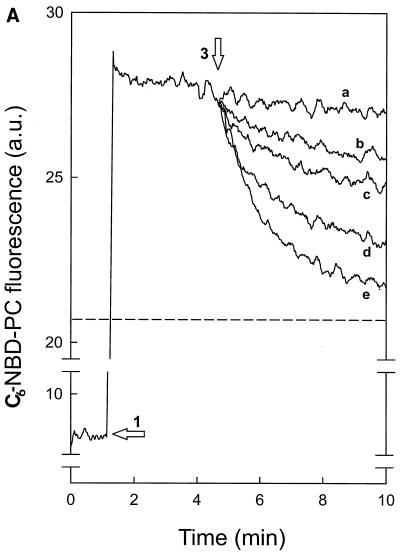
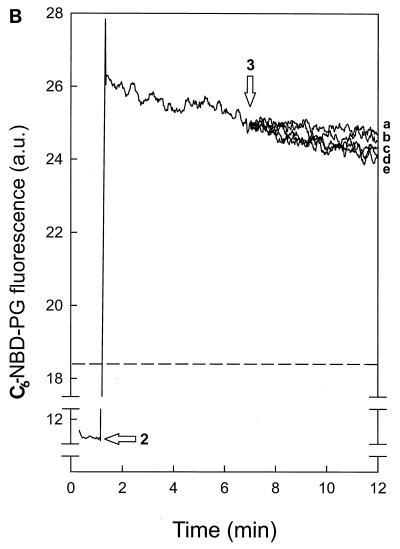
FIG. 1.
Nisin-induced transmembrane movement of C6-NBD-PC in DOPG vesicles. (A and B) DOPG (58 μM) vesicles, which contained either C6-NBD-PC (arrow 1 in panel A) or C6-NBD-PG (arrow 2 in panel B) exclusively in the inner leaflet, were added to DOPC (1 mM) vesicles that contained Rh-PE (2% [wt/wt]). Addition of 166 nM (b), 500 nM (c), 1,000 nM (d), and 1,500 nM (e) nisin (arrows 3 in panels A and B) caused a decrease in C6-NBD-PC (A) and C6-NBD-PG (B) fluorescence. Lines a were obtained after addition of buffer. In a separate measurement, C6-NBD-PC (A) and C6-NBD-PG (B) were incorporated in DOPC–Rh-PE vesicles: fluorescence values of 20.7 (A) and 18.4 (B) correspond to 100% transfer (horizontal broken lines) of C6-NBD-phospholipid to DOPC–Rh-PE vesicles. a.u., arbitrary units. (C) DOPG (58 μM) vesicles with either C6-NBD-PC (▴) or C6-NBD-PG (□) exclusively in the inner leaflet or with DOPC (58 μM) vesicles with C6-NBD-PC (•) exclusively in the inner leaflet. Nisin-induced NBD fluorescence decrease followed migration of the C6-NBD-phospholipids to DOPC (1 mM) vesicles with Rh-PE (2% [wt/wt]) and was measured as a percentage at the point at which a stable level of fluorescence decrease was reached.
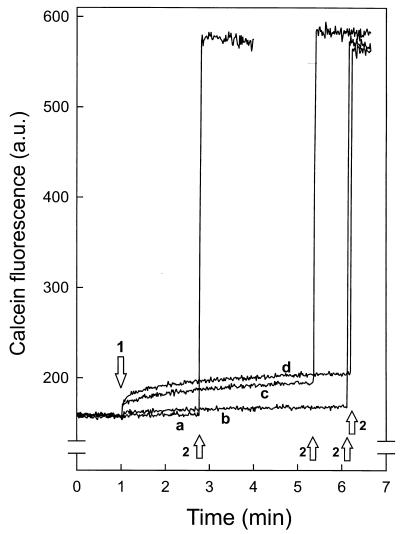
To exclude the possibility that the C6-NBD-phospholipids in the inner leaflet diffused to the acceptor vesicles through an aqueous pore formed by nisin instead of via a transmembrane movement, the integrity of the vesicles was determined by following the nisin-induced leakage of the fluorophore calcein. Under conditions where extensive transmembrane movement of C6-NBD-PC took place, nisin-induced calcein leakage was only moderate and limited to around 10% at the highest nisin concentration (Fig. 2). This demonstrated that the decrease in C6-NBD-PC fluorescence, as observed in Fig. 1, was the result of a transmembrane movement of the C6-NBD-PC. The small decrease in C6-NBD-PG fluorescence (Fig. 1C) was, however, only slightly larger than that observed for calcein leakage. Therefore, no conclusion could be drawn on the nature of the nisin-induced C6-NBD-PG movement. As observed previously (10), control experiments with phospholipid vesicles containing the C6-NBD-phospholipids exclusively in the outer leaflet showed in the absence of nisin a rapid maximal decrease in fluorescence due to diffusion of the C6-NBD-phospholipid to the quenching Rh-PE–DOPC acceptor vesicles.
FIG. 2.
Nisin-induced release of calcein from phospholipid vesicles. DOPG (58 μM) vesicles loaded with calcein were incubated with nisin. At arrow 1, nisin at either 166 (a), 500 (b), 1,000 (c), or 1,500 (d) nM was added, followed by addition of 0.2% Triton X-100 (arrows 2). a.u., arbitrary units.
In order to enable a more direct analysis of the transmembrane movement, experiments were performed with DOPG vesicles containing the C6-NBD-phospholipid in the inner leaflet, without the addition of Rh-PE–DOPC acceptor vesicles. In this case, nisin-induced movement of C6-NBD-phospholipid to the outer leaflet caused an increase in NBD fluorescence. This is due to shielding of the NBD group from the aqueous environment by the membrane-bound nisin (9). The NBD group in C6-NBD-phospholipids loops back to the phospholipid head group (1, 6, 7). Indeed, addition of nisin to DOPG vesicles with C6-NBD-PC (Fig. 3A and B) or with C6-NBD-PG (data not shown) exclusively in the inner leaflet caused an increase in the NBD fluorescence. Analyses of the kinetics of the fluorescence increase revealed that at all nisin concentrations, the rate constant k was lower for C6-NBD-PC (Fig. 3C) than for C6-NBD-PG; in the latter case, the rate constant ranged from 0.046 ± 0.007 s−1 at 166 nM nisin to 0.194 ± 0.032 s−1 at 1.5 μM nisin (data not shown). In contrast, addition of the nisin to vesicles with symmetric distribution of C6-NBD-PC or C6-NBD-PG resulted in a very rapid increase in fluorescence, which reached a higher plateau within less than a second. This was observed previously for vesicles with symmetric distribution of head group-labelled NBD-phosphatidylethanolamine (9).
FIG. 3.
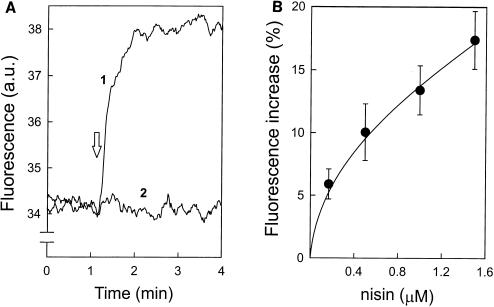
Nisin induced a transmembrane movement of C6-NBD-PC. In panel A, at the arrow, 500 nM nisin (line 1) or buffer (line 2) was added to DOPG (58 μM) vesicles with C6-NBD-PC exclusively in the inner leaflet, and the increase in the NBD fluorescence was recorded. The same experiment was performed with various concentrations of nisin. At each nisin concentration a different plateau level of fluorescence increase (B) and a different rate constant (C) were measured.
Nisin pore formation is known to depend on the proton motive force. We investigated the effect of a valinomycin-induced membrane potential (ΔΨ) on transmembrane movement of C6-NBD-PC. Nisin was added to DOPG vesicles with C6-NBD-phospholipid exclusively in the inner leaflet, and the fluorescence increase was recorded. Neither for C6-NBD-PC nor for C6-NBD-PG was any effect of the ΔΨ on the extent or rate of fluorescence increase observed (data not shown). Under the conditions used, nisin-induced dissipation of the ΔΨ was much faster than the rate of nisin-induced NBD fluorescence increase (data not shown). The rapid dissipation of the ΔΨ therefore probably prevented the detection of a possible effect of the ΔΨ on nisin-induced transmembrane movement of C6-NBD-phospholipid.
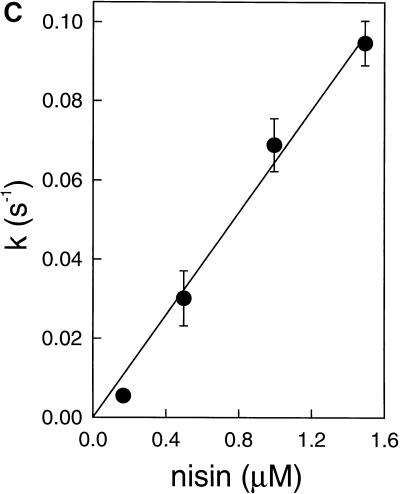
The effect of temperature on the transmembrane movement of C6-NBD-phospholipid was investigated by using the assay with the C6-NBD-phospholipid exclusively in the inner leaflet. The nisin-induced transmembrane movement of C6-NBD-PC was not affected by temperature (Fig. 4). As a control, the temperature dependence of the transfer of C6-NBD-PC from C6-NBD-PC–Rh-PE–DOPG vesicles to DOPG vesicles was measured. In contrast to the rate constant of transmembrane movement, the rate constant of the movement of C6-NBD-PC from vesicle to vesicle was strongly affected by temperature (Fig. 4). Also, the nisin-induced C6-NBD-PG movement observed when nisin is added to vesicles in which the inner leaflet contains C6-NBD-PG was dependent on temperature. At 500 nM nisin, k increased from 0.017 ± 0.003 s−1 at 5°C to 0.066 ± 0.012 s−1 at 30°C (data not shown), which further concurs with the suggestion that the C6-NBD-PG movement occurs via a mechanism that is different from that of the C6-NBD-PC transmembrane movement.
FIG. 4.
Temperature dependence of the rate constants of transmembrane movement and intervesicle movement of C6-NBD-PC. Nisin (500 nM) was added to DOPG (58 μM) vesicles, with C6-NBD-PC (■) exclusively in the inner leaflet. Intervesicle movement was measured by incubating DOPG (58 μM–Rh-PE–C6-NBD-PC vesicles with pure DOPG (174 μM) vesicles (○). Lines were obtained by regression. Standard deviations of the absolute values of the rate constants were around 17% for transmembrane movement and around 2% for intervesicle movement.
DISCUSSION
The present work shows that nisin induces movement of a fluorescent phospholipid, C6-NBD-PC, from the inner leaflet of unilamellar phospholipid vesicles to the outer leaflet. Nisin is a pore-forming antimicrobial peptide. However, various lines of evidence indicate that C6-NBD-PC undergoes transmembrane movement and does not pass through an aqueous pore formed by nisin. First, the extent of calcein leakage was very low compared to the transmembrane movement of C6-NBD-PC. Nisin Z forms an anion-selective pore in DOPG vesicles (3). This excludes the theoretical possibility that the negative charge of calcein (and/or C6-NBD-PG) might limit its passage through a nisin pore due to electrostatic repulsion between DOPG and calcein (and/or C6-NBD-PG). The calcein leakage experiments also demonstrated that the vesicles remained intact under the conditions employed. Second, movement of C6-NBD-PG was much more restricted than that of C6-NBD-PC (Fig. 1C), whereas the rate constant of C6-NBD-PG movement was higher than that of C6-NBD-PC. Third, the rate constant of transmembrane movement of C6-NBD-PC markedly increased with the nisin concentration (Fig. 3C). Similarly, another study (35) showed that the (low) rate constant of transmembrane movement of lysophospholipid in erythrocytes increased with cytotoxin (of S. aureus and of P. aeruginosa) concentration. Finally, the rate constant of intervesicle movement of C6-NBD-PC increased strongly with the temperature, while the rate constant of transmembrane movement of C6-NBD-PC was not affected by temperature. Similar results were found in experiments on flippase activity in B. megaterium membrane vesicles. In that study, the rate constant of transmembrane movement of phospholipid was largely independent of temperature, whereas the rate constant of intervesicle transport was dependent on temperature (13).
Why was the nisin-induced transbilayer movement of lipids restricted to C6-NBD-PC without having an equal effect on the movement of C6-NBD-PG? The lack of the C6-NBD-PG movement was possibly due to the immobilization of the anionic PG by the cationic nisin as previously suggested by 31P nuclear magnetic resonance experiments (9). The relative nisin-induced C6-NBD-PG movement hardly exceeded the calcein leakage. Therefore, the nature of this limited movement is not clear at this time.
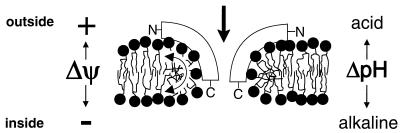
What is the mechanism of nisin-induced transmembrane movement of C6-NBD-PC? In all likelihood, transmembrane movement of C6-NBD-PC is the consequence of insertion of part(s) of the nisin molecule into the membrane with concomitant disturbance of the membrane (Fig. 5). Insertion of nisin into lipid monolayers (3, 8) has been demonstrated, and the C terminus of nisin is known to insert deeply into the membrane (23). A study in which a His-tagged nisin was used indicated that the C-terminal part of nisin had the ability to translocate across the membrane (44). Physicochemical measurements on the interaction of nisin with Listeria lipid vesicles also indicated an insertion of the C-terminal part of nisin into the membrane (45). In equilibrium, however, nisin seemed to be oriented parallel with the membrane surface, assuming a surface-bound state (4). The wedge-like model (Fig. 5) invokes a proton motive force-driven insertion of the C-terminal part of nisin (9, 26). It could well be that the insertion of the C-terminal region of nisin induced temporary fusion joints that permit transmembrane movements.
FIG. 5.
Model of altered phospholipid organisation after insertion of nisin.
In the experiments in which C6-NBD-PC moves to the outer leaflet and subsequently to Rh-PE-containing vesicles, nisin concentration-dependent stable levels of movement were reached after 5 min (data not shown). Supposing a surface area of 0.716 nm2/DOPG molecule (37) and using a phospholipid/nisin ratio that ranges from 39 to 438, the number of molecules per vesicle was between 1,602 and 18,000. Therefore, the observed saturation of the fluorescence decrease was probably not due to a low nisin/phospholipid ratio. Apparently, nisin only transiently induced transmembrane movement of C6-NBD-phospholipid. Within the range used, the rate constant of transmembrane movement increased with the nisin concentration (Fig. 3C). This confirms that nisin indeed bound to all vesicles and that binding of additional nisin cooperatively enhanced transmembrane movement of C6-NBD-PC. This phenomenon might be the result of aggregation of nisin molecules.
In conclusion, the present study shows for the first time that nisin transiently induces rapid transmembrane movement of C6-NBD-PC. This nisin-induced transmembrane movement indicates that a transient disturbance of the phospholipid organization of the membrane takes place locally during nisin-induced membrane permeabilization.
ACKNOWLEDGMENT
Margaret Mullaly is gratefully acknowledged for reading the manuscript.
REFERENCES
- 1.Abrams F S, London E. Extension of the parallax analysis of membrane penetration depth to the polar region of model membranes: use of fluorescence quenching by a spin-label attached to the phospholipid polar headgroup. Biochemistry. 1993;32:10826–10831. doi: 10.1021/bi00091a038. [DOI] [PubMed] [Google Scholar]
- 2.Batzri S, Korn E D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973;298:1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- 3.Breukink E, van Kraaij C, Demel R A, Siezen R J, Kuipers O P, de Kruijff B. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry. 1997;36:6968–6976. doi: 10.1021/bi970008u. [DOI] [PubMed] [Google Scholar]
- 4.Breukink E, van Kraaij C, van Dalen A, Demel R A, Siezen R J, de Kruijff B, Kuipers O P. The orientation of nisin in membranes. Biochemistry. 1998;37:8153–8162. doi: 10.1021/bi972797l. [DOI] [PubMed] [Google Scholar]
- 5.Bruno M E C, Kaiser A, Montville T J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992;58:2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chattopadhyay A. Chemistry and biology of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) labelled lipids: fluorescent probes of biological and model membranes. Chem Phys Lipids. 1990;53:1–15. doi: 10.1016/0009-3084(90)90128-e. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay A, London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labelled phospholipids. Biochemistry. 1987;26:39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- 8.Demel R A, Peelen T, Siezen R J, de Kruijff B, Kuipers O P. Nisin Z, mutant nisin Z and lacticin 481 interactions with anionic lipids correlate with antimicrobial activity. A monolayer study. Eur J Biochem. 1996;235:267–274. doi: 10.1111/j.1432-1033.1996.00267.x. [DOI] [PubMed] [Google Scholar]
- 9.Driessen A J M, van den Hooven H W, Kuiper W, van de Kamp M, Sahl H-G, Konings R N H, Konings W N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry. 1995;34:1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- 10.Fattal E, Nir S, Parente R A, Szoka F C., Jr Pore-forming peptides induce rapid phospholipid flip-flop in membranes. Biochemistry. 1994;33:6721–6731. doi: 10.1021/bi00187a044. [DOI] [PubMed] [Google Scholar]
- 11.Gao F H, Abee T, Konings W N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991;57:2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haest C W M. Interactions between membrane skeleton proteins and the intrinsic domain of the erythrocyte membrane. Biochim Biophys Acta. 1982;694:331–352. doi: 10.1016/0304-4157(82)90001-6. [DOI] [PubMed] [Google Scholar]
- 13.Hrafnsdóttir S, Nichols J W, Menon A K. Transbilayer movement of fluorescent phospholipids in Bacillus megaterium membrane vesicles. Biochemistry. 1997;36:4969–4978. doi: 10.1021/bi962513h. [DOI] [PubMed] [Google Scholar]
- 14.Huijbregts R P H, de Kroon A I P M, de Kruijff B. Rapid transbilayer movement of C6-NBD-labelled phospholipids across the inner membrane of Escherichia coli. Biochim Biophys Acta. 1996;1280:41–50. doi: 10.1016/0005-2736(95)00272-3. [DOI] [PubMed] [Google Scholar]
- 15.Kordel M, Sahl H-G. Susceptibility of bacterial eukaryotic and artificial membranes to the disruptive action of the cationic peptides Pep5 and nisin. FEMS Microbiol Lett. 1986;34:139–144. [Google Scholar]
- 16.Kuipers O P, Yap W M G J, Rollema H S, Beerthuizen M M, Siezen R J, de Vos W M. Expression of wild-type and mutant nisin genes in Lactococcus lactis. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 250–259. [Google Scholar]
- 17.Kuipers O P, Rollema H S, Yap W M G J, Boot H J, Siezen R J, de Vos W M. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J Biol Chem. 1992;267:24340–24346. [PubMed] [Google Scholar]
- 18.Kuipers O P, Beerthuyzen M M, Siezen R J, de Vos W M. Characterization of the nisin gene cluster nis ABTCIPR of Lactococcus lactis. Requirement of expression of the nis A and nis I genes for the development of immunity. Eur J Biochem. 1993;216:281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers O P, Beerthuyzen M M, de Ruyter P G G A, Luesink E J, de Vos W M. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem. 1995;45:27299–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- 20.Kuipers O P, Bierbaum G, Ottenwälder B, Dodd H M, Horn N, Metzger J, Kupke T, Gnau V, Bongers R, van den Boogaard P, Kosters H, Rollema H S, de Vos W, Siezen R J, Jung G, Götz F, Sahl H-G, Gasson M J. Protein engineering of lantibiotics. Antonie Leeuwenhoek. 1996;69:161–170. doi: 10.1007/BF00399421. [DOI] [PubMed] [Google Scholar]
- 21.Lian L Y, Chan W C, Morley S D, Roberts G C K, Bycroft B W, Jackson D. Solution structures of nisin A and its two major degradation products determined by NMR. Biochem J. 1992;283:413–420. doi: 10.1042/bj2830413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludtke S J, He K, Heller W T, Harroun T A, Yang L, Huang H W. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- 23.Martin I, Ruysschaert J M, Sanders D, Giffard C. Interaction of the lantibiotic nisin with membranes revealed by fluorescence quenching of an introduced tryptophan. Eur J Biochem. 1996;239:156–164. doi: 10.1111/j.1432-1033.1996.0156u.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzaki K, Yoneyama S, Murase O, Miyajima K. Transbilayer transport of ions and lipids coupled with mastoparan X translocation. Biochemistry. 1996;35:8450–8456. doi: 10.1021/bi960342a. [DOI] [PubMed] [Google Scholar]
- 25.Moll G N, Roberts G C K, Konings W N, Driessen A J M. Mechanism of lantibiotic-induced pore-formation. Antonie Leeuwenhoek. 1996;69:185–191. doi: 10.1007/BF00399423. [DOI] [PubMed] [Google Scholar]
- 26.Moll G N, Clark J, Chan W C, Bycroft B W, Roberts G C K, Konings W N, Driessen A J M. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J Bacteriol. 1997;179:135–140. doi: 10.1128/jb.179.1.135-140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okereke A, Montville T J. Nisin dissipates the proton motive force of the obligate anaerobe Clostridium sporogenes PA 3679. Appl Environ Microbiol. 1992;58:2463–2467. doi: 10.1128/aem.58.8.2463-2467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Op den Kamp J A F. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 29.Roelofsen B, Op den Kamp J A F. Plasma membrane phospholipid asymmetry and its maintenance: the human erythrocyte as a model. Curr Top Membr. 1994;40:7–46. [Google Scholar]
- 30.Rouser G, Fleischer S, Yamamoto A. Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 31.Ruhr E, Sahl H-G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985;27:841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahl H-G, Kordel M, Benz R. Voltage dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol. 1987;149:120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- 33.Sahl H-G. Pore formation in bacterial membranes by cationic lantibiotics. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 347–358. [Google Scholar]
- 34.Sahl H-G, Jack R W, Bierbaum G. Lantibiotics: biosynthesis and biological activities of peptides with unique post-translational modifications. Eur J Biochem. 1995;230:827–853. doi: 10.1111/j.1432-1033.1995.tb20627.x. [DOI] [PubMed] [Google Scholar]
- 35.Schneider E, Haest C W M, Plasa G, Deuticke B. Bacterial cytotoxins, amphotericin B and local anesthetics enhance transbilayer mobility of phospholipids in erythrocyte membrane. Consequences for phospholipid asymmetry. Biochim Biophys Acta. 1986;855:325–336. doi: 10.1016/0005-2736(86)90078-7. [DOI] [PubMed] [Google Scholar]
- 36.Seigneuret M, Devaux P F. ATP-dependent asymmetric distribution of spinlabeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci USA. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smaal E, Mandersloot J, Demel R A, de Kruijff B, de Gier J. Consequences of calcium with dioleoylphosphatidate-containing model membranes: calcium-membrane, membrane-membrane interactions. Biochim Biophys Acta. 1987;897:180–190. doi: 10.1016/0005-2736(87)90326-9. [DOI] [PubMed] [Google Scholar]
- 38.Szoka F C, Olsen F, Heath T, Vail W, Mayhew E, Papahadjopoulos D. Preparation of unilamellar liposomes of intermediate size by a combination of reverse phase evaporation and extrusion through polycarbonate membranes. Biochim Biophys Acta. 1980;601:559–571. doi: 10.1016/0005-2736(80)90558-1. [DOI] [PubMed] [Google Scholar]
- 39.Van den Hooven H W, Fogolari F, Rollema H S, Konings R N H, Hilbers C W, van De Ven F J M. NMR and circular dichroism studies of the lantibiotic nisin in non-aqueous environments. FEBS Lett. 1993;319:189–194. doi: 10.1016/0014-5793(93)80065-3. [DOI] [PubMed] [Google Scholar]
- 40.Van den Hooven H W, Doeland C C M, van de Kamp M, Konings R N H, Hilbers C W, van De Ven F J M. Three-dimensional structure of the lantibiotic nisin in the presence of membrane-mimetic micelles of dodecylphosphocholine and of sodium dodecylsulphate. Eur J Biochem. 1996;235:382–393. doi: 10.1111/j.1432-1033.1996.00382.x. [DOI] [PubMed] [Google Scholar]
- 41.Van den Hooven H W, Spronk C A E M, van de Kamp M, Konings R N H, Hilbers C W, van de Ven F J M. Surface location and orientation of the lantibiotic nisin bound to membrane-mimicking micelles of dodecylphosphocholine and of sodium dodecylsulphate. Eur J Biochem. 1996;235:394–403. doi: 10.1111/j.1432-1033.1996.00394.x. [DOI] [PubMed] [Google Scholar]
- 42.van De Ven F J M, van den Hooven H W, Konings R N H, Hilbers C W. NMR and circular dichroism studies of the lantibiotic nisin in non aqueous environments. Eur J Biochem. 1991;202:1181–1188. [Google Scholar]
- 43.van Helvoort A, Smith A J, Sprong H, Fritzshe I, Schinkel A H, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 44.van Kraaij C, Breukink E, Kuipers O P, de Kruijff B. Third Lantibiotics Meeting, 5 to 8 April 1998, Blaubeuren, Germany. 1998. Pore formation by nisin involves translocation of the C-terminal part across the membrane. [DOI] [PubMed] [Google Scholar]
- 45.Winkowsky K, Ludescher R D, Montville T J. Physicochemical characterization of the nisin-membrane interaction with liposomes derived from Listeria monocytogenes. Appl Environ Microbiol. 1996;62:323–327. doi: 10.1128/aem.62.2.323-327.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]