Abstract
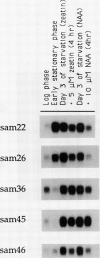
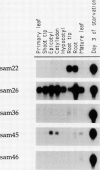
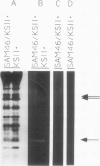
We report the isolation of five cDNA clones whose corresponding mRNAs accumulate in cultured soybean cells (Glycine max cv Mandarin) during cytokinin or auxin starvation. The levels of three of these mRNAs decrease rapidly after addition of 5 micromolar zeatin to cytokinin-starved cells or after addition of 10 micromolar α-naphthaleneacetic acid to auxin-starved cells. These mRNAs also exhibit various patterns of accumulation in the tissues of intact soybean plants. Partial nucleotide sequence analysis demonstrates that one of the cDNAs in the collection, called SAM46, is 46% identical at the amino acid level to the iron superoxide dismutase gene of Escherichia coli. Expression of this cDNA in Escherichia coli cells results in detectable iron superoxide dismutase activity, confirming the identity of the cDNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Chen C. M., Leisner S. M. Cytokinin-modulated gene expression in excised pumpkin cotyledons. Plant Physiol. 1985 Jan;77(1):99–103. doi: 10.1104/pp.77.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton J. L., Ross C. W., Byrne D. H., Colbert J. T. Down-regulation of phytochrome mRNA abundance by red light and benzyladenine in etiolated cucumber cotyledons. Plant Mol Biol. 1990 May;14(5):707–714. doi: 10.1007/BF00016503. [DOI] [PubMed] [Google Scholar]
- Crowell D. N., Kadlecek A. T., John M. C., Amasino R. M. Cytokinin-induced mRNAs in cultured soybean cells. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8815–8819. doi: 10.1073/pnas.87.22.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- John M. C., Amasino R. M. Expression of an Agrobacterium Ti plasmid gene involved in cytokinin biosynthesis is regulated by virulence loci and induced by plant phenolic compounds. J Bacteriol. 1988 Feb;170(2):790–795. doi: 10.1128/jb.170.2.790-795.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R. A., John M. C., Amasino R. M. Deoxyribonuclease I sensitivity of the T-DNA ipt gene is associated with gene expression. Biochemistry. 1988 Jul 26;27(15):5748–5754. doi: 10.1021/bi00415a053. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kuroda H., Tanaka T., Machida Y., Takebe I., Nagata T. Isolation of an auxin-regulated gene cDNA expressed during the transition from G0 to S phase in tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9279–9283. doi: 10.1073/pnas.86.23.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker J. C., Key J. L. Isolation of cloned cDNAs to auxin-responsive poly(A)RNAs of elongating soybean hypocotyl. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]