Abstract
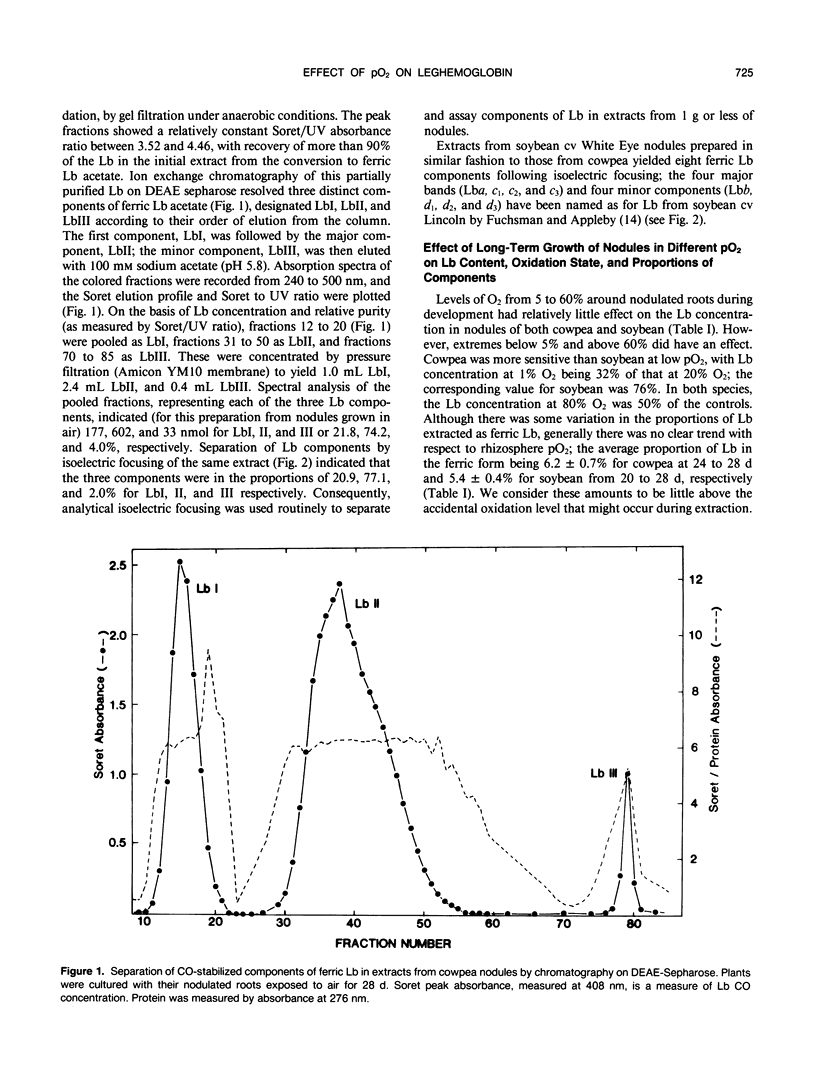
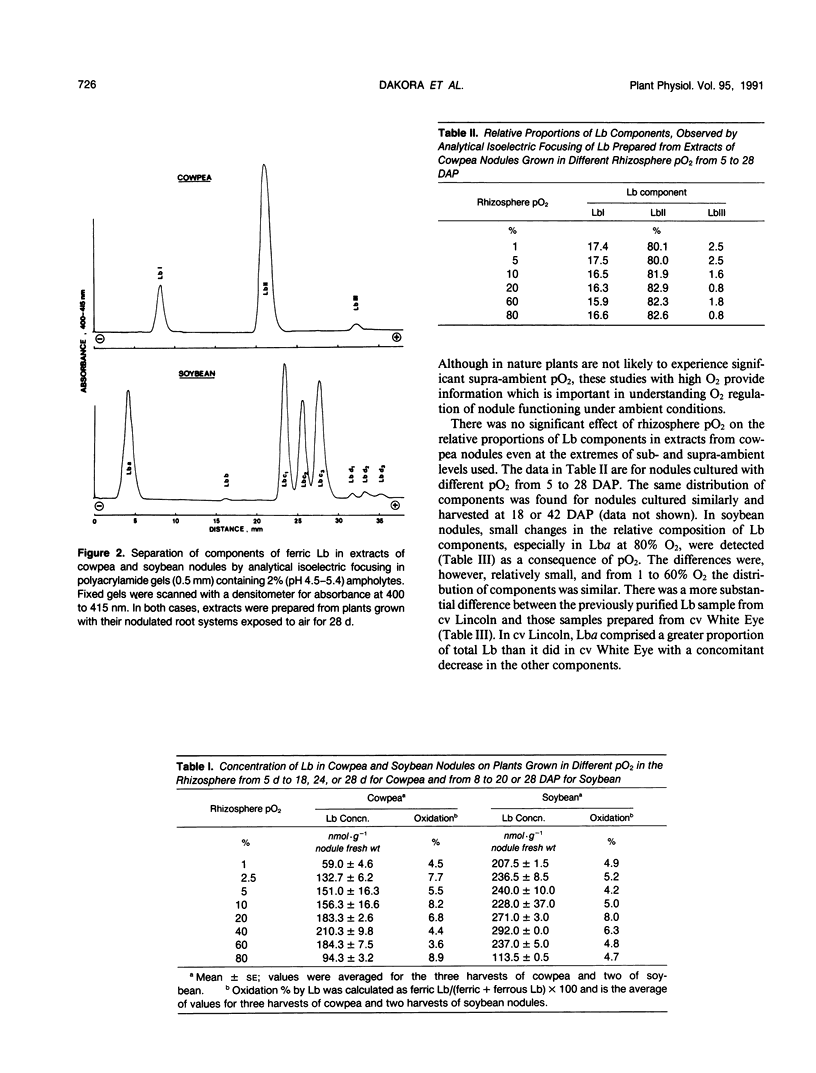
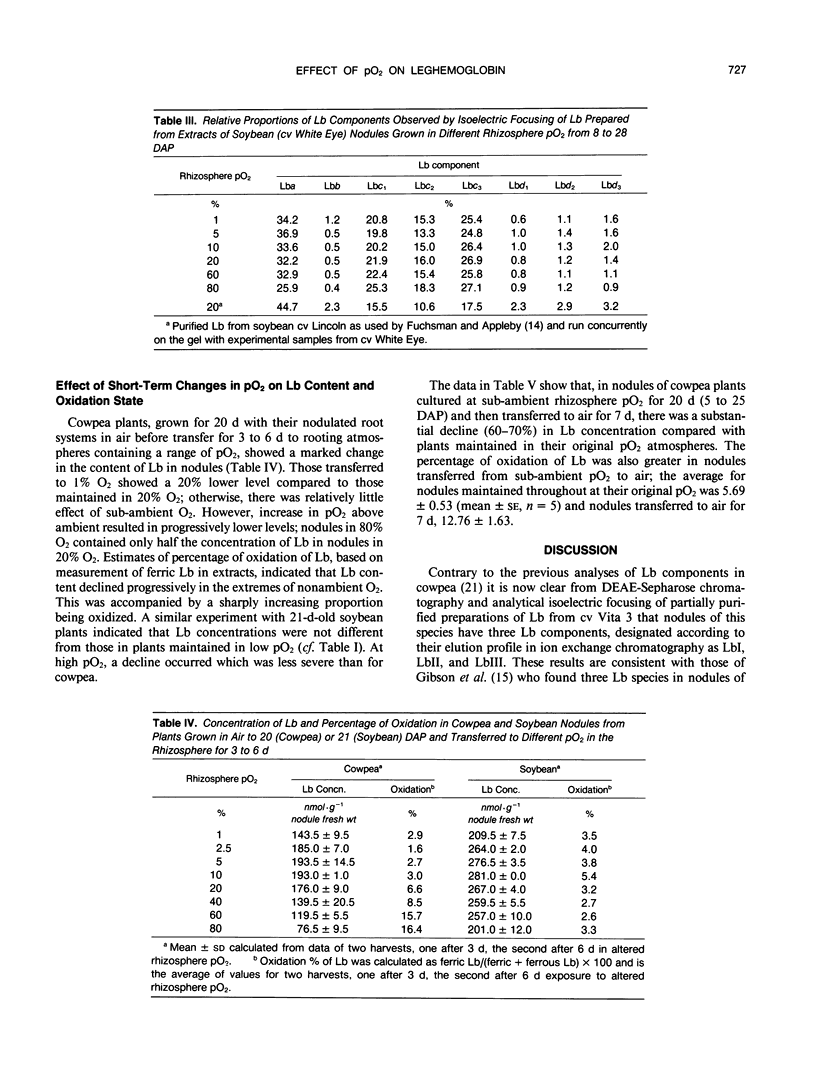
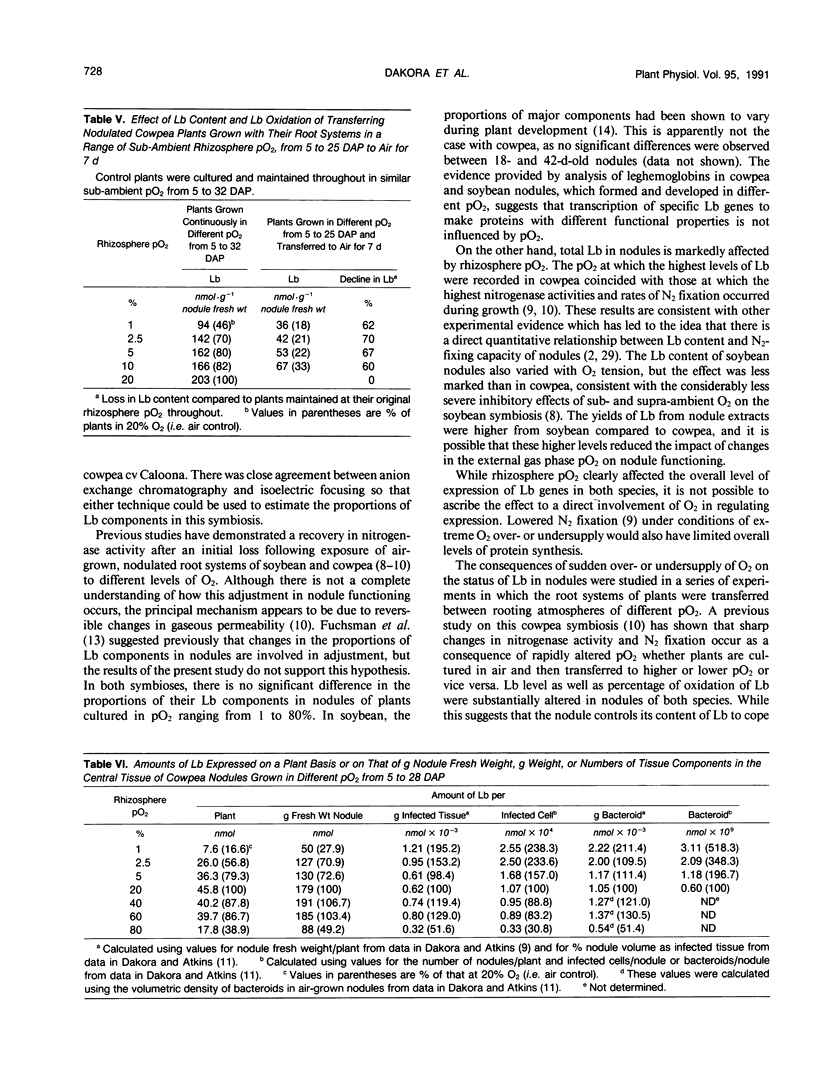
Nodulated cowpea (Vigna unguiculata [L.] Walp. cv Vita 3: Bradyrhizobium strain CB756) and soybean (Glycine max [L.] Merr. cv White Eye: Bradyrhizobium strain CB1809) were grown with their root systems maintained in a flowing gas stream containing a range of pO2 (1-80%, v/v) in N2 for up to 28 days after planting. At the extremes of sub- and supra-ambient pO2, the levels of leghemoglobin (Lb) in nodules were reduced. However, neither the proportional composition of Lb component proteins (eight in soybean, three in cowpea) nor their oxidation state was affected by pO2. Short-term changes in pO2 (transferring plants grown with sub- or supra-ambient pO2 in the rhizosphere to air or vice versa) caused a significant decline in Lb content and, in cowpea but not soybean, where pO2 was increased, a higher percentage of oxidation of Lb. Combining data on changes in Lb level of cowpea nodules grown in sub-ambient pO2 with those for their structural adaptation to an under supply of O2 indicated that, despite the nodules having a lower level of Lb, the amount per infected cell was increased by up to twofold and per bacteroid up to fivefold (in those from 1% O2) compared to those grown in air. Progressive decline in pO2 resulted in a progressive increase on this basis, indicating a close relationship between Lb content and the adaptation of nodule functioning to external O2 level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dakora F. D., Atkins C. A. Effect of pO(2) during Growth on the Gaseous Diffusional Properties of Nodules of Cowpea (Vigna unguiculata L. Walp.). Plant Physiol. 1990 Jul;93(3):956–961. doi: 10.1104/pp.93.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakora F. D., Atkins C. A. Effect of pO(2) on Growth and Nodule Functioning of Symbiotic Cowpea (Vigna unguiculata L. Walp.). Plant Physiol. 1990 Jul;93(3):948–955. doi: 10.1104/pp.93.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979 Aug 28;579(2):314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Fuchsman W. H., Barton C. R., Stein M. M., Thompson J. T., Willett R. M. Leghemoglobin: different roles for different components? Biochem Biophys Res Commun. 1976 Jan 26;68(2):387–392. doi: 10.1016/0006-291x(76)91157-8. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H., Wittenberg J. B., Wittenberg B. A., Bogusz D., Appleby C. A. The kinetics of ligand binding to plant hemoglobins. Structural implications. J Biol Chem. 1989 Jan 5;264(1):100–107. [PubMed] [Google Scholar]
- Keithly J. H., Nadler K. D. Protoporphyrin formation in Rhizobium japonicum. J Bacteriol. 1983 May;154(2):838–845. doi: 10.1128/jb.154.2.838-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Klucas R. V. Reduction of ferric leghemoglobin in soybean root nodules. Plant Physiol. 1984 Apr;74(4):984–988. doi: 10.1104/pp.74.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem Y. Y. Plant senescence processes and free radicals. Free Radic Biol Med. 1988;5(1):39–49. doi: 10.1016/0891-5849(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Maskall C. S., Gibson J. F., Dart P. J. Electron-paramagnetic-resonance studies of leghaemoglobins from soya-bean and cowpea root nodules. Identification of nitrosyl-leghaemoglobin in crude leghaemoglobin preparations. Biochem J. 1977 Nov 1;167(2):435–445. doi: 10.1042/bj1670435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D. T., Schulman H. M. The absence of oxidized leghemoglobin in soybean root nodules during nodule development. Biochem Biophys Res Commun. 1976 Feb 9;68(3):781–785. doi: 10.1016/0006-291x(76)91213-4. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Stacey G., Tandon S. R., Silver L. E., Brill W. J. Rhizobium japonicum mutants defective in symbiotic nitrogen fixation. J Bacteriol. 1982 Oct;152(1):485–494. doi: 10.1128/jb.152.1.485-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari L. L., Klucas R. V. Nonenzymatic reduction of ferric leghemoglobin. Biochim Biophys Acta. 1987 Apr 8;912(2):198–202. doi: 10.1016/0167-4838(87)90089-6. [DOI] [PubMed] [Google Scholar]


