Abstract
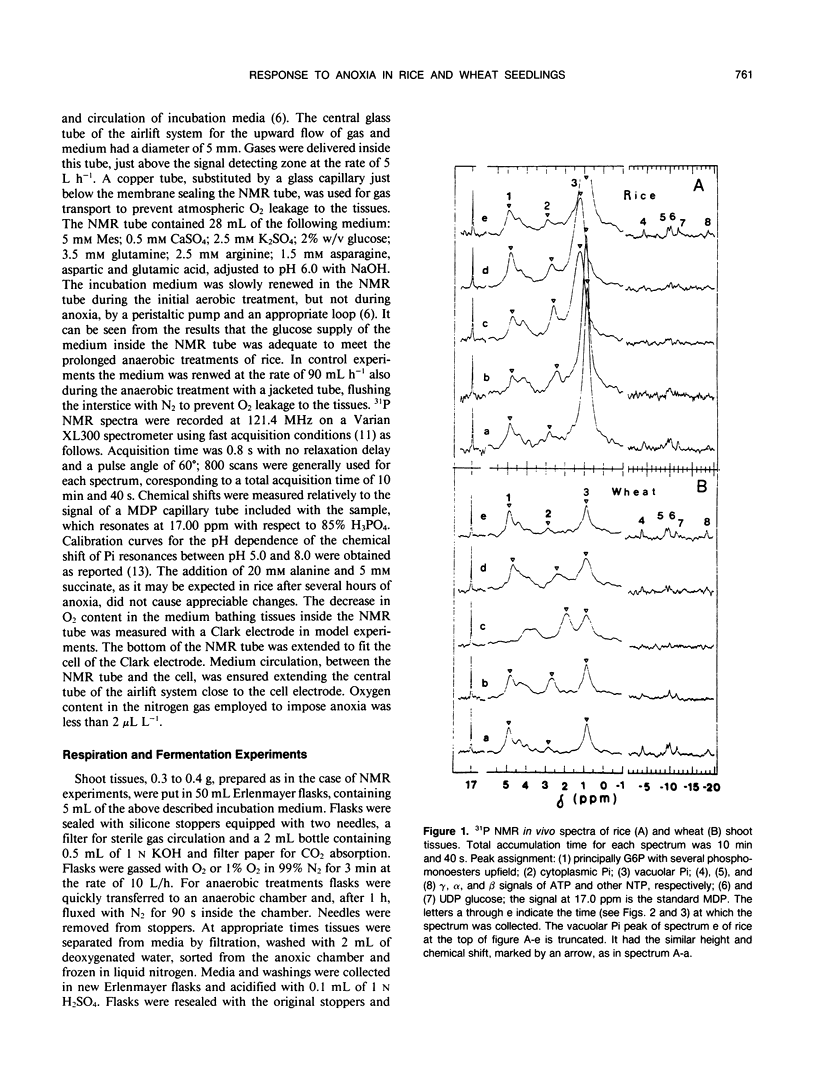
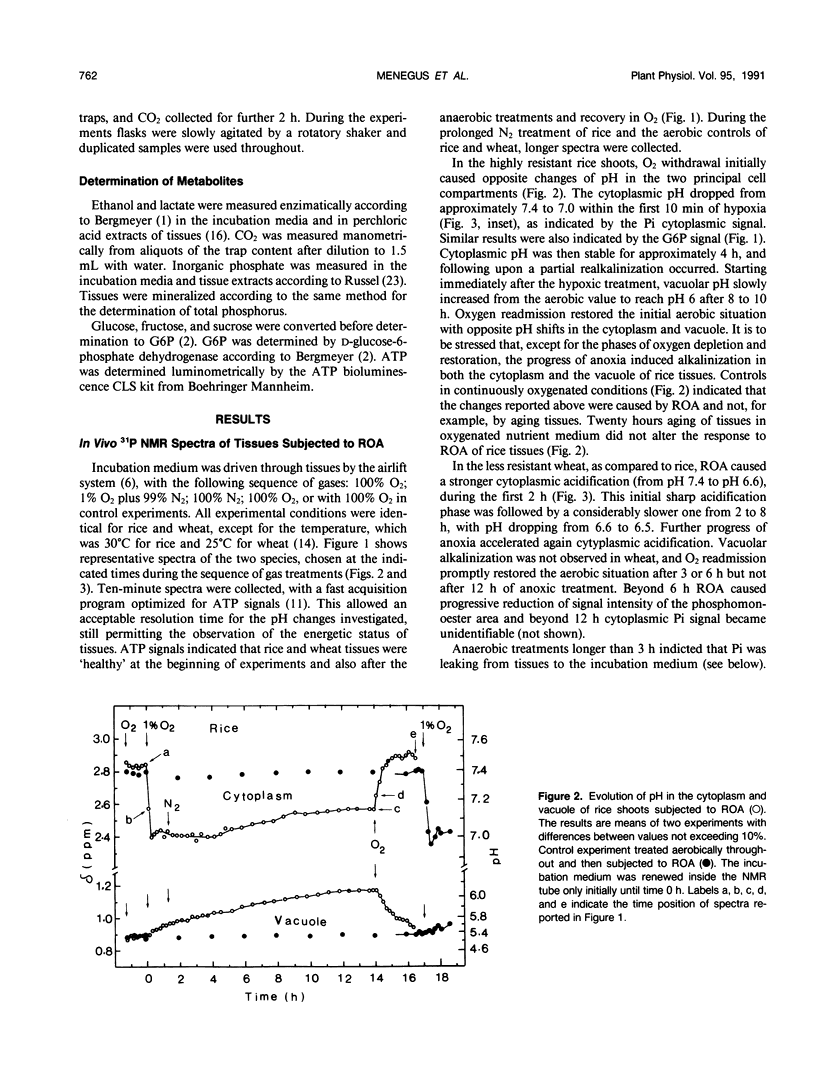
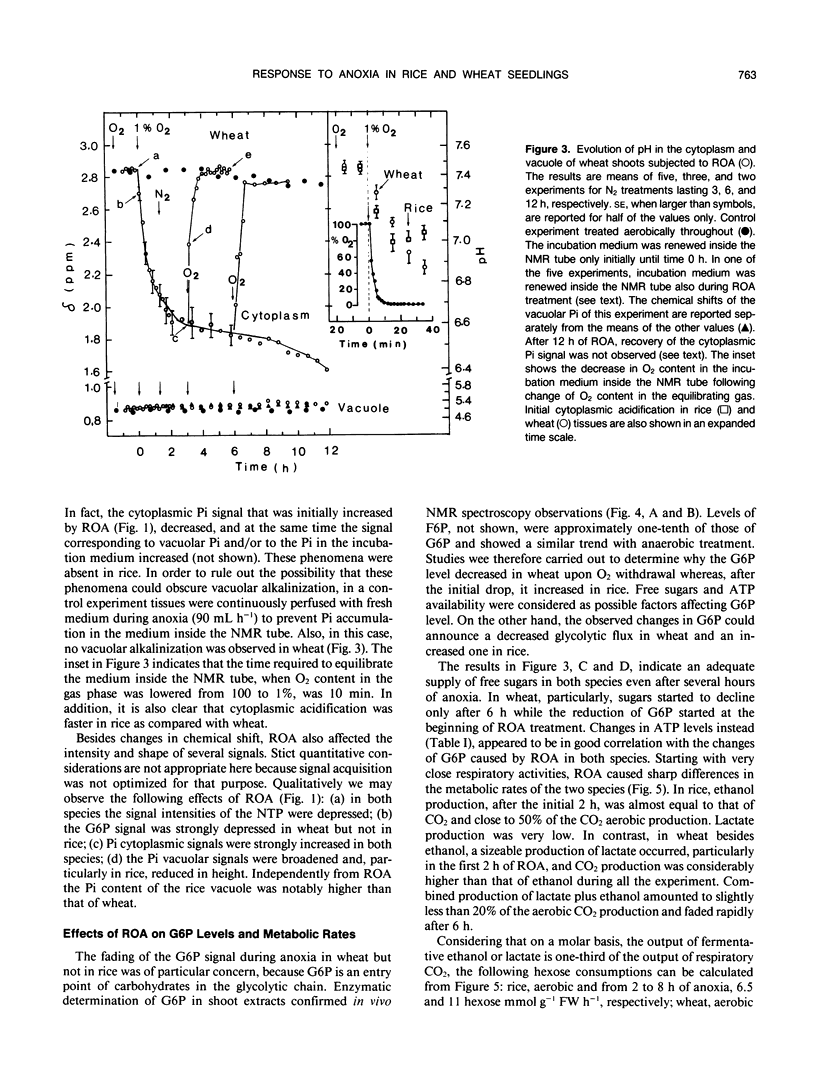
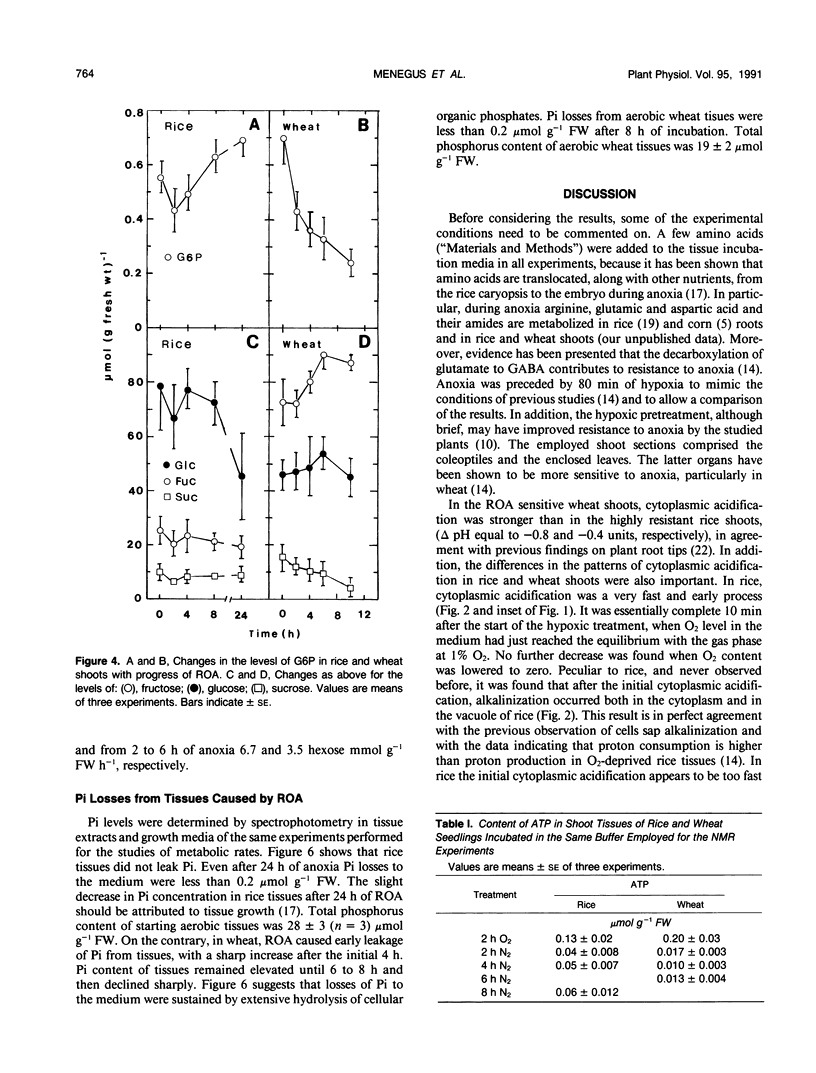
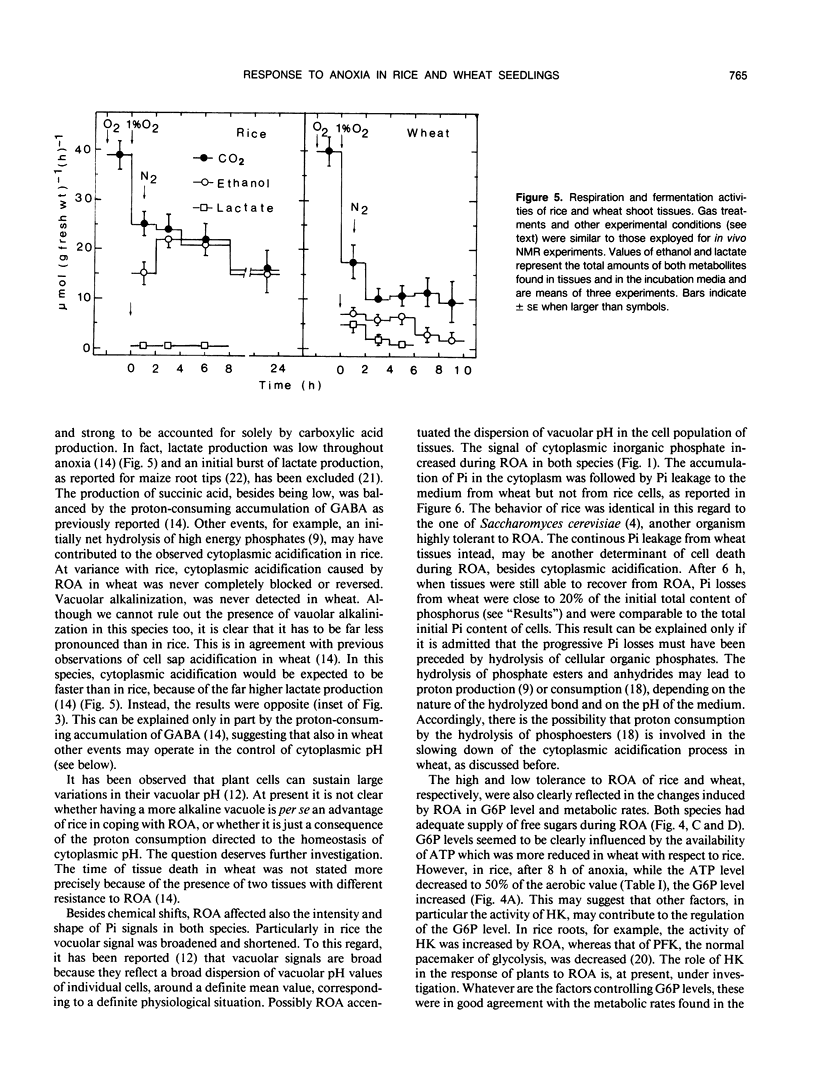
31P nuclear magnetic resonance spectroscopy was used to measure intracellular pH in living tissues. Oxygen deprivation caused fast cytoplasmic acidification from pH 7.4 to 7.0 in shoots of rice, Oryza sativa L. var arborio, a species highly resistant to anoxia. Acidification was complete after 10 minutes of anoxia. Alkalinization of both cytosplasm and vacuole followed thereafter. In the anoxia intolerant wheat shoots, Triticum aestivum L. var MEK, the same treatment caused a sharper cytoplasmic acidification, from pH 7.4 to 6.6, which occurred during a period of 2 hours. Cytoplasmic acidification continued with progress of anoxia and there was no vacuolar alkalinization comparable to the one observed in rice. In wheat oxyen, withdrawal also caused the reduction of both glucose-6-phosphate level and of metabolic rate. It also induced heavy losses of inorganic phosphate from tissues. Conversely, in rice, glucose-6-phosphate level and metabolic rate were increased and inorganic phosphate leakage from tissues was completely absent. These results are discussed in relation to the mechanisms of plant resistance to anoxia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T. W., Higashi R. M., Lane A. N. An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Arch Biochem Biophys. 1988 Nov 1;266(2):592–606. doi: 10.1016/0003-9861(88)90292-5. [DOI] [PubMed] [Google Scholar]
- Good A. G., Crosby W. L. Induction of alcohol dehydrogenase and lactate dehydrogenase in hypoxically induced barley. Plant Physiol. 1989 Jul;90(3):860–866. doi: 10.1104/pp.90.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V. Control of lactate dehydrogenase, lactate glycolysis, and alpha-amylase by o(2) deficit in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegus F., Cattaruzza L., Chersi A., Fronza G. Differences in the Anaerobic Lactate-Succinate Production and in the Changes of Cell Sap pH for Plants with High and Low Resistance to Anoxia. Plant Physiol. 1989 May;90(1):29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murataliev M. B., Vulfson E. N. A pH-metrical method of determining glucose-6-phosphatase activity. Anal Biochem. 1985 Nov 15;151(1):24–27. doi: 10.1016/0003-2697(85)90047-8. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Brown T. R., Shulman R. G. Phosphorus-31 nuclear magnetic resonance studies of the effect of oxygen upon glycolysis in yeast. Biochemistry. 1981 Sep 29;20(20):5871–5880. doi: 10.1021/bi00523a034. [DOI] [PubMed] [Google Scholar]


