Abstract
Background
In people with portal hypertension, gastric varices are less prevalent than oesophageal varices. The risk of bleeding from gastric varices seems to be lower than from oesophageal varices; however, when gastric varices bleed, it is often severe and associated with higher mortality. Endoscopic sclerotherapy of bleeding gastric varices with N‐butyl‐2‐cyanoacrylate glue (cyanoacrylate) is considered the best haemostasis with a lower risk of re‐bleeding compared with other endoscopic methods. However, there are some inconsistencies between trials regarding mortality, incidence of re‐bleeding, and adverse effects.
Objectives
To assess the benefits and harms of sclerotherapy using cyanoacrylate compared with other endoscopic sclerotherapy procedures or with variceal band ligation for treating acute gastric variceal bleeding with or without vasoactive drugs in people with portal hypertension and to assess the best dosage of cyanoacrylate.
Search methods
We searched the Cochrane Hepato‐Biliary Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and Science Citation Index Expanded from inception to September 2014 and reference lists of articles. We included trials irrespective of trial setting, language, publication status, or date of publication.
Selection criteria
Randomised clinical trials comparing sclerotherapy using cyanoacrylate versus other endoscopic methods (sclerotherapy using alcohol‐based compounds or endoscopy band ligation) for acute gastric variceal bleeding in people with portal hypertension.
Data collection and analysis
We performed the review following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Hepato‐Biliary Module.
We presented results as risk ratios (RR) with 95% confidence intervals (CI), with I2 statistic values as a measure of intertrial heterogeneity. We analysed data with both fixed‐effect and random‐effects models, and reported the results with random‐effects models. We performed subgroup, sensitivity, and trial sequential analyses to evaluate the robustness of the overall results, risk of bias, sources of intertrial heterogeneity, and risk of random errors.
Main results
We included six randomised clinical trials with three different comparisons: one trial compared two different doses of cyanoacrylate in 91 adults, bleeding actively from all types of gastric varices; one trial compared cyanoacrylate versus alcohol‐based compounds in 37 adults with active or acute bleeding from isolated gastric varices only; and four trials compared cyanoacrylate versus endoscopic band ligation in 365 adults, with active or acute bleeding from all types of gastric varices. Main outcomes in the included trials were bleeding‐related mortality, failure of intervention, re‐bleeding, adverse events, and control of bleeding. Follow‐up varied from six to 26 months. The participants included in these trials had chronic liver disease of different severities, were predominantly men, and most were from Eastern countries. We judged all trials at high risk of bias. Application of quality criteria for all outcomes yielded very low quality grade of the evidence in the three analyses, except for the low quality evidence rated for the re‐bleeding outcome in the cyanoacrylate versus endoscopic band ligation comparison.
Two different doses of cyanoacrylate: we found very low quality evidence from one trial for the effect of 0.5 mL compared with 1.0 mL of cyanoacrylate on all‐cause mortality (20/44 (45.5%) with 0.5 mL versus 21/47 (45%) with 1.0 mL; RR 1.02; 95% CI 0.65 to 1.60), 30‐day mortality (RR 1.07; 95% CI 0.41 to 2.80), failure of intervention (RR 1.07; 95% CI 0.56 to 2.05), prevention of re‐bleeding (RR 1.30; 95% CI 0.73 to 2.31), adverse events reported as fever (RR 0.56; 95% CI 0.32 to 0.98), and control of bleeding (RR 1.04; 95% CI 0.78 to 1.38).
Cyanoacrylate versus alcohol‐based compounds: we found very low quality evidence from one trial for the effect of cyanoacrylate versus alcohol‐based compounds on 30‐day mortality (2/20 (10%) with cyanoacrylate versus 4/17 (23.5%) with alcohol‐based compound; RR 0.43; 95% CI 0.09 to 2.04), failure of intervention (RR 0.36; 95% CI 0.09 to 1.35), prevention of re‐bleeding (RR 0.85; 95% CI 0.30 to 2.45), adverse events reported as fever (RR 0.43; 95% CI 0.22 to 0.80), and control of bleeding (RR 1.79; 95% CI 1.13 to 2.84).
Cyanoacrylate versus endoscopic band ligation: we found very low quality evidence for the effect of cyanoacrylate versus endoscopic band ligation on bleeding‐related mortality (44/185 (23.7%) with cyanoacrylate versus 50/181 (27.6%) with endoscopic band ligation; RR 0.83; 95% CI 0.52 to 1.31), failure of intervention (RR 1.13; 95% CI 0.23 to 5.69), complications (RR 2.81; 95% CI 0.69 to 11.49), and control of bleeding (RR 1.07; 95% CI 0.90 to 1.27). There was low quality evidence for the prevention of re‐bleeding (RR 0.60; 95% CI 0.41 to 0.88). Trial sequential analysis showed that the analyses were underpowered (diversity‐adjusted required information size was 5290 participants for bleeding‐related mortality).
Authors' conclusions
This review suggests that endoscopic sclerotherapy using cyanoacrylate may be more effective than endoscopic band ligation in terms of preventing re‐bleeding from gastric varices. However, due to the very low quality of the evidence, we are very uncertain about our estimates on all‐cause and bleeding‐related mortality, failure of intervention, adverse events, and control of bleeding. The trials were at high risk of bias; the number of the included randomised clinical trials and number of participants included in each trial was small; and there was evidence of internal heterogeneity across trials, indirectness of evidence in terms of population, and possible publication bias.
The effectiveness of different doses of cyanoacrylate and the comparison of cyanoacrylate versus alcohol compounds to treat variceal bleeding in people with portal hypertension is uncertain due to the very low quality of the evidence.
The shortcomings mentioned call for more evidence from larger trials that need to be conducted according to the SPIRIT statement and reported according to CONSORT guidelines.
Plain language summary
Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension
Background
Acute bleeding from ruptured gastric varices (enlarged veins), the most severe consequence of portal hypertension (that is increased pressure in the veins leading to the liver), is associated with high death rates. The most promising treatment for this condition is considered to be endoscopic sclerotherapy (passing a flexible tube with a camera at the end down the oesophagus (swallowing tube) allowing direct visualisation and treatment of bleeding varices) with N‐butyl‐2‐cyanoacrylate (cyanoacrylate), which is a glue that causes blood clots to form and stops the bleeding. However, incidence of re‐bleeding and complications have opened a debate on when this glue should be used compared with other endoscopic procedures.
Characteristic of included studies
This review includes six trials (following search of scientific databases through to September 2014) of three different comparisons regarding the use of cyanoacrylate: comparison of different dosages of cyanoacrylate (one trial, 91 participants), cyanoacrylate compared with alcohol‐based compounds (one trial, 37 participants), and cyanoacrylate compared with endoscopic band ligation (where enlarged veins are tied off using elastic bands; four trials, 366 participants). Risk of bias (that is overestimation of benefits and underestimation of harms) was high in all trials. Outcomes assessed included death, bleeding‐related death, treatment failure, re‐bleeding, side effects, and bleeding control. Follow‐up of people varied from six to 26 months. All people included in these trials had chronic liver disease of different severities and were predominantly men. Most of the trials came from Eastern countries, although it must be noted that prevalence of chronic liver disease is fairly similar worldwide, with differences in causes that may have no effect on variceal bleeding.
Results
One trial showed that death was similar between the group of people who received the lower dose (0.5 mL) of cyanoacrylate and people who received a higher dose (1.0 mL), but fewer people who were given the lower dose had fewer complications. However, because the trial was small, we cannot be certain that the doses have the same effect. One trial implied that cyanoacrylate may be better than endoscopic sclerotherapy using alcohol‐based compounds in terms of bleeding control, control of bleeding in fundal varices (enlarged veins at the base of the oesophagus), and complications, but the trial was too small to be certain about this effect. Results from four trials suggested that cyanoacrylate may be better than endoscopic band ligation regarding re‐bleeding, and that it seems as effective as endoscopic band ligation regarding bleeding control, treatment failure, and prevention of death.
Quality of evidence
The quality of evidence ranged from very low to low. The main reasons for downgrading the quality of evidence included high likelihood of bias (due to small numbers of participants), imprecision of results, and differences in populations studied in the trials.
Summary of findings
Summary of findings for the main comparison. Cyanoacrylate versus band ligation for acute bleeding gastric varices in people with portal hypertension.
| Cyanoacrylate versus endoscopic band ligation for acute bleeding gastric varices in people with portal hypertension | ||||||
| Patient or population: acute bleeding gastric varices in people with portal hypertension Settings: endoscopy room Intervention: cyanoacrylate Control: endoscopic band ligation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control: endoscopic band ligation | Intervention: cyanoacrylate | |||||
| Mortality Total of deaths and the end of follow‐up. Follow‐up: 6 to 14 months | Study population | RR 0.83 (0.52 to 1.31) | 365 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,5,6 | Counts for the total deaths at the end of follow‐up. Included 30‐day mortality (not available for all trials), mortality from bleeding, and other causes. | |
| 278 per 1000 | 231 per 1000 (144 to 364) | |||||
| Moderate | ||||||
| 277 per 1000 | 230 per 1000 (144 to 363) | |||||
| Failure of intervention Continuous variceal bleeding after intervention Follow‐up: mean 1 days | Study population | RR 1.13 (0.23 to 5.69) | 264 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5,6 | The numbers represents only the trials considering active bleeding at the moment of intervention. | |
| 62 per 1000 | 70 per 1000 (14 to 353) | |||||
| Moderate | ||||||
| 40 per 1000 | 45 per 1000 (9 to 228) | |||||
| Re‐bleeding Re‐bleeding after the bleeding was controlled in the first intervention Follow‐up: mean 7 days | Study population | RR 0.6 (0.41 to 0.88) | 360 (4 studies) | ⊕⊕⊝⊝ low1,2,5,6 | Trial sequential analysis suggested that cyanoacrylate superiority was not likely to be due to random error. | |
| 299 per 1000 | 180 per 1000 (123 to 264) | |||||
| Moderate | ||||||
| 326 per 1000 | 196 per 1000 (134 to 287) | |||||
| Complications (general) Number of total complications Follow‐up: 6 to 14 months | Study population | RR 2.81 (0.69 to 11.49) | 307 (3 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5,6 | Heterogeneity between trials about the complications detected. The 2 common complications (and the assessed ones) were pain and fever. | |
| 112 per 1000 | 314 per 1000 (77 to 1000) | |||||
| Moderate | ||||||
| 67 per 1000 | 188 per 1000 (46 to 770) | |||||
| Control of bleeding Success in control variceal bleeding Follow‐up: mean 30 days | Study population | RR 1.07 (0.9 to 1.27) | 264 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5,6 | Mixed risk of bias and small total numbers. | |
| 837 per 1000 | 896 per 1000 (753 to 1000) | |||||
| Moderate | ||||||
| 873 per 1000 | 934 per 1000 (786 to 1000) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed control risk: mean baseline risk of the trials. 2 Downgraded on level due to serious risk of bias (we rated the four trials as high risk of bias). 3 Downgraded one level due to imprecision (264 to 365 participants in the five outcomes). 4 Downgraded on level to moderate heterogeneity (moderate to high I2). 5 Downgraded one level due to serious indirectness (only one type of population).
6 Downgraded one level due to likely publication bias (only four trials found).
Summary of findings 2. Cyanoacrylate 1 mL versus cyanoacrylate 0.5 mL for acute bleeding gastric varices in people with portal hypertension.
| Cyanoacrylate 1 mL versus cyanoacrylate 0.5 mL for acute bleeding gastric varices in people with portal hypertension | ||||||
| Patient or population: acute bleeding gastric varices in people with portal hypertension Settings: endoscopy room Intervention: cyanoacrylate 1 mL Control: cyanoacrylate 0.5 mL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control: cyanoacrylate 0.5 mL | Intervention: cyanoacrylate 1 mL | |||||
| Total mortality Total deaths and the end of follow‐up Follow‐up: mean 26 months | Study population | RR 1.02 (0.65 to 1.60) | 91 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 447 per 1000 | 438 per 1000 (277 to 693) | |||||
| Moderate | ||||||
| 447 per 1000 | 438 per 1000 (277 to 693) | |||||
| 30 day ‐ mortality Mortality due to bleeding Follow‐up: mean 30 days | Study population | RR 1.07 (0.41 to 2.8) | 91 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 149 per 1000 | 159 per 1000 (61 to 417) | |||||
| Moderate | ||||||
| 149 per 1000 | 159 per 1000 (61 to 417) | |||||
| Failure of intervention Continuous bleeding after intervention Follow‐up: mean 1 day. | Study population | RR 1.07 (0.56 to 2.05) | 91 (1 study) | ⊕⊕⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 277 per 1000 | 296 per 1000 (155 to 567) | |||||
| Moderate | ||||||
| 277 per 1000 | 296 per 1000 (155 to 568) | |||||
| Complications (fever) Presence of fever Follow‐up: mean 26 months | Study population | RR 0.56 (0.32 to 0.98) | 91 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 489 per 1000 | 387 per 1000 (50 to 154) | |||||
| Moderate | ||||||
| 489 per 1000 | 386 per 1000 (50 to 154) | |||||
| Re‐bleeding Bleeding after initial success in the intervention Follow‐up: mean 1 weeks | Study population | RR 1.3 (0.73 to 2.31) | 91 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 298 per 1000 | 387 per 1000 (217 to 688) | |||||
| Moderate | ||||||
| 298 per 1000 | 387 per 1000 (218 to 688) | |||||
| Control of bleeding Success in control the active variceal bleeding Follow‐up: mean 26 months | Study population | RR 1.04 (0.78 to 1.38) | 25 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 867 per 1000 | 901 per 1000 (676 to 1000) | |||||
| Moderate | ||||||
| 867 per 1000 | 902 per 1000 (676 to 1000) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed control risk: equates control group risk from the trial. 2 Downgraded one level due to serious risk of bias (only one trial rated as high risk of bias for unclear performance bias). 3 Downgraded two levels due to serious imprecision (only one trial with 91 participants in total, few events, 95% CI included appreciable benefit and harm). 4 Downgraded one level due to serious indirectness (only one type of population). 5 Downgraded one level due to likely publication bias (only one trial found).
Summary of findings 3. Cyanoacrylate versus alcohol for acute bleeding gastric varices in people with portal hypertension.
| Cyanoacrylate versus alcohol for acute bleeding gastric varices in people with portal hypertension | ||||||
| Patient or population: acute bleeding gastric varices in people with portal hypertension Settings: endoscopy room Intervention: cyanoacrylate Control: absolute alcohol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control: absolute alcohol | Intervention: cyanoacrylate | |||||
| Mortality Total deaths at 30 days Follow‐up: mean 14 months | Study population | RR 0.43 (0.09 to 2.04) | 37 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 235 per 1000 | 101 per 1000 (21 to 480) | |||||
| Moderate | ||||||
| 235 per 1000 | 101 per 1000 (21 to 479) | |||||
| Failure of intervention Follow‐up: mean 1 days | Study population | RR 0.36 (0.09 to 1.35) | 17 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 625 per 1000 | 225 per 1000 (56 to 844) | |||||
| Moderate | ||||||
| 625 per 1000 | 225 per 1000 (56 to 844) | |||||
| Complications (fever) Presence of fever Follow‐up: mean 14 months | Study population | RR 0.43 (0.22 to 0.8) | 37 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 824 per 1000 | 354 per 1000 (181 to 659) | |||||
| Moderate | ||||||
| 824 per 1000 | 354 per 1000 (181 to 659) | |||||
| Re‐bleeding Re‐bleeding after intervention Follow‐up: 1 to 4 weeks | Study population | RR 0.85 (0.3 to 2.45) | 37 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 294 per 1000 | 250 per 1000 (88 to 721) | |||||
| Moderate | ||||||
| 294 per 1000 | 250 per 1000 (88 to 720) | |||||
| Control of bleeding Success in controlling the active variceal bleeding Follow‐up: mean 14 months | Study population | RR 1.79 (1.13 to 2.84) | 37 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4,5 | Only 1 trial. | |
| 529 per 1000 | 948 per 1000 (598 to 1000) | |||||
| Moderate | ||||||
| 529 per 1000 | 947 per 1000 (598 to 1000) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed control risk: equates control group risk from the trial. 2 Downgraded one level due to serious risk of bias (only one trial rated as high risk of bias for unclear selection, performance, and detection bias). 3 Downgraded two levels due to serious imprecision (only one trial with 37 participants in total, few events, 95% CI includes appreciable benefit and harm). 4 Downgraded one level due to serious indirectness (only one type of population). 5 Downgraded one level due to likely publication bias (only one trial found).
Background
Description of the condition
Acute bleeding from ruptured gastro‐oesophageal varices is the most severe consequence of portal hypertension. It is associated with high mortality in people with cirrhosis and other diseases (Sharara 2001). Although gastric varices are less prevalent than oesophageal varices (5% to 33%), their actual magnitude is not well known and their risk of bleeding seems to be lower, but such bleeding is severe and the mortality associated with it is higher than bleeding oesophageal varices (Sarin 1992). The incidence of bleeding in gastric varices is 25%, with re‐bleeding rates as high as 40% and mortality rates as high as 50% (Soehendra 1986; Greig 1990). Early re‐bleeding in gastric varices is associated with increased risk of death, and usually a 'second try' is not attempted in the endoscopic treatment.
The prevalence of gastric varices seems to be similar worldwide, despite the fact that different countries present different aetiologies for portal hypertension, and different aetiologies for liver cirrhosis (e.g., alcohol being more prevalent in some countries of South America, parasites in other South American and African countries, and hepatitis C in Asian countries). However, gastric varices are more common in people with non‐cirrhotic portal hypertension and extrahepatic portal vein obstruction (Sarin 1992). It has been suggested that gastric varices may bleed with lower portal pressure gradients than those of oesophageal varices as a consequence of large splenorenal shunts (Irani 2011).
Gastric varices can be: gastro‐oesophageal, also called cardial varices (type I, GOV) or isolated gastric varices (type II, IGV). GOV can be GOV1 (extension of oesophageal varices along lesser curve) or GOV2 (extension towards fundus). IGV can be IGV1 (isolated varices in the fundus) or IGV2 (isolated varices anywhere in the stomach). Bleeding associated with type 2 varices is more severe and has lower rates of treatment success (Sarin 1992). Most of the available data comes from studies of IGV1, GOV1, and GOV2. There are few data from varices type IGV2 due to their low prevalence, although their management is similar to IGV1 (Garcia‐Pagán 2013).
Description of the intervention
Although there are few studies of the specific management of the gastric varices, their initial workout is similar to that of oesophageal variceal bleeding. Treatment includes the use of prophylactic antibiotics, replacement of volaemia using a restrictive transfusion policy, and the use of vasoactive drugs given intravenously (such as terlipressin, somatostatin, or somatostatin analogues), which may be effective in oesophageal varices but less so in gastric varices (Wu 2002; Evrard 2003). Consensus and guidelines on gastric variceal bleeding recommend concomitant use of vasoactive drugs with endoscopic therapy. Some people require rescue therapy, such as transjugular intrahepatic portosystemic shunt (TIPS) both in people with oesophageal and gastric varices (McCormick 1994), and some people require derivative surgery. In massive bleeding, when it is not possible to perform endoscopy or any other intervention, balloon tamponade may potentially be used as a temporary treatment for a maximum of 24 hours. At deflation, re‐bleeding could be higher than 50%.
Endoscopic interventions are the preferred emergency treatment for bleeding gastric varices. These procedures are similar to those used in oesophageal varices bleeding, though with dissimilar results. For instance, endoscopic sclerosis using ethanolamine oleate, polidocanol, and sodium tetradecyl is less effective in the control of bleeding from gastric varices than from oesophageal varices in uncontrolled series (Korula 1991; Ogawa 1999; Huang 2000; Akahoshi 2002; Cheng 2007). Similarly, endoscopic band ligation, despite the favourable results reported in the treatment of oesophageal varices, is associated with a high re‐bleeding rate in gastric varices (Takeuchi 1996; Harada 1997). Other treatments involve loop ligation and endoscopic sclerotherapy with thrombin, which have been tested in some centres with good initial results (Kitano 1989; Yoshida 1999; Yang 2002).
Injection of N‐butyl‐2‐cyanoacrylate (cyanoacrylate) is considered the best endoscopic treatment for gastric varices, achieving better haemostasis and lower re‐bleeding rates than other sclerosants and band ligation. However, inconsistencies among studies exist (Oho 1995; Sarin 2001), and serious complications have been reported (Rosch 1998; Turler 2001). Cyanoacrylate is widely used around the world despite requiring skilled personnel for its administration. However, it has not been approved in the US because of reports of embolism to distal organs, which is the most serious complication associated with its use (Rosch 1998; Huang 2000; Turler 2001; Upadhyay 2005; Alexander 2006; Bonilha 2011). In Canada, 2‐octylcyanoacrylate, a compound similar to cyanoacrylate, is used (Rengstorff 2004; Belletrutti 2008).
The most usual protocol uses cyanoacrylate and lipiodol in a 1 : 1 ratio, injecting 0.5 to 1.0 mL of cyanoacrylate into the varix in every injection. A proper dosage has not been established (Hou 2009), and it is usually decided by the endoscopist at the time of intervention, taking into account the size of the gastric varices and the initial success in arresting bleeding, considering that larger doses could increase the risk of embolism to distal organs.
How the intervention might work
Cyanoacrylate is a monomer in a liquid form that lends itself to variceal injection. On contact with hydroxyl ions in water or blood, cyanoacrylate undergoes rapid polymerisation into a hard plastic or glue, acting as a chemical tissue adhesive and leading to haemostasis of the varix. Endoscopic injection of this monomer is achieved through a standard forward‐viewing endoscope using a disposable sclerotherapy needle, alone or in combination with a contrast agent (e.g., lipiodol) to facilitate X‐ray visualisation during or after the procedure (Sarin 2001; Akahoshi 2002). Cyanoacrylate is used to arrest active bleeding, and subsequently, to obliterate and eventually eradicate the varices. It takes several months to expel the hard plastic inside the varix.
Endoscopy sclerotherapy with cyanoacrylate glue has achieved the best haemostasis in people with bleeding gastric varices (up to 90% of people) and is associated with lower incidence of re‐bleeding compared with other sclerosants (Oho 1995; Ogawa 1999; Huang 2000; Sarin 2001; Akahoshi 2002; Rengstorff 2004; Cheng 2007), and with endoscopic band ligation (Takeuchi 1996; Harada 1997; Tan 2006). Many of these studies are non‐randomised studies or only small randomised trials. Embolism of cyanoacrylate to distal organs is the worst complication, and has been described in several observational studies (Rosch 1998; Huang 2000; Turler 2001; Upadhyay 2005; Alexander 2006; Bonilha 2011).
Why it is important to do this review
We have been unable to identify meta‐analyses or systematic reviews on this topic. There is scant evidence on the proper treatment and management of gastric varices, since they are less frequent than oesophageal varices. Consequently, it is not clear whether sclerotherapy with cyanoacrylate is more effective than other endoscopic treatments, whether there will be fewer complications, or whether the combination of cyanoacrylate with vasoactive drugs is useful.
Objectives
To assess the benefits and harms of sclerotherapy with cyanoacrylate compared with other endoscopic sclerotherapy procedures or with variceal band ligation for treating acute gastric variceal bleeding with or without vasoactive drugs in people with portal hypertension and to assess the best dosage of cyanoacrylate.
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria for benefits and harms
Randomised clinical trials regardless of publication status, blinding, or language.
Inclusion criteria for harms
Observational studies and studies using quasi‐randomisation methods, for example, day of birth or date of admission.
Types of participants
Participants with endoscopically verified acute bleeding from gastric varices regardless of the underlying aetiology of the portal hypertension, and not treated previously with endoscopic sclerotherapy, surgery, or TIPS.
Types of interventions
Experimental treatment
Endoscopic sclerotherapy of gastric varices with cyanoacrylate glue alone or combined with systemic vasoactive drugs such as:
vasopressin with or without nitroglycerin;
terlipressin;
somatostatin;
octreotide; or
vapreotide.
Control treatment
Endoscopic sclerotherapy, no intervention, emergency ligation (band or loop), or sclerotherapy with alcohol‐based sclerosants or injection of thrombin, alone or combined with the same vasoactive drugs used in the experimental group.
We allowed concomitant interventions such as use of systematic vasoactive drugs, proton pump inhibitors, prophylactic antibiotics, and use of vasoactive drugs if administered equally in all trial intervention groups.
Types of outcome measures
Primary outcomes
All‐cause mortality at maximum follow‐up (see Differences between protocol and review).
Bleeding‐related mortality: number of people who died from uncontrolled variceal bleeding at medium term (approximately one month) (see Differences between protocol and review).
Failure of intervention: number of people in which the intervention was unable to control active or acute bleeding within 24 hours, triggering a need to change treatment or repeat endoscopy (active: endoscopy evidence of current bleeding; acute: endoscopy evidence of recent bleeding stigmata without current bleeding) (see Differences between protocol and review).
Re‐bleeding: number of people in which the intervention was unable to prevent re‐bleeding at short term (approximately one week) (see Differences between protocol and review).
-
Adverse events:
number of people with pulmonary embolism caused by cyanoacrylate (measured by radiological and clinical criteria) or with cyanoacrylate embolism in other organs such as brain and spleen;
number of people who developed septicaemia after intervention;
number of people with other serious adverse effects according to the International Conference on Harmonization Guidelines (ICH‐GCP 1997) (see Differences between protocol and review).
Secondary outcomes
Control of bleeding: number of people in which the intervention was able to control bleeding in the first intervention.
Number of transfusions: number of packed red cell transfusions while in hospital (see Differences between protocol and review).
Quality of life (see Differences between protocol and review).
TIPS or surgery: number of people who underwent TIPS or surgery (see Differences between protocol and review).
Search methods for identification of studies
Electronic searches
We performed electronic searches of The Cochrane Hepato‐Biliary Controlled Trials Register (Gluud 2015), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003) for randomised clinical trials to September 2014. We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/en/). The search strategies with the time spans of the searches are given in Appendix 1.
Searching other resources
We reviewed the reference lists of the retrieved articles for potentially relevant studies on benefits and harms, including review articles on the topic. We attempted to contact the corresponding authors of relevant studies identified from the initial search and experts in the field to request information on unpublished articles.
We also tried to contact the authors of the publications of interest if further clarification was necessary. We made a search of the proceedings of the most important conferences related to digestive endoscopy for unpublished trials.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and The Cochrane Hepato‐Biliary Module (Gluud 2015).
Selection of studies
Two review authors (ER, PS) undertook the trial selection process. They were unblinded with regard to names of the authors, investigators, institutions, and results. The review authors independently extracted data to assess whether trials met the inclusion criteria. We resolved discrepancies by discussion and involvement of a third review author (JG) when necessary.
Data extraction and management
We designed standardised extraction sheets and pilot‐tested them before use. We extracted the following data:
trial characteristics: risk of bias, design, number of intervention groups, number of participants with missing data, and length of follow‐up;
participant characteristics: number of participants randomised to each intervention group, mean (or median) age, number of males and females, severity of bleeding (according to haemoglobin level, arterial pressure, heart rate), stage of liver compromise according to Child‐Pugh and model for end‐stage liver disease (MELD) classifications, main diagnosis or cause of portal hypertension, time from beginning of bleeding to treatment, factors precipitating bleeding, and type of gastric varices;
intervention characteristics: type and dose of the experimental and control interventions, duration of therapy, mode of administration, type and dose of additional interventions, obliteration, and eradication of varices, or both, if reported.
We also recorded if intention‐to‐treat analysis was implemented, if blinded assessment of outcome measures was conducted, and if a sample‐size calculation was performed before the trial started.
Two review authors (ER, PS) independently extracted relevant data from the studies. The review authors were unblinded with regard to names of the authors, investigators, institutions, and results. We resolved discrepancies by discussion and involvement of a third review author (JG) when necessary.
Assessment of risk of bias in included studies
Randomised clinical trials with high risk of bias may lead to overestimation or underestimation of intervention effects (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savović 2012a; Savović 2012b). Usually, such bias risks are associated with overestimation of benefits and underestimation of harms if an experimental intervention is compared with placebo or no intervention. When two 'active' interventions are compared, it becomes more difficult to know in which direction bias will lead to overestimation of benefits and underestimation of harms. To assess risk of bias in a trial, we have used a set of bias risk domains relevant for our review (see below) (Higgins 2011).
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (e.g., if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias on the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes was likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: outcome assessment was carried out blinded for all relevant outcomes, and the method of blinding was described, so that knowledge of allocation was prevented.
Unclear risk of bias: blinding of outcome assessment was not described, or the outcome assessment was described as blinded, but the method of blinding was not described, so that knowledge of allocation was possible.
High risk of bias: outcome assessment was not blinded, so that the allocation was known to outcome assessors.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, was employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether the missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were pre‐defined and reported, or all clinically relevant and reasonably expected outcomes were reported.
Uncertain risk of bias: it is unclear whether all pre‐defined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conductance, or results of the trial.
Uncertain risk of bias: the trial may or may not have been free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other bias domains that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
We considered trials at low risk of bias if they were classified as 'low risk of bias' in all of the individual domains specified above. We considered trials at 'high risk of bias' if we judged the risk of bias as high or uncertain in any of the individual domains specified above.
Measures of treatment effect
We used relative risks (RR) with 95% confidence intervals (CI) (Higgins 2011). We determined absolute measures of effect by calculating absolute risk reduction, number needed to treat for an additional beneficial outcome (NNTB), and number needed to treat for an additional harmful outcome (NNTH) whenever results were statistically significant. For continuous data, we calculated the mean difference (MD) with 95% CI.
Unit of analysis issues
Participants in the individual randomised trials.
Dealing with missing data
We conducted all analyses using the intention‐to‐treat principle by including all randomised participants irrespective of compliance or follow‐up. We did not detect relevant missing data in the full‐article papers, as all expected results were accounted for. However, there were participants lost to follow‐up after the main measures had been taken.
We attempted to contact the authors of the publication in an abstract form included in this review. However, we received no response.
Assessment of heterogeneity
We examined statistical heterogeneity between results of different trials by checking the test statistic (Cochrane's Q), with significance set at P value < 0.1. We also calculated inconsistency (I2 statistic) with an I2 of 50% judged as high heterogeneity (Higgins 2003).
Assessment of reporting biases
We did not assess reporting biases by means of a funnel plot as we did not have the minimum of 10 trials needed to construct it (Egger 1997).
Data synthesis
Meta‐analysis
We performed statistical analyses following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and using Review Manager 5 (RevMan 2014).
We used mean and standard deviations to derive an MD for continuous data, as well as RRs and CI values for dichotomous data.
When possible, we meta‐analysed data using both random‐effects and fixed‐effect models to ensure robustness of the results. In case of differences in findings regarding significance of the intervention effect using the two models, we presented the results with both methods. When there were no differences in the results, we presented only the random‐effects model (Higgins 2011).
Trial sequential analyses
Cumulative meta‐analyses are prone to produce high risk of type I and type II errors due to sparse data and repetitive testing of cumulative data (Wetterslev 2008; Thorlund 2011). We performed trial sequential analysis (TSA) to control such random errors (Thorlund 2011; TSA 2011). The outcomes analysed using TSA were from comparisons including more than one trial (i.e., cyanoacrylate versus band ligation). We used the meta‐analytic estimate of the control event proportion (Pc) of the trials as the control event proportion in the TSAs. We planned to use the intervention effect estimated in the meta‐analysis of trials with low risks of bias but, as we found none, we conducted the TSAs using an a priori intervention effect of 20% risk ratio reduction. For one outcome (treatment failure), this effect did not result in an intelligible TSA figure (the accrued information was too small a fraction of the required information size), which is why we increased the risk ratio reduction to 40%. For each TSA performed, we calculated a diversity‐adjusted required information size based on the intervention effect of 20% (or 40%) risk ratio reduction, a risk of type I error of 5%, and a risk of type II error of 20% (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). Diversity adjustment was performed with the observed diversity adjustment factor (1/(1 ‐ D2) using the diversity estimate (D2) among all trials in the meta‐analysis (Wetterslev 2009). We had planned to use the intervention effects estimated in trials with low risk of bias; however, all trials were at high risk of bias and this is planned should we include more trials in future updates of this review.
Subgroup analysis and investigation of heterogeneity
When possible, we performed the following subgroup analyses.
Trials at low risk of bias compared to trials at high risk of bias.
Trials with co‐interventions compared to trials without co‐interventions (use of vasoactive drugs).
Comparison of people with different type of varices.
Comparison of trials including participants with hepatocarcinoma compared to trials without inclusion of such participants.
We grouped trials according to severity of the underlying disease using Child‐Pugh and MELD scores when available.
Sensitivity analysis
We included or excluded individual trials during the review process to determine whether the conclusions were robust. We examined the following factors in the sensitivity analyses:
full texts versus abstracts;
trials with unclear risk of bias versus trials with high risk of bias;
trials with shorter versus longer follow‐up periods;
trials with only GOV1 versus other type of gastric varices;
trials with inclusion of hepatocarcinoma versus exclusion hepatocarcinoma, and
trials with concomitant use of vasoactive drugs.
Summary of findings' tables
We used 'Summary of findings' tables, constructed using GRADEPro software, to present our assessment of the body of evidence associated with the primary and some secondary outcomes in our review (GRADEpro 2008; Guyatt 2008; Higgins 2011).
The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers five factors regarding limitations in the design and implementation of available studies: high likelihood of bias: indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results (wide confidence intervals); and high probability of publication bias (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013).
Results
Description of studies
See: Characteristics of included studies table.
Results of the search
From 256 identified studies, we removed 98 duplicates. We analysed the abstracts of the remaining 158 publications and eliminated 136 references that did not refer to randomised trials. We assessed the full‐text versions of the 22 remaining publications in depth. Of these, we excluded all references dealing with primary or secondary prevention of bleeding. Six trials described in six publications met our inclusion criteria and were included in the analysis (Figure 1).
1.
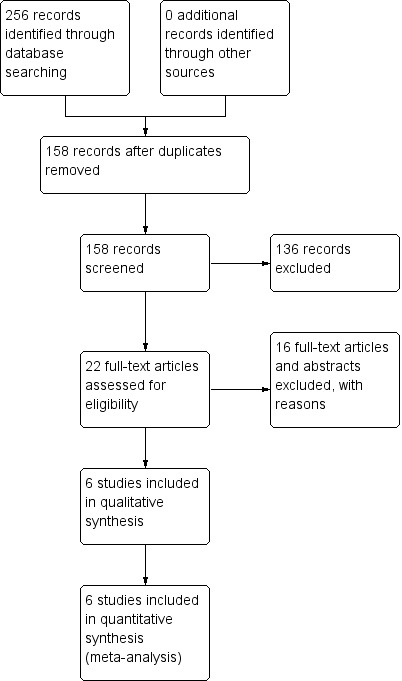
Study flow diagram.
Included studies
Descriptive statistics for the whole group of trials
Trials were performed in Egypt (one trial), Taiwan (one trial), Republic of China (two trials), Taipei (one trial), and India (one trial). Five trials were conducted at a single clinical site, whereas one trial was conducted at three clinical sites. Five trials were published as full papers and one in abstract form, all within the period of 2001 to 2012. The trial published as an abstract had few data (Zheng 2012).
Inclusion criteria were people with portal hypertension, clinical signs of bleeding, endoscopic signs of bleeding, written consent (participant or relative), and adult age. Exclusion criteria were undetermined source of bleeding, previous history of any endoscopy or shunt treatment, encephalopathy, hepatorenal syndrome, non‐consent, terminal illness, major organ system disease, life expectancy of 24 hours or less, portal thrombosis, and gastric varices without stigmata of bleeding. One trial excluded participants with hepatocarcinoma, whereas two excluded only the advanced type, and two included all types of hepatocarcinoma (no data in the abstract).
Underlying liver disease was diagnosed based on clinical, biochemical, or histological signs. Most of the aetiology underlying the hepatic disease was post‐viral hepatitis (59%), with alcoholic liver disease being the least common (17%). The stage of liver involvement according to the Child‐Pugh classification score for all participants (available data in four of six trials) was: Child A: 90 participants (26.1%); Child B: 171 participants (49.7%); and Child C: 83 participants (24.1%). Only one trial used the MELD classification. All trials classified varices according to Sarin's classification (Sarin 1992). Three trials focused on all types of gastric varices, whereas one trial focused only on isolated varices (IGV1), and one trial focused on cardial varices (GOV1). Concomitant oesophageal varices were treated with band ligation during the first endoscopy session in all trials.
The mean sample size was 82 people (range 37 to 150). Three trials included a mix of participants with active and acute bleeding, whereas three trials included only participants with acute bleeding. One trial compared two different doses of cyanoacrylate, one trial compared cyanoacrylate versus alcohol‐based compounds (absolute alcohol), and four trials compared cyanoacrylate versus endoscopic band ligation.
The mean age of all included participants was 53.4 years (range 22 to 75), whereas mean age for participants randomised to cyanoacrylate was 54.6 years (range 24 to 75), band ligation was 56.2 years (range 42 to 74), and alcohol‐based compounds was 35 years (range 22 to 48). The male : female ratio was 322 : 113 (65% male) overall, 67% male for participants randomised to cyanoacrylate, 72% male for participants randomised to alcohol‐based compounds, and 66% male for participants randomised to band ligation.
All trials assessed bleeding‐related mortality, treatment failure, re‐bleeding, and complications. Timing for the outcomes varied across trials. Trials involving cyanoacrylate versus band ligation also assessed variceal obliteration. Mean time of total follow‐up was 16.3 months (range six to 26).
The criteria used for assessing active or acute bleeding involved clinical signs of bleeding, endoscopic signs of bleeding, adherent clot, white nipple or variceal erosion, large varices with red spots or wale marking, and absence of other causes of bleeding.
A mean of 5.2 units of blood was used in all participants, 5.8 units in the cyanoacrylate group and 4.6 units in the band ligation group (data available from two trials). TIPS was offered after second endoscopy treatment failure in one trial (no numbers available). Surgery was conducted in one trial after second endoscopy treatment failure (one after cyanoacrylate failure, four after band ligation failure). Vasoactive drugs were used in four trials.
Cyanoacrylate was administered by intravariceal injection in all trials, starting near the bleeding point. Each injection was composed of 0.5 mL of N‐butyl‐2‐cyanoacrylate and 0.5 to 1.8 mL of lipiodol, using a 21‐ to 23‐gauge needle (range one to six injections). Sessions were repeated at one to four weeks until varix eradication. Participants were then followed up three to six months after treatment; cyanoacrylate injection was repeated in cases of variceal recurrence. The mean number of sessions needed to obliterate varices was 1.98.
Band ligation was performed with one shooter and over tube in one trial and with a multi‐band shooter (standard or pneumoactive ligator) in five trials. Four to 10 bands were used in each session. Sessions were repeated at one to four weeks until varix eradication. Subsequently, participants were followed at three to six months after treatment; banding was repeated in case of variceal recurrence. The mean number of sessions needed to obliterate varices was 2.1. In five participants (one in one trial, four in one trial) treatment was switched from band ligation to cyanoacrylate after the first treatment failure.
Description of the individual comparisons in the trials
There were three different comparisons in the six trials. One trial compared two different doses of cyanoacrylate (Hou 2009); one trial compared cyanoacrylate versus alcohol‐based compounds (Sarin 2002); and four trials compared cyanoacrylate versus endoscopic band ligation (Lo 2001; Tan 2006; El Amin 2010; Zheng 2012).
Two different doses of cyanoacrylate
One trial compared two different doses of cyanoacrylate, 0.5 mL versus 1.0 mL (Hou 2009). This single‐centre trial from China randomised 91 adults bleeding actively from all types of gastric varices (proportion with type GOV and IGV1 similar in both groups). Demographics and clinical characteristics in both intervention groups were similar. We judged randomisation and allocation sequence generation as adequate. Participants and personnel conducting the intervention were not blinded, but personnel conducting the corresponding assessment were blinded, but blinding methods were not described. Sample size calculation was performed. Intention‐to treat was applied. Control of active bleeding, re‐bleeding, bleeding‐related mortality, and complications were measured. Total length of follow‐up was 26 months. There were two participants lost to follow‐up in the 0.5 mL group and three participants in the 1.0 mL group, but their outcomes had already been measured. We considered this trial at high risk of bias.
Cyanoacrylate versus alcohol‐based compounds
Only one randomised trial compared cyanoacrylate versus alcohol‐based compounds (Sarin 2002). This single‐centre trial from India randomised 37 adults, with active or acute bleeding (17 active, 20 acute) from isolated gastric varices only (IGV1). Demographics and clinical characteristics in both intervention groups were similar. We judged randomisation and allocation sequence generation as adequate. Participants or personnel conducting the intervention or assessing outcomes were not blinded. Sample size calculations were not reported, and intention to treat was not declared. Cyanoacrylate 0.5 mL plus lipiodol 0.7 mL versus absolute alcohol 2 to 9 mL were used. All participants with acute bleeding were treated with somatostatin or octreotide before and after the intervention. Control of active bleeding, re‐bleeding, bleeding‐related mortality, complications, failure of treatment and variceal obliteration were reported. Length of follow‐up was (mean ± standard deviation) 14.4 ± 3.7 months. There was one participant in each group lost to follow‐up. We considered this trial at high risk of bias.
Cyanoacrylate versus endoscopic band ligation
Four trials compared cyanoacrylate versus endoscopic band ligation. Three were full‐text articles, while one was an abstract from the proceedings of an international meeting (Zheng 2012).
One randomised trial compared cyanoacrylate versus endoscopic band ligation in bleeding GOV1‐type only gastric varices (El Amin 2010). This multicentric trial from Egypt randomised 150 adults who were bleeding actively and excluded people with advanced hepatocarcinoma. Demographics and clinical characteristics in both intervention groups were similar. Randomisation method was adequate. Participants and the personnel conducting the intervention or assessing outcomes were not blinded. Sample size calculation was not described and intention‐to‐treat analysis was not declared. Cyanoacrylate 0.5 mL plus 0.7 mL of lipiodol versus endoscopic band ligation using a six shooter device were used. Vasoactive drugs and non‐selective beta‐blockers were not used before or after the procedure in either group. Concurrent oesophageal varices in both groups were treated by band ligation in the same endoscopy session. Control of active bleeding (initial haemostasis), re‐bleeding, bleeding‐related mortality, survival time, complications, failure of treatment, and obliteration were measured. Length of follow‐up was six months. One participant having band ligation was switched to cyanoacrylate after treatment failure with band ligation. We considered this trial at high risk of bias.
One randomised trial compared cyanoacrylate versus endoscopic band ligation in bleeding gastric varices of all types (Lo 2001). This single‐centre trial from China randomised 60 adults bleeding actively or recently and included people with hepatocarcinoma. Demographics and clinical characteristics in both groups were similar. Allocation sequence generation and concealment were adequate. Participants and the personnel conducting the intervention or assessing outcomes were not blinded. Sample size calculation is described (originally 242 participants in each group were needed, but after 3 years, interim analyses reached significance) and intention‐to‐treat analysis was applied. Cyanoacrylate 0.5 mL plus 1.5 mL of lipiodol versus endoscopic band ligation using a pneumatic ligator device plus over tube were used. Vasoactive drugs and non‐selective beta‐blockers were not used before or after the procedure in either group. Concurrent oesophageal varices in both groups were treated by endoscopic band ligation in the same endoscopy session. Control of active bleeding (initial haemostasis), re‐bleeding, bleeding‐related mortality, complications, and failure of treatment were measured. Length of follow‐up was 14 months for cyanoacrylate and nine months for band ligation. One participant in each group was lost to follow‐up and one participant in band ligation was switched to cyanoacrylate. We considered this trial at high risk of bias.
One randomised trial compared cyanoacrylate versus band ligation in bleeding gastric varices of all types (Tan 2006). This single‐centre trial from Taiwan randomised 97 adults with active or acute bleeding (30 active, 66 acute) from all types of gastric varices and included people with hepatocarcinoma. Demographics and clinical characteristics in both groups were similar. Allocation sequence generation and concealment were adequate. Participants or the personnel conducting the intervention were not blinded, but the personnel conducting assessments were blinded. Sample size calculation was described and a modified intention‐to‐treat was applied. Cyanoacrylate 0.5 mL, mixed with 0.5 mL of lipiodol versus band ligation using a pneumoactive ligator were used. Vasoactive drugs were used in both groups before the procedure. Concurrent oesophageal varices in both groups were treated by band ligation in the same endoscopy session. Control of active bleeding, re‐bleeding, bleeding‐related mortality, complications, and failure of treatment were measured. Length of follow‐up was six months. Four participants (two in each group) were lost to follow‐up and four participants were switched from endoscopic band ligation to cyanoacrylate. We considered this trial at high risk of bias.
One trial was presented at a meeting and was published as an abstract (Zheng 2012). We tried on several occasions, with no success, to contact the authors in order to locate the full‐text paper. This single‐centre trial from China randomised 58 adults bleeding actively from gastric varices. Data on randomisation, allocation sequence generation and concealment, or blinding of personnel were not available. There were no available data on sample size calculations or intention‐to‐treat analyses. Cyanoacrylate 0.5 mL mixed with 0.5 mL of lipiodol versus endoscopic band ligation were used. Vasoactive drugs were used in all participants before endoscopic treatment. Concurrent oesophageal varices in both groups were treated by endoscopic band ligation in the same endoscopy session. Somatostanin and proton pump inhibitors were used in all participants before endoscopic treatment. Control of active bleeding, re‐bleeding, survival rates, and complications were measured. There were no available data on length of or loss to follow‐up. We considered this trial at high risk of bias.
Excluded studies
See: Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
Four trials reported adequate allocation sequence generation (Lo 2001; Sarin 2002; Tan 2006; Hou 2009), whereas in two trials, allocation sequence generation was unclear (El Amin 2010; Zheng 2012). Four trials reported adequate allocation concealment (Lo 2001; Tan 2006; Hou 2009; El Amin 2010), whereas two trials had unclear allocation concealment (Sarin 2002; Zheng 2012).
Blinding
Due to the nature of the intervention, participants and treatment providers were not blinded in any of the trials. Two trials reported some form of blinded outcome assessment (Tan 2006; Hou 2009).
Incomplete outcome data
Three trials reported intention‐to‐treat analyses that counted for all randomised participants (Lo 2001; Tan 2006; Hou 2009), one of them used a modified intention‐to‐treat analysis (inclusion criteria were applied only after randomisation) (Tan 2006). Two trials did not specifically report intention‐to‐treat analysis (Sarin 2002; El Amin 2010), and there were no available data on this matter in the article, which was in abstract form (Zheng 2012).
In four trials, the methods used to account for participants with missing data appeared to be correct (Lo 2001Tan 2006Hou 2009; Sarin 2002). In one trial there were no participants lost to follow‐up (El Amin 2010), and, in another trial, participants lost to follow‐up were equally distributed among groups. For the one trial in abstract form, there was not enough data to assess incomplete outcome data (Zheng 2012).
Selective reporting
With the exception of the trial published as abstract only (Zheng 2012), all trials reported bleeding‐related mortality, treatment failure, re‐bleeding, adverse events, and control of bleeding in both groups. Definition of time of mortality and re‐bleeding varied across trials. It was possible to extract data on adverse events, despite the fact that definitions also varied across trials. Pain, fever, and embolism were nonetheless, common to all trials.
Other potential sources of bias
It was unclear if the industry had any influence in all the trials.
Three trials reported a sample size calculation (Lo 2001; Tan 2006; Hou 2009). One of these was terminated after three years at the point when interim analyses reached significant differences (level not reported) (Lo 2001). Three trials did not report sample size calculations or whether trials were terminated at any arbitrary point (Sarin 2002; El Amin 2010; Zheng 2012). None of the trials reported clear differences between baseline characteristics of participants randomised to cyanoacrylate or the alternative intervention. Severity of the underlying hepatic disease measured by the Child‐Pugh classification showed uniformity across all trials. Major differences between trials were the inclusion or exclusion of participants with hepatocarcinoma, type of gastric varices, length of follow‐up, use of vasoactive drugs, and active (endoscopic evidence of active bleeding) or acute bleeding (endoscopic evidence of recent bleeding without active bleeding at the moment).
Figure 2 shows the 'Risk of bias' graph and Figure 3 shows the 'Risk of bias' summary.
2.
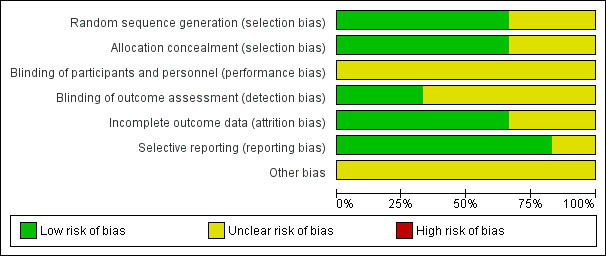
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
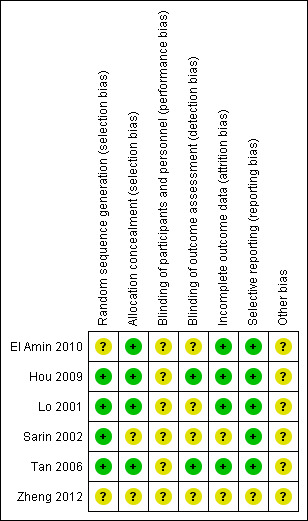
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Accordingly, we considered all six trials at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Two different doses of cyanoacrylate
One trial compared two different doses of cyanoacrylate, 0.5 mL versus 1.0 mL (Hou 2009).
All‐cause mortality at maximum follow‐up
Overall mortality from all causes at the end of the observation period was 20/44 in the 0.5 mL group versus 21/47 in the 1.0 mL group with no statistically significant differences (RR 1.02; 0.65 to 1.60) (Analysis 1.1).
1.1. Analysis.
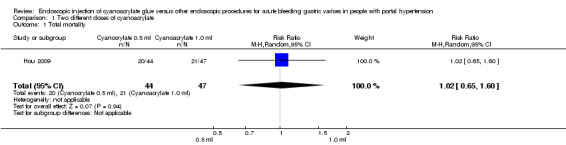
Comparison 1 Two different doses of cyanoacrylate, Outcome 1 Total mortality.
Bleeding‐related mortality (30 day‐mortality)
A total of 7/44 participants (15.9%) treated with 0.5 mL of cyanoacrylate had died by day 30 (bleeding‐related mortality) versus 7/47 participants (14.9%) treated with 1.0 mL. The Analysis showed no difference between the groups (RR 1.07; 95% CI 0.41 to 2.80) (Analysis 1.2).
1.2. Analysis.
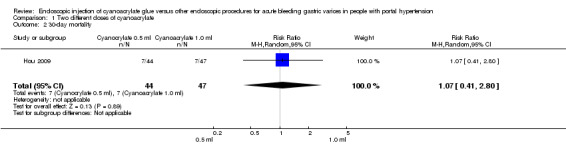
Comparison 1 Two different doses of cyanoacrylate, Outcome 2 30‐day mortality.
Failure of intervention
Thirteen of 44 participants (29.5%) treated with 0.5 mL of cyanoacrylate presented continuous bleeding after the procedure versus 13/47 participants (27.6%) treated with 1.0 mL. Analysis showed no difference between the groups (RR 1.07; 95% CI 0.56 to 2.05) (Analysis 1.3).
1.3. Analysis.
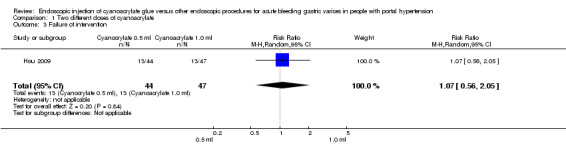
Comparison 1 Two different doses of cyanoacrylate, Outcome 3 Failure of intervention.
Re‐bleeding
In 17/44 participants (38.6%) treated with 0.5 mL of cyanoacrylate, re‐bleeding occurred during the defined time after procedure versus 14/47 participants (29.8%) treated with 1.0 mL. Analysis showed no difference between the groups (RR 1.30; 95% CI 0.73 to 2.31) (Analysis 1.4).
1.4. Analysis.
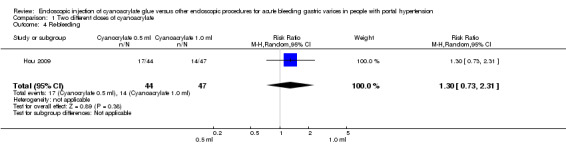
Comparison 1 Two different doses of cyanoacrylate, Outcome 4 Re‐bleeding.
Adverse events (complications: fever)
Twelve of 44 participants (27.2%) treated with 0.5 mL of cyanoacrylate presented fever after the procedure versus 23/47 participants (48.9%) treated with 1.0 mL. Analysis showed a statistically significant difference between the groups (RR 0.56; 95% CI 0.32 to 0.98) (Analysis 1.5).
1.5. Analysis.
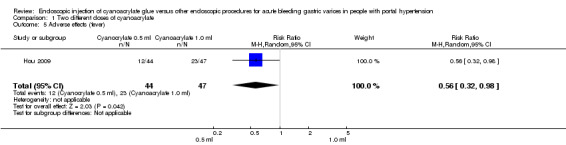
Comparison 1 Two different doses of cyanoacrylate, Outcome 5 Adverse effects (fever).
One participant had a pulmonary embolism in the 0.5 mL group. One participant in each group had portal vein thrombosis.
Control of bleeding
In 9/10 participants (90%) with active bleeding treated with 0.5 mL of cyanoacrylate, bleeding was controlled versus 13/15 participants (86.6%) treated with 1.0 mL. Analysis showed no difference between the groups (RR 1.04; 95% CI 0.78 to 1.38) (Analysis 1.6).
1.6. Analysis.
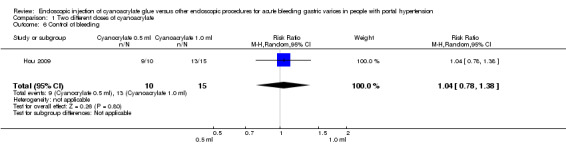
Comparison 1 Two different doses of cyanoacrylate, Outcome 6 Control of bleeding.
Number of transfusions
A total of 4.42 units were used in the 0.5 mL of cyanoacrylate group versus 4.11 units used in the 1.0 mL group. There was no difference between the groups (P value = 0.68).
Quality of life
The trial did not report quality of life.
Transjugular intrahepatic portosystemic shunt and surgery
Both procedures were offered to the participant in case of failure, but actual numbers were not provided.
We considered the quality of evidence in this comparison very low. We found only one trial with high risk of bias, which included high imprecision due to the limited number of participants, risk of indirectness (only one type of population was studied), and uncertain risk of publication bias (Table 2).
All the above‐mentioned Review Manager analysis, results were in agreement with the results produced with the Fisher's exact test.
Cyanoacrylate versus alcohol‐based compounds
One randomised trial compared cyanoacrylate versus alcohol‐based compounds (Sarin 2002).
All‐cause mortality
The trial did not report all‐cause mortality.
Bleeding‐related mortality (30 day‐mortality)
Two of 20 participants (10%) died from bleeding after 30 days in the cyanoacrylate group versus 4/17 (23.5%) in the alcohol‐based compounds group. Analysis showed no difference between the groups (RR 0.43; 95% CI 0.09 to 2.04) (Analysis 2.1).
2.1. Analysis.
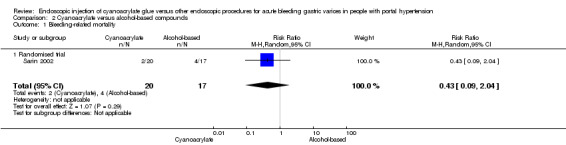
Comparison 2 Cyanoacrylate versus alcohol‐based compounds, Outcome 1 Bleeding‐related mortality.
Failure of intervention
Only participants with acute bleeding were considered for this analysis. In 2/9 participants (22.2%), cyanoacrylate did not control bleeding versus 5/8 (62.5%) in the alcohol‐based compounds group. Analysis showed no difference between the groups (RR 0.36; 95% CI 0.09 to 1.35) (Analysis 2.2).
2.2. Analysis.
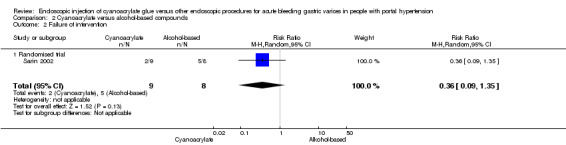
Comparison 2 Cyanoacrylate versus alcohol‐based compounds, Outcome 2 Failure of intervention.
Re‐bleeding
Five of 20 participants (25%) presented re‐bleeding (defined as bleeding one to four weeks after first treatment) using cyanoacrylate versus 5/17 (29.4%) using alcohol‐based compounds. Analysis showed no difference between the groups (RR 0.85; 95% CI 0.30 to 2.45) (Analysis 2.3).
2.3. Analysis.
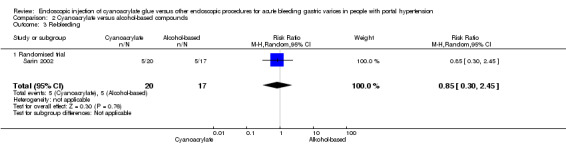
Comparison 2 Cyanoacrylate versus alcohol‐based compounds, Outcome 3 Re‐bleeding.
Adverse events
A total of 7/20 participants (35%) had post‐procedure fever in the cyanoacrylate group during the observation period versus 14/17 (82.3%) in the alcohol‐based compounds group. The difference between the groups was statistically significant (RR 0.43; 95% CI 0.22 to 0.80) (Analysis 2.4). A total of 13/20 participants presented ulceration in the site of injection using cyanoacrylate versus 14/17 using alcohol‐based compounds. There was no difference between the groups (RR 0.79; 95% CI 0.53 to 1.17) (Analysis 2.5). No cases of distant embolism were reported.
2.4. Analysis.
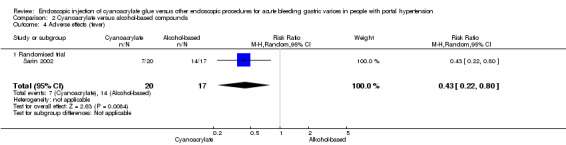
Comparison 2 Cyanoacrylate versus alcohol‐based compounds, Outcome 4 Adverse effects (fever).
2.5. Analysis.
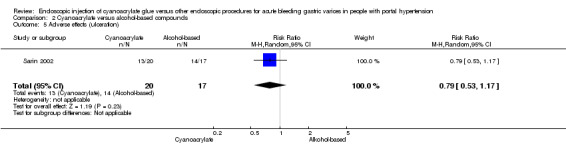
Comparison 2 Cyanoacrylate versus alcohol‐based compounds, Outcome 5 Adverse effects (ulceration).
Control of bleeding
Control of gastric variceal bleeding was achieved in 19/20 participants (95%) using cyanoacrylate versus 9/17 participants (52.9%) using alcohol‐based compounds. The difference between the groups was statistically significant (RR 1.79; 95% CI 1.13 to 2.84) (Analysis 2.6).
2.6. Analysis.
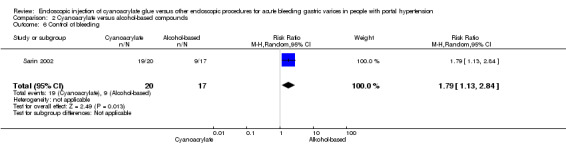
Comparison 2 Cyanoacrylate versus alcohol‐based compounds, Outcome 6 Control of bleeding.
Number of transfusions
The trial did not report number of transfusions.
Quality of life
The trial did not report quality of life.
Transjugular intrahepatic portosystemic shunt and surgery
The trial did not report use of TIPS. In the acute variceal bleeding participants subgroup, 1/9 participants (11%) in the cyanoacrylate group versus 4/8 participants (50%) in the alcohol group underwent surgery. There was no difference between the groups (RR 0.22; 95% CI 0.03 to 1.6).
We considered the quality of the evidence very low. We found only one trial with high risk of bias, including high imprecision due to the limited number of participants, risk of indirectness (only one type of population was studied), and uncertain risk of publication bias (Table 3).
All the above‐mentioned Review Manager analysis results were in agreement with the results produced with the Fisher's exact test.
Cyanoacrylate versus endoscopic band ligation
Four trials compared cyanoacrylate versus endoscopic band ligation (Lo 2001; Tan 2006; El Amin 2010; Zheng 2012). Although we considered all as having high risk of bias, one of them scored low risk in all the items, except performance bias in which it scored unclear (Tan 2006). The result of this trial with unclear risk of bias (potentially lower risk of bias) was compared to the other trials for every outcome. All the analysis are reported using random‐effect model.
All‐cause mortality
Only one trial reported all‐cause mortality (Lo 2001), and there are no complete data in the others.
Bleeding‐related mortality
A total of 44/185 participants (23.7%) using cyanoacrylate died a bleeding‐related death during the observation period compared with 50/181 participants (27.6%) using endoscopic band ligation. Random‐effects model meta‐analysis found no statistically significant differences between groups (RR 0.83; 95% CI 0.52 to 1.31). There was evidence of internal heterogeneity (I2 = 29%) (Analysis 3.1).
3.1. Analysis.

Comparison 3 Cyanoacrylate versus band ligation, Outcome 1 Bleeding‐related mortality.
Subgroup analyses
When the trials with unclear versus high risk of bias were compared, the results were not statistically significant with higher heterogeneity (Analysis 3.2). Results were similar when only full‐text articles were taken into account. They did not reflect superiority for cyanoacrylate although heterogeneity did go up (Analysis 3.4). Results were no different when controlling for GOV1 type only varices, or when taking into account only trials that included people with hepatocarcinoma (Analysis 3.3). Trials using vasoactive drugs showed a lower mortality rate for cyanoacrylate, although results were not statistically significant (Analysis 3.5). When stratifying by length of follow‐up, there were no differences between shorter or longer follow‐up periods.
3.2. Analysis.
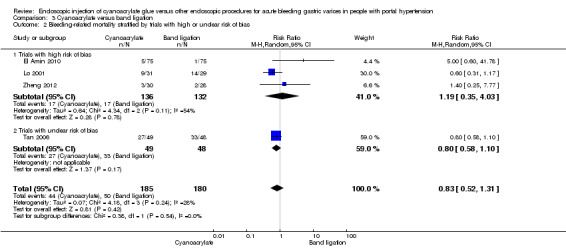
Comparison 3 Cyanoacrylate versus band ligation, Outcome 2 Bleeding‐related mortality stratified by trials with high or unclear risk of bias.
3.4. Analysis.
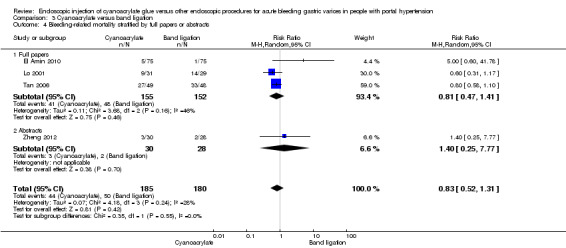
Comparison 3 Cyanoacrylate versus band ligation, Outcome 4 Bleeding‐related mortality stratified by full papers or abstracts.
3.3. Analysis.
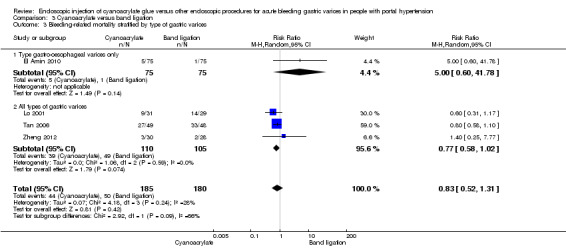
Comparison 3 Cyanoacrylate versus band ligation, Outcome 3 Bleeding‐related mortality stratified by type of gastric varices.
3.5. Analysis.
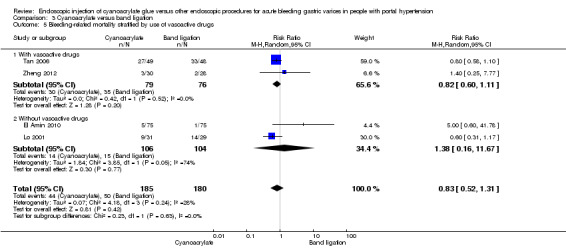
Comparison 3 Cyanoacrylate versus band ligation, Outcome 5 Bleeding‐related mortality stratified by use of vasoactive drugs.
Trial sequential analyses
TSA showed a diversity‐adjusted required information size (DARIS) of 5290 participants. The cumulative Z‐curve did not cross either the conventional or the trial sequential monitoring boundaries, showing that none of the interventions reached superiority and that the limits of futility were not reached (Figure 4).
4.
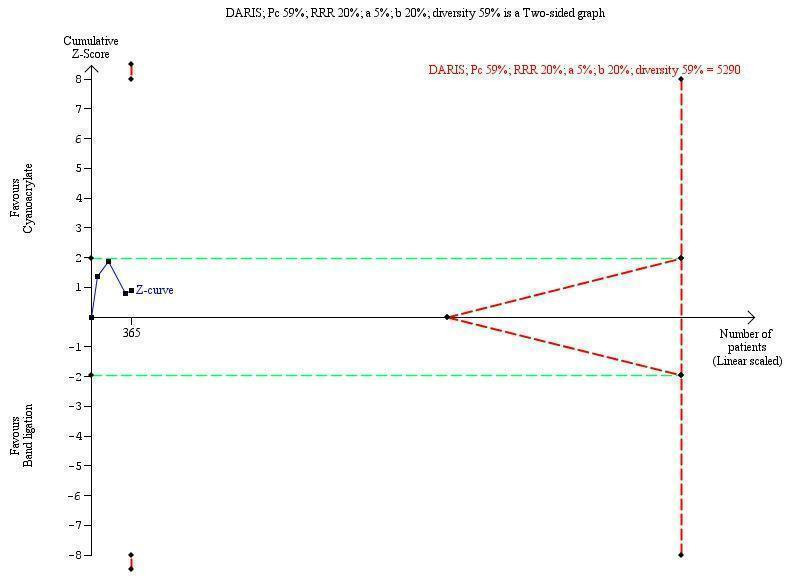
Trial sequential analysis of cyanoacrylate versus band ligation for acute bleeding in people with gastric varices on the outcome bleeding‐related mortality. The diversity‐adjusted required information size (DARIS) is 5290 participants. The calculation is based on a proportion of people dying in the control group (Pc) of 59%; a relative risk reduction (RRR) of 20% based on the intervention effect in trials with a high risk of bias; an alpha (a) of 5%; a beta (b) of 20%; and diversity of 59%. The red lines sloping towards a Z‐value of 1.96 and ‐1.96 are the trial sequential monitoring boundaries. The blue line is the cumulative Z‐curve that does not cross the trial sequential monitoring boundaries for benefit, harm, or futility of cyanoacrylate.
Failure of intervention
In 9/135 participants (6.6%) with acute bleeding cyanoacrylate did not arrest bleeding versus 8/129 participants (6.2%) using endoscopic band ligation. Random‐effects model meta‐analysis showed no difference between the groups (RR 1.13; 95% CI 0.23 to 5.69) with moderate evidence of internal heterogeneity (I2 = 53%) (Analysis 3.6).
3.6. Analysis.
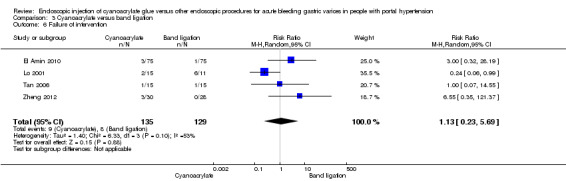
Comparison 3 Cyanoacrylate versus band ligation, Outcome 6 Failure of intervention.
Subgroup analyses
When taking into account trials with unclear versus high risk of bias, the results were not statistically significant (Analysis 3.7). When taking into account only full‐text papers, the results were very similar, and without statistically significant differences (Analysis 3.8). This last result came also the two trials that treated all types of varices and that included people with hepatocarcinoma.
3.7. Analysis.
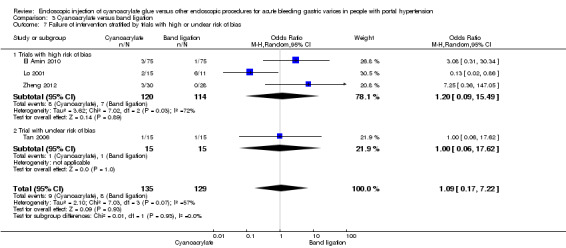
Comparison 3 Cyanoacrylate versus band ligation, Outcome 7 Failure of intervention stratified by trials with high or unclear risk of bias.
3.8. Analysis.
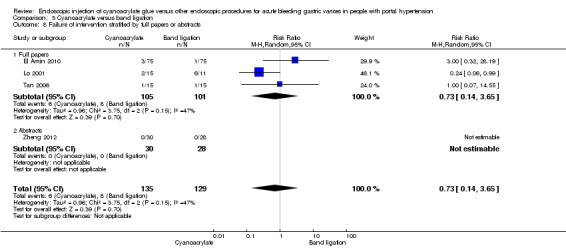
Comparison 3 Cyanoacrylate versus band ligation, Outcome 8 Failure of intervention stratified by full papers or abstracts.
Trial sequential analyses
TSA showed that DARIS of 4098 participants. The cumulative Z‐curve cross the conventional boundaries briefly during the first trial to fell under the conventional boundaries during the second trial and remaining there, showing that none of the interventions reached superiority and that the trial sequential monitoring boundaries of futility were not reached (Figure 5).
5.
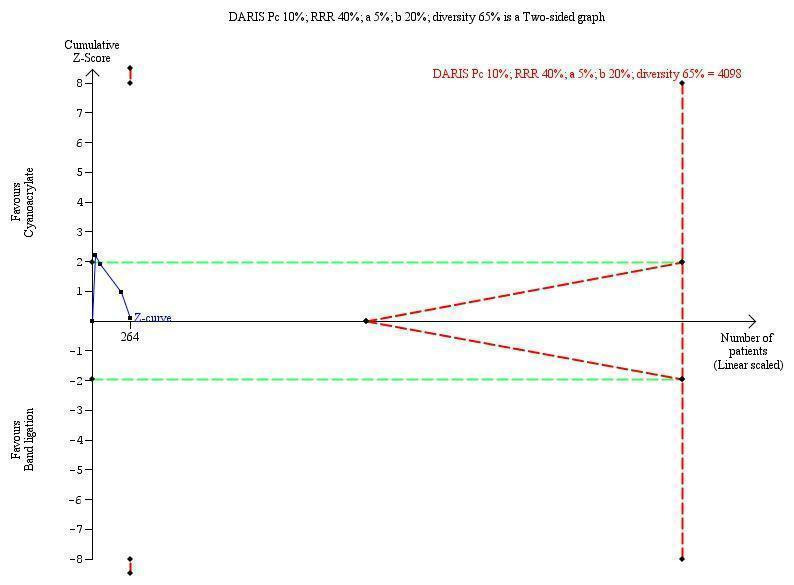
Trial sequential analysis of cyanoacrylate versus band ligation for acute bleeding in people with gastric varices on the outcome failure of intervention. The diversity‐adjusted required information size (DARIS) is 4098 participants. The calculation is based on a proportion of people with failure of the intervention in the control group (Pc) of 10%; a relative risk reduction (RRR) of 40% based on the intervention effect in trials with a high risk of bias; an alpha (a) of 5%; a beta (b) of 20%; and diversity of 65%. The red lines sloping towards a Z‐value of 1.96 and ‐1.96 are the trial sequential monitoring boundaries. The blue line is the cumulative Z‐curve that crosses the conventional boundaries after the first trial and fell under the conventional boundaries and remained there after the second trial. The cumulative Z‐curve does not cross the trial sequential monitoring boundaries for benefit, harm, or futility of cyanoacrylate.
Re‐bleeding
Re‐bleeding occurred in 33/183 participants (18%) using cyanoacrylate versus 53/177 participants (29.9%) using endoscopic band ligation. Random‐effects model meta‐analysis showed a statistically significant difference between groups (RR 0.60; 95% CI 0.41 to 0.88) with little evidence of internal heterogeneity (I2 = 6%) (Analysis 3.9).
3.9. Analysis.
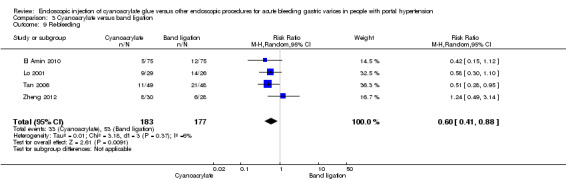
Comparison 3 Cyanoacrylate versus band ligation, Outcome 9 Re‐bleeding.
Subgroup analyses
When taking into account trials with unclear versus high risk of bias, the results were statistically significant in both subgroups with low heterogeneity (Analysis 3.10), with no differences between them. Similar results were found when only full‐text articles were taken into account, there was a small increase in the benefit of cyanoacrylate, reaching statistical significance and displaying lower heterogeneity (RR 0.52; 95% CI 0.35 to 0.78; I2 = 0%) (Analysis 3.11). Stratified by type of varices, the results favoured cyanoacrylate for all types and GOV1‐only type of varices, almost reaching statistical significance (Analysis 3.12). Stratified by use of vasoactive drugs, trials not using them achieved better results for cyanoacrylate (Analysis 3.13). Regarding length of follow‐up, both the shorter trials and the longer trials showed statistical significance in favour of cyanoacrylate.
3.10. Analysis.
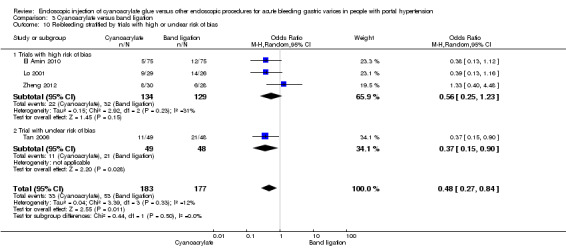
Comparison 3 Cyanoacrylate versus band ligation, Outcome 10 Re‐bleeding stratified by trials with high or unclear risk of bias.
3.11. Analysis.
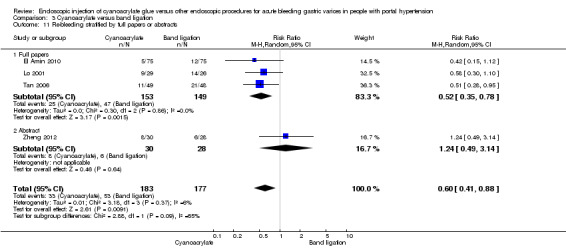
Comparison 3 Cyanoacrylate versus band ligation, Outcome 11 Re‐bleeding stratified by full papers or abstracts.
3.12. Analysis.
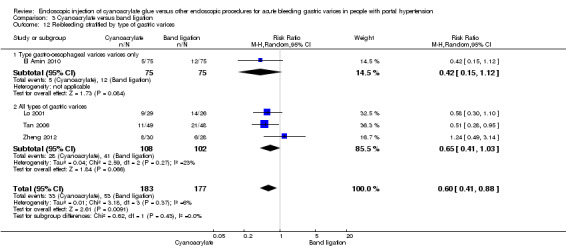
Comparison 3 Cyanoacrylate versus band ligation, Outcome 12 Re‐bleeding stratified by type of gastric varices.
3.13. Analysis.
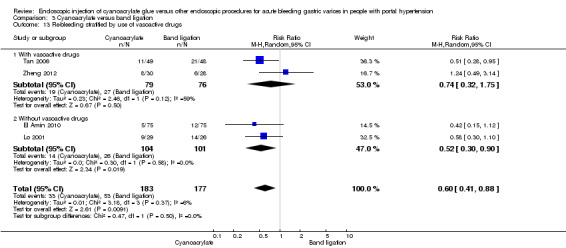
Comparison 3 Cyanoacrylate versus band ligation, Outcome 13 Re‐bleeding stratified by use of vasoactive drugs.
Trial sequential analyses
TSA showed a DARIS of 1840 participants. The cumulative Z‐curve crossed the conventional boundary after the second trial (155 participants), and approached the trial sequential monitoring boundary for benefit of cyanoacrylate. These results suggest that the superiority of cyanoacrylate when it comes to preventing re‐bleeding may be achieved after further trials (Figure 6).
6.
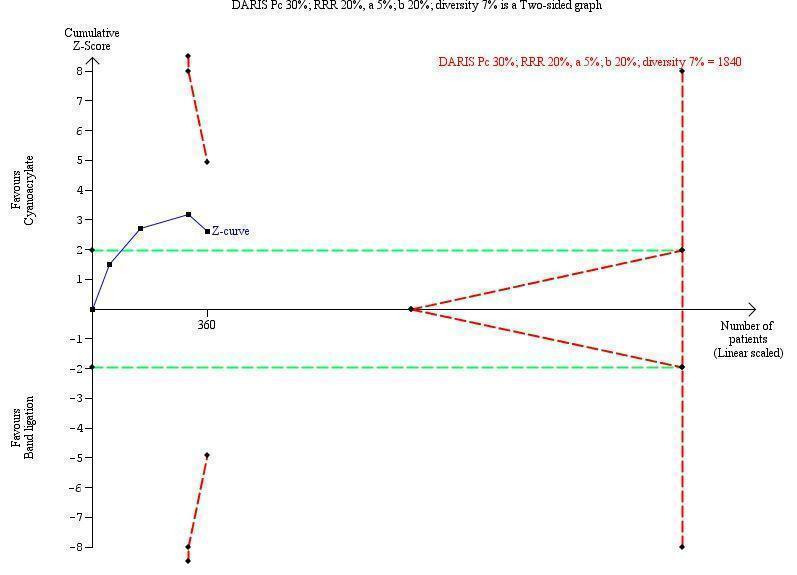
Trial sequential analysis of cyanoacrylate versus band ligation for acute bleeding in people with gastric varices on the risk of the outcome re‐bleeding. The diversity‐adjusted required information size (DARIS) was 1840 participants. The calculation is based on a proportion of people re‐bleeding in the control group (Pc) of 30%; a relative risk reduction (RRR) of 20%; an alpha (a) of 5%; a beta (b) of 20%; and diversity of 7%. The blue line is the cumulative Z‐curve that crosses the conventional boundarie for benefit during the second trial and remained there adding the third and fourth trials.
Adverse events
A total of 45/155 participants (29.0%) who received cyanoacrylate presented with some form of complication (complications were defined differently in each trial, therefore we used total number of complications) versus 17/152 participants (11.1%) using endoscopic band ligation. Random‐effects model meta‐analysis showed fewer complications in the band ligation group, although statistical significance was not achieved (RR 2.81; 95% CI 0.69 to 11.49) and there was high evidence of internal heterogeneity (I2 = 80%) (Analysis 3.14). These data came only from full‐text papers because information associated with complications was not available in the paper found only in abstract form.
3.14. Analysis.
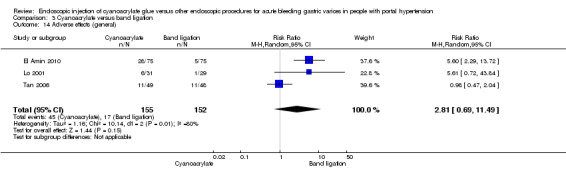
Comparison 3 Cyanoacrylate versus band ligation, Outcome 14 Adverse effects (general).
Subgroup analyses
When taking into account trials with unclear compared to high risk of bias, the results were not statistically significant (Analysis 3.15). Similar results were found when we compared full‐text papers and abstracts (Analysis 3.16). Stratified by use of vasoactive drugs, band ligation showed fewer complications, though without reaching statistical significance (Analysis 3.17).
3.15. Analysis.
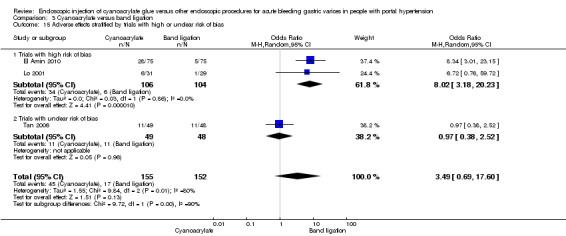
Comparison 3 Cyanoacrylate versus band ligation, Outcome 15 Adverse effects stratified by trials with high or unclear risk of bias.
3.16. Analysis.
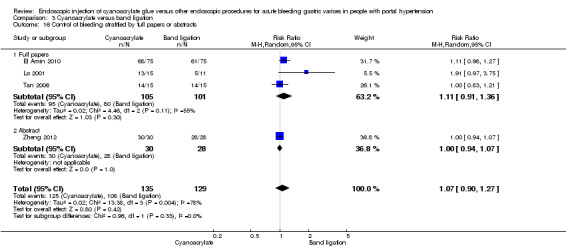
Comparison 3 Cyanoacrylate versus band ligation, Outcome 16 Control of bleeding stratified by full papers or abstracts.
3.17. Analysis.
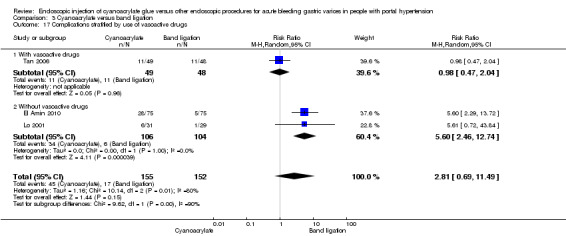
Comparison 3 Cyanoacrylate versus band ligation, Outcome 17 Complications stratified by use of vasoactive drugs.
Embolism to distal organs, which is the major complication associated with cyanoacrylate, occurred in only one of the participants (endoscopic band ligation group).
Control of bleeding
Control of gastric variceal bleeding was achieved in 125/135 participants (92.5%) using cyanoacrylate versus 108/129 participants (83.7%) using endoscopic band ligation. Random‐effects model meta‐analysis showed no difference between groups (RR 1.07; 95% CI 0.90 to 1.27) with major evidence of internal heterogeneity (I2 = 78%) (Analysis 3.18).
3.18. Analysis.
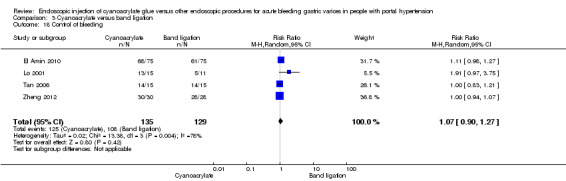
Comparison 3 Cyanoacrylate versus band ligation, Outcome 18 Control of bleeding.
Subgroup analyses
When taking into account trials with unclear versus high risk of bias, the results were not statistically significant (Analysis 3.19). There were no statistically significant differences between groups when only full‐text articles were taken into account or when the two trials that treated all types of varices and included people with hepatocarcinoma were analysed. When trials were stratified according to use of vasoactive drugs, there were better results for cyanoacrylate in the absence of vasoactive drugs, although statistical significance was not achieved (Analysis 3.20).
3.19. Analysis.
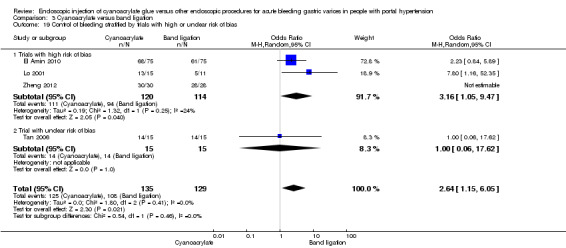
Comparison 3 Cyanoacrylate versus band ligation, Outcome 19 Control of bleeding stratified by trials with high or unclear risk of bias.
3.20. Analysis.
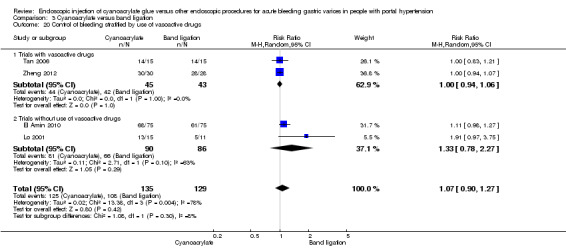
Comparison 3 Cyanoacrylate versus band ligation, Outcome 20 Control of bleeding stratified by use of vasoactive drugs.
Trial sequential analyses
TSA showed a DARIS of 534 participants. The cumulative Z‐curve did not cross either the conventional or the trial sequential monitoring boundaries, showing that none of the interventions reached superiority and that the trial sequential monitoring boundaries of futility were not reached (Figure 7).
7.
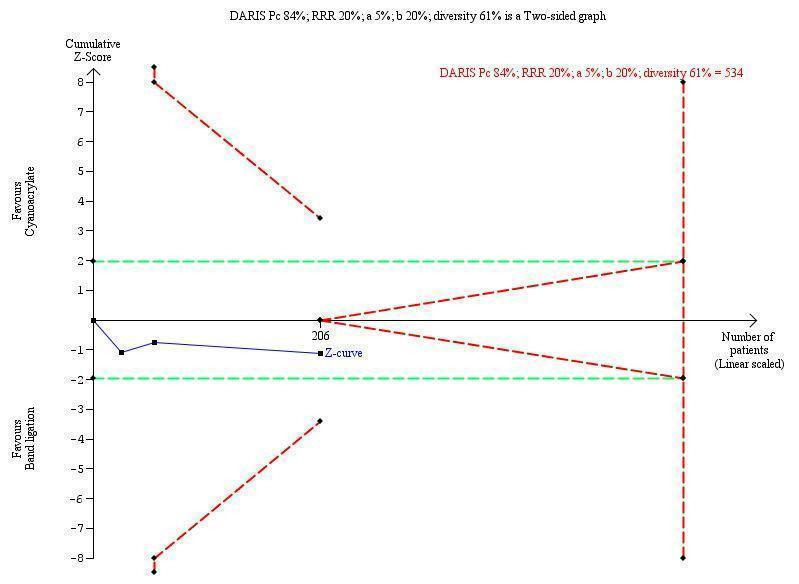
Trial sequential analysis of cyanoacrylate versus band ligation for acute bleeding in people with gastric varices on the outcome control of bleeding. The diversity‐adjusted required information size (DARIS) is 534 participants. The calculation is based on a proportion of people with control of bleeding in the control group (Pc) of 84%; a relative risk reduction (RRR) of 20%; an alpha (a) of 5%; a beta (b) of 20%; and diversity of 61%. The red lines sloping towards a Z‐value of 1.96 and ‐1.96 are the trial sequential monitoring boundaries for benefit or harm. The blue line is the cumulative Z‐curve that does not cross the conventional boundaries or the trial sequential monitoring boundaries for benefit, harm, or futility of cyanoacrylate.
Number of transfusions
Only two trials reported number of transfusions (Lo 2001; Tan 2006), and there were no complete data in the others.
Quality of life
None of the trials reported quality of life.
Transjugular intrahepatic portosystemic shunt and surgery
TIPS and surgery were offered in case of treatment failure, but actual numbers were not provided.
We considered the quality of the evidence in this comparison very low. All the trials presented high risk of bias, although the risk of performance bias in one was unclear and others biases were low. However, on the outcome of re‐bleeding, imprecision seemed to be low, and the number of participants adequate according to the TSA; there was risk of indirectness (only one type of population was studied) and uncertain risk of publication bias (Table 1).
Discussion
The present review compared the effects of endoscopic sclerotherapy with cyanoacrylate versus endoscopic sclerotherapy with alcohol‐based compounds or versus endoscopy band ligation in adults with active or acute, or both, gastric variceal bleeding. Two different doses of cyanoacrylate were also compared.
One of the main findings of this review was that there are few randomised clinical trials available on the endoscopic treatment of acute bleeding gastric varices. This is due to several factors, including low prevalence of this type of varices compared to oesophageal varices (Korula 1991; Sarin 1992). This can explain the fact that after several years, even the largest centres had treated only a limited number of gastric varices, generally below the required number of participants needed to fulfil sample size calculation requirements associated with the research projects. In addition, given that bleeding associated with gastric varices is usually severe, decisions must be made based on conditions that may vary greatly between centres, for example, ability of performing therapeutic endoscopies, availability of resources, expertise of the attending physician, and a series of participant‐dependent variables such as basal disease, degree of severity of the underlying hepatic disease and their complications, existence of hepatocellular carcinoma or portal vein thrombosis (many of them are reported factors that cause more severe variceal bleeding), or a combination of these. Other variables include, but are not limited to, size and type of the varices, (IGV being more ominous than GOV), and pre‐ and post‐endoscopy treatments, such as use of different resuscitation schemes, use of vasoactive drugs, proton pump inhibitors, and the liberal or restrictive use of blood transfusions. As a result, the available trials on gastric varices are scarce and heterogeneous. Blinding of personnel is not feasible for endoscopic interventions, raising the risk of performance bias, although this is debatable given the objective nature of the outcomes associated with this treatment.
Two different doses of cyanoacrylate
We found only one randomised trial that compared different doses of cyanoacrylate (Hou 2009). This trial, assessed as at high risk bias due to unclear performance bias, showed that 0.5 and 1.0 mL doses of cyanoacrylate seemed similar in terms of reducing mortality, treatment failure, bleeding control, and preventing re‐bleeding. However, there were fewer reported adverse effects (only minor) in the 0.5 mL group. The fundamental characteristic of this comparison lies in the amount of cyanoacrylate present inside each varix after each injection, since the total amount used depends on the number of varices, their size, and the success controlling bleeding and achieving obliteration. Although the total amount of cyanoacrylate administered varied among included trials, in this specific trial the total dose of cyanoacrylate used when the 1.0 mL dose was applied was only 0.5 mL more compared with when using the lower dosage. Other studies (not comparing different doses) used up to double this amount in individual injections of 0.5 mL (Lo 2001; Sarin 2002). The final issue when dealing with varying the amount of cyanoacrylate in each injection is the capacity of the injected cyanoacrylate to obliterate the entire varix, and the likelihood that cyanoacrylate could enter the blood stream and cause an embolism. This complication occurred only in one patient (with the lesser dose) in (Hou 2009), and was observed rarely in the remaining trials of this review (Lo 2001; Tan 2006). Other small adverse effects were more common with the higher dosage. Since these results came from only one trial with high risk of bias, imprecision, indirectness, and publication bias (Hou 2009), it is difficult to draw firm conclusions concerning which volume of cyanoacrylate to use.
Cyanoacrylate versus alcohol‐based compounds
Alcohol‐based compounds (ethanolamine maleate, absolute alcohol, and polidocanol) have been used for many years for the management of oesophageal varices (Grace 1997; Sarin 1997; Garcia‐Tsao 2007; Garcia‐Tsao 2008). They became less popular with the advent of endoscopic band ligation, which showed comparative advantages (Laine 1995; D'Amico 2010; Gluud 2012). Alcohol‐based compounds were never too common in the management of gastric varices due to the large size of these varices and the need of large volumes of alcohol‐based compounds for treatment (such as in the trial included in this review). Their efficacy compared with other endoscopy treatments in one randomised trial (Sarin 2002) and in non‐randomised studies (Schuman 1987; Gimson 1991; Oho 1995; Ogawa 1999) showed less efficacy in controlling acute bleeding, as well as higher incidences of re‐bleeding.
Only one randomised trial with high bias risk was available for comparing cyanoacrylate versus absolute alcohol (Sarin 2002). This trial suggested that cyanoacrylate was superior to absolute alcohol in terms of bleeding control and adverse events, but there were no apparent differences in bleeding‐related mortality, failure to control bleeding, and prevention of re‐bleeding. There were no reported baseline differences between intervention groups regarding prognostic factors such as the inclusion of participants with hepatocellular carcinoma, severity of liver disease, or use of vasoactive drugs. It must be highlighted also that these results came from only one trial with only 37 participants that were furthermore divided into people with acute and active bleedings, therefore presenting limited evidence. As these results came from only one trial with high risk of bias, imprecision, indirectness, and risk of publication bias (Hou 2009), it was difficult to draw firm conclusions on which sclerosant to use.
Cyanoacrylate versus endoscopic band ligation
Several non‐randomised studies discussed the advantages of cyanoacrylate over endoscopic band ligation in controlling bleeding and preventing re‐bleeding and mortality (Takeuchi 1996; Huang 2000; Akahoshi 2002; Kim 2006; Sugimoto 2007; Mishra 2010). However, the randomised clinical trials included in this systematic review reported improvement only in prevention of re‐bleeding (Lo 2001; Tan 2006; El Amin 2010; Zheng 2012). They showed no advantages of cyanoacrylate in terms of decreasing bleeding‐related mortality and complications, better control of acute bleeding, or failure of intervention.
These randomised clinical trials presented similar trial designs, sclerotherapy procedures for cyanoacrylate and band ligation, grades of liver compromise according to the Child‐Pugh classification, and outcomes. Nevertheless, there were some differences that could compromise the results of this review. The first difference has to do with the type of gastric varices. It is known that type 1 gastric varices (GOV or cardiac varices) are always associated with oesophageal varices and could be a continuation of the oesophageal variceal column, which is in clear contrast to gastric varices type 2 (IGV1, fundal or isolated varices), which are separated and often found without the presence of concomitant oesophageal varices. IGV1 varices could present more severe bleeding than GOV1 according to the literature. In this review, one of the four trials comparing cyanoacrylate with band ligation dealt exclusively with GOV1 varices (El Amin 2010). The remaining three trials dealt with all types of gastric varices (Lo 2001; Tan 2006; Zheng 2012). When stratification was done separating trials with all types of varices and the cardiac type alone trial (El Amin 2010), both treatments fared similarly and without statistically significant differences, suggesting that when it comes to prevention of re‐bleeding, type of varices may be irrelevant. However, mortality increases when cyanoacrylate is used in GOV1 varices and when band ligation is used in IGV1 varices. The random‐effects meta‐analysis showed a significant difference in prevention of re‐bleeding in favour of cyanoacrylate; this difference did not change when trials with unclear risk of bias were compared to trials with high risk of bias, when articles reported in full were compared to abstracts, or type of varices were considered.
The use of vasoactive drugs yielded no statistically differences. Two trials used vasoactive drugs (Tan 2006; Zheng 2012) and two did not (Lo 2001; El Amin 2010). Due to the low number of participants, it is not possible to exclude or accept a modifying influence of vasoactive drugs in the intervention effect of cyanoacrylate.
Length of follow‐up was different in the included trials, varying from six to 26 months. This could skew results, particularly when short‐term trials (Lo 2001; El Amin 2010) were compared to long‐term trials (Tan 2006). Given the nature of the disease, re‐bleeding and mortality could be under‐represented in short‐term trials and over‐represented in long‐term trials. Nonetheless, we observed no differences between long‐term and short‐term trials.
Data regarding units of blood used were available from only two trials (Lo 2001; Tan 2006), with a trend that suggested lesser usage in the cyanoacrylate group. In addition, re‐bleeding was significantly lower in these two trials.
Future work is needed to clarify these points, including the completion of studies with large numbers of participants and proper stratification of severity of the basic disease, type and size of varices, presence of hepatocarcinoma, and use of vasoactive drugs. It would also be important to standardise measurements related to time to acute bleeding, re‐bleeding, and mortality rates due to bleeding. In the meantime, and in light of the results of this review, it seems sensible to use cyanoacrylate in the treatment of gastric varices, particularly IGV1 varices, although treatment with band ligation is also an option, mainly for GOV1 type varices.
It must be noted that the results of the comparisons between cyanoacrylate and band ligation came from studies that had 365 participants in total. The apparent superiority of cyanoacrylate to prevent re‐bleeding may still be due to random error according to the random‐effects model and TSA. In addition, high risk of bias, heterogeneity, indirectness, and publication bias make it difficult to draw firm conclusions on the studied outcomes. The worst possible adverse effect associated with the use of cyanoacrylate (i.e., embolism) was rarely presented (in one case embolism was observed in the non‐cyanoacrylate group). There were a few minor adverse effects, especially in the band ligation group.
Summary of main results
Taking into account the overall low quality of the evidence due to the high risk of bias in the trials, significant imprecision due to small number of participants included in the trials identified for this review, presence of heterogeneity, and indirectness (only Asiatic participants in the trials), our results suggested that, when treating gastric varices, cyanoacrylate appeared to be superior to band ligation in terms of re‐bleeding, particularly in IGV1 type varices, but cyanoacrylate appeared fairly similar regarding bleeding control, treatment failure, and mortality. In addition, it could be reasonable to recommend cyanoacrylate in volumes of 0.5 mL. Lastly, cyanoacrylate appears to be superior to alcohol‐based compounds.
Overall completeness and applicability of evidence
Two different doses of cyanoacrylate
Based on only one trial comprising 91 participants, 0.5 mL of cyanoacrylate seemed to be associated with fewer complications than 1.0 mL of cyanoacrylate. The evidence identified was not enough to accomplish the objectives of the review on this issue. The proposed dose of 0.5 mL of cyanoacrylate is the dose most used in the current practice.
Cyanoacrylate versus alcohol‐based compounds
Based on only one trial comprising 37 participants, cyanoacrylate seemed more effective than alcohol‐based compounds regarding control of bleeding in fundal varices and complications, but cyanoacrylate did not differ from sclerotherapy with alcohol‐based compounds in decreasing mortality, arresting bleeding, and reducing complications. The evidence identified was not enough to achieve the objectives of the review on this issue.
Cyanoacrylate versus endoscopic band ligation
Based on four trials comprising 365 participants, the use of cyanoacrylate seemed superior to endoscopic band ligation only in terms of preventing re‐bleeding, particularly in IGV1 varices. Band ligation could still be a viable treatment, particularly in GOV1 type varices. The evidence identified was not complete to reach the objectives of the review on this point, especially due to heterogeneity and low quality of the evidence, although results in the outcome re‐bleeding seemed to be robust to random errors. The lower risk of re‐bleeding is the main reason to prefer the use of cyanoacrylate over the use of band ligation in current practice.
Quality of the evidence
Two different doses of cyanoacrylate
Data for this analysis came from only one trial. The quality of the evidence was very low due to the high risk of bias, imprecision, indirectness, and possible risk of publication bias. The evidence identified did not allow a robust conclusion regarding this review objective.
Cyanoacrylate versus alcohol‐based compounds
Data for this analysis came from only one trial. The quality of the evidence was very low due to high risk of bias, imprecision, indirectness, and possible risk of publication bias. The evidence identified did not allow a robust conclusion regarding this review objective.
Cyanoacrylate versus endoscopic band ligation
The results came from three full‐text trials and one abstract. The trials all had high risk of bias. The quality of the general evidence was very low due to the high risk of bias, heterogeneity, indirectness, and possible risk of publication bias. From the several outcomes studied, the meta‐analysis demonstrated differences in favour of cyanoacrylate in only one outcome (re‐bleeding). The identified evidence did not allow a robust conclusion regarding several objectives of this review, but concerning the outcome re‐bleeding, TSA suggested that cyanoacrylate superiority was not likely to be due to random error.
Potential biases in the review process
Two different doses of cyanoacrylate
We had not planned this outcome in the protocol. We found no other trial dealing with this question despite the comprehensive literature search in English and Spanish. We could have missed some trials in other languages, such as French, or trials published as abstracts. Not all the planned outcomes were present in the assessed trial.
Cyanoacrylate versus alcohol‐based compounds
There were several observational studies dealing with this comparison, but the comprehensive literature search located no other randomised trial. We could have missed some trials in different languages, such as French or other (abstracts or articles). Not all the planned outcomes were present in the assessed trial.
Cyanoacrylate versus endoscopic band ligation
One potential source of bias was the inclusion of an article in abstract form for this comparison (Zheng 2012). It was not possible to retrieve all the needed data on the respective trial, despite several attempts to contact the authors. We calculated the results for this comparison with and without this trial, and also stratified according to the possible selection bias and the differences were mainly not statistically significant. Heterogeneity was low to moderate, although there were many differences between trials regarding type of varices, use of vasoactive drugs, and inclusion of participants with hepatocarcinoma. Time to defined outcomes was also different across included studies. The literature search was comprehensive in English and Spanish, but we could have missed some trials in different languages, such as French or other (abstracts or articles).
Agreements and disagreements with other studies or reviews
Two different doses of cyanoacrylate
There seems to be no major disagreements with other studies on this matter. Most of the included trials used 0.5 mL of cyanoacrylate.
Cyanoacrylate versus alcohol‐based compounds
There are non‐randomised trials and case series that compared cyanoacrylate to alcohol‐based compounds (Schuman 1987; Gimson 1991; Oho 1995; Sarin 1997; Ogawa 1999). These studies reported that alcohol‐based compounds were associated with inferior results regarding initial haemostasis, incidence of re‐bleeding, varix obliteration, and complications. There are no studies that concluded that alcohol‐based compounds were better than cyanoacrylate for any outcome of interest.
Cyanoacrylate versus endoscopic band ligation
There are randomised (Bazeed 2013; Shiha 2010) (see Excluded studies) and non‐randomised studies, and case series on different methods of band ligation using the classic, new, or combined techniques (Chun 1995; Cipolletta 1998; Shiha 1999; Yoshida 1999; Lee 2002; Arakaki 2003). Their results are more optimistic than the results of this review. There are no studies that concluded that band ligation was superior to cyanoacrylate for any outcome.
Authors' conclusions
Implications for practice.
Taking into account that there was only one randomised trial for different doses of cyanoacrylate, one trial for the comparison of cyanoacrylate versus alcohol‐based compounds, and four randomised trials for the comparison of cyanoacrylate versus endoscopic band ligation, this systematic review has found evidence of very low quality showing that endoscopic sclerotherapy may be more effective than endoscopic band ligation in terms of preventing re‐bleeding from gastric varices, particularly the isolated (IGV1) type, using doses of 0.5 mL each. Endoscopic band ligation seems to be a viable treatment for all types of gastric varices, especially the cardiac (GOV1) type, although with an expected increase in incidence of re‐bleeding rates. The quality of the evidence is limited by the high risk of bias of the included studies, imprecision arising from small samples, heterogeneity, and indirectness of most of the evidence, as well as potential risks of publication bias. Caution must be applied until further evidence is gathered.
Implications for research.
Large, long‐term, randomised clinical trials with low risks of bias that compare cyanoacrylate versus band ligation for active or acute gastric variceal bleeding in adults are needed as well as trials comparing different doses of cyanoacrylate. These trials should include all types of gastric varices, people with hepatocellular carcinoma, consider the use of vasoactive drugs, and should use standardised times to assess outcomes according to the latest Baveno guidelines (de Franchis 2010). Such randomised clinical trials need to be designed and conducted according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement and reported according to CONSORT (Consolidated Standards of Reporting Trials) guidelines (www.equator‐network.org/).
Acknowledgements
Department of Internal Medicine, La Frontera University, Temuco (Chile) (Dr. Edmundo Hofmann, director) for providing funding for this project. Marta Roqué from Universitat Autònoma de Barcelona, Spain and Sergio Muñoz from La Frontera University, for helping in the statistical analyses needed to complete this review. Héctor Pardo from Universitat Autònoma de Barcelona, for editing the manuscript, Dimitrinka Nikolova from the Editorial Team Office of Cochrane Hepato‐Biliary for correcting and editing the manuscript. Sarah L Klingenberg for the literature search and updates. Christian Gluud for helping in the TSA analysis and correcting and editing the manuscript.
Peer Reviewers: Naoki Hosoe, Japan; Andreas Cardenas, Spain.
Contact Editors: Stefano Trastulli, Italy; Luit Penninga, Denmark; Christian Gluud, Denmark.
Eddy Ríos is a PhD student of the Paediatrics, Obstetrics and Gynaecology and Preventive Medicine Department, Universitat Autónoma de Barcelona, Barcelona, Spain.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Cochrane Hepato‐Biliary Controlled Trials Register | September 2014. | (cyanoacrylat* OR cyanoacrilat*) AND (varic* AND (bleed* OR hemmorhage*)) |
| Cochrane Central Register of Controlled Trials (CENTRAL) | Issue 9 of 12, 2014. | #1 MeSH descriptor Cyanoacrylatesexplode all trees #2 cyanoacr*lat* #3 (#1 OR #2) #4 MeSH descriptor Esophageal and Gastric Varices explode all trees #5 (varic* AND (bleed* OR hemmorhage*)) #6 (#4 OR #5) #7 (#3 AND #6) |
| MEDLINE (OvidSP) | 1946 to September 2014. | 1. exp Cyanoacrylates/ 2. cyanoacr*lat*.mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. exp "Esophageal and Gastric Varices"/ 5. (varic* and (bleed* or hemmorhage*)).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 7 and 8 |
| EMBASE (OvidSP) | 1974 to September 2014. | 1. exp cyanoacrylate/ 2. cyanoacr*lat*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp stomach varices/ 5. (varic* and (bleed* or hemmorhage*)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 and 8 |
| Science Citation Index Expanded | 1900 to September 2014. | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analysis) #3 #2 AND #1 #2 TS=(varic* AND (bleed* OR hemmorhage*)) #1 TS=cyanoacr*lat* |
Data and analyses
Comparison 1. Two different doses of cyanoacrylate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.60] |
| 2 30‐day mortality | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.41, 2.80] |
| 3 Failure of intervention | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.56, 2.05] |
| 4 Re‐bleeding | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.73, 2.31] |
| 5 Adverse effects (fever) | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.98] |
| 6 Control of bleeding | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.78, 1.38] |
Comparison 2. Cyanoacrylate versus alcohol‐based compounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding‐related mortality | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.09, 2.04] |
| 1.1 Randomised trial | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.09, 2.04] |
| 2 Failure of intervention | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.09, 1.35] |
| 2.1 Randomised trial | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.09, 1.35] |
| 3 Re‐bleeding | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.30, 2.45] |
| 3.1 Randomised trial | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.30, 2.45] |
| 4 Adverse effects (fever) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.80] |
| 4.1 Randomised trial | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.80] |
| 5 Adverse effects (ulceration) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.17] |
| 6 Control of bleeding | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.13, 2.84] |
Comparison 3. Cyanoacrylate versus band ligation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bleeding‐related mortality | 4 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.31] |
| 2 Bleeding‐related mortality stratified by trials with high or unclear risk of bias | 4 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.31] |
| 2.1 Trials with high risk of bias | 3 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.35, 4.03] |
| 2.2 Trials with unclear risk of bias | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.10] |
| 3 Bleeding‐related mortality stratified by type of gastric varices | 4 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.31] |
| 3.1 Type gastro‐oesophageal varices only | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.60, 41.78] |
| 3.2 All types of gastric varices | 3 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 4 Bleeding‐related mortality stratified by full papers or abstracts | 4 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.31] |
| 4.1 Full papers | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.47, 1.41] |
| 4.2 Abstracts | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.25, 7.77] |
| 5 Bleeding‐related mortality stratified by use of vasoactive drugs | 4 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.31] |
| 5.1 With vasoactive drugs | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.60, 1.11] |
| 5.2 Without vasoactive drugs | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.16, 11.67] |
| 6 Failure of intervention | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.23, 5.69] |
| 7 Failure of intervention stratified by trials with high or unclear risk of bias | 4 | 264 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.17, 7.22] |
| 7.1 Trials with high risk of bias | 3 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.09, 15.49] |
| 7.2 Trial with unclear risk of bias | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 17.62] |
| 8 Failure of intervention stratified by full papers or abstracts | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.14, 3.65] |
| 8.1 Full papers | 3 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.14, 3.65] |
| 8.2 Abstracts | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Re‐bleeding | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.88] |
| 10 Re‐bleeding stratified by trials with high or unclear risk of bias | 4 | 360 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.84] |
| 10.1 Trials with high risk of bias | 3 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.23] |
| 10.2 Trial with unclear risk of bias | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.15, 0.90] |
| 11 Re‐bleeding stratified by full papers or abstracts | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.88] |
| 11.1 Full papers | 3 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.35, 0.78] |
| 11.2 Abstract | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.49, 3.14] |
| 12 Re‐bleeding stratified by type of gastric varices | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.88] |
| 12.1 Type gastro‐oesophageal varices varices only | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.15, 1.12] |
| 12.2 All types of gastric varices | 3 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.03] |
| 13 Re‐bleeding stratified by use of vasoactive drugs | 4 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.41, 0.88] |
| 13.1 With vasoactive drugs | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.75] |
| 13.2 Without vasoactive drugs | 2 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.30, 0.90] |
| 14 Adverse effects (general) | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.69, 11.49] |
| 15 Adverse effects stratified by trials with high or unclear risk of bias | 3 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 3.49 [0.69, 17.60] |
| 15.1 Trials with high risk of bias | 2 | 210 | Odds Ratio (M‐H, Random, 95% CI) | 8.02 [3.18, 20.23] |
| 15.2 Trials with unclear risk of bias | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.38, 2.52] |
| 16 Control of bleeding stratified by full papers or abstracts | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.90, 1.27] |
| 16.1 Full papers | 3 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.91, 1.36] |
| 16.2 Abstract | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.94, 1.07] |
| 17 Complications stratified by use of vasoactive drugs | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.69, 11.49] |
| 17.1 With vasoactive drugs | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.47, 2.04] |
| 17.2 Without vasoactive drugs | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 5.60 [2.46, 12.74] |
| 18 Control of bleeding | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.90, 1.27] |
| 19 Control of bleeding stratified by trials with high or unclear risk of bias | 4 | 264 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [1.15, 6.05] |
| 19.1 Trials with high risk of bias | 3 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [1.05, 9.47] |
| 19.2 Trial with unclear risk of bias | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 17.62] |
| 20 Control of bleeding stratified by use of vasoactive drugs | 4 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.90, 1.27] |
| 20.1 Trials with vasoactive drugs | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.94, 1.06] |
| 20.2 Trials without use of vasoactive drugs | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.78, 2.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
El Amin 2010.
| Methods | N‐butyl‐2‐cyanoacrylate vs. band ligation for acute bleeding from junctional gastric varices (GOV1 type). Jan 2008 to September 2009. Generation of allocation sequence: unclear (randomisation done by assistant); concealment of allocation sequence, sealed opaque envelopes. Blinding: participants and personnel not blinded. Intention‐to treat: no. Interim analysis: none. Follow‐up period: 6 months. |
|
| Participants | Egypt. 3‐centre trial. 150 participants, randomised into 75 in each group. Active bleeding from GOV1 only gastric varices probed by endoscopy. Cirrhosis of the liver (mostly post‐viral hepatitis). Treatment performed 24 hours after admission. Similar demographics and clinical characteristics in both groups. Same general treatment (blood, frozen plasma, fluids, antibiotics, and lactulose) in both groups. |
|
| Interventions | Experimental: cyanoacrylate group: 0.5 mL cyanoacrylate + 0.7 mL lipiodol. 21‐gauge needle. Intravariceal injection. Control: band ligation, 6 shooter. Concurrent oesophageal varices for both groups: band ligation in the same session. Number of sessions to eradicate (mean ± SD): cyanoacrylate: 1.3 ± 0.6; band ligation: 2.3 ± 0.7. Follow‐up endoscopy: every 2 weeks by same method until obliteration. Follow‐up post obliteration: every 6 months. Treatment of re‐bleeding: same as first session. |
|
| Outcomes | Initial haemostasis. Survival time. Complications. Mortality. Re‐bleeding. Treatment failure. |
|
| Notes | All adverse effects were reported. Gastric varices were limited to type GOV1. 1 participant randomised to band ligation was switched to cyanoacrylate upon failure. We attempted to contact the authors (23 July 2013), but received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Low risk | Concealment: consecutively numbered opaque sealed envelopes. "Eligible patients were randomised into two groups using consecutively numbered opaque‐sealed envelopes containing the treatment assignment to receive either endoscopic variceal ligation or endoscopic cyanoacrylate injection", p. 280. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. Methods of blinding personnel were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Treatment completed by protocol 100%. Trial profile, p. 280. |
| Selective reporting (reporting bias) | Low risk | All primary (initial haemostasis) and secondary (survival time, complications, and death) endpoints were measured and informed. p. 281, Table 2 and Table 3 and in Figure 2, p. 283. |
| Other bias | Unclear risk | 1 case randomised to band ligation was switched to cyanoacrylate upon failure. "Except one case in the EVL group where cyanoacrylate was used as a rescue procedure to control bleeding", p. 283. |
Hou 2009.
| Methods | 2 different doses of N‐butyl‐2‐cyanoacrylate for active bleeding from gastric varices of all types (0.5 mL vs. 1 mL). September 2005 to August 2007. Generation of allocation sequence: generated by computer‐allocated random digits; concealment of allocation sequence, sealed opaque envelopes. Blinding: participants not blinded. Trained nurses and physicians blinded to group assignment conducted the assessments. Intention‐to‐treat: yes. Interim analysis: none. Follow‐up period: 26 months. |
|
| Participants | Taiwan. Single‐centre randomised clinical trial. 91 participants, randomised to 44 and 47 in each group. Active bleeding from all types of gastric varices probed by endoscopy. Cirrhosis of the liver (diagnosed by needle biopsy or clinical, biochemical, and radiology) with or without hepatocellular carcinoma. Treatment within 24 hours from bleeding. Similar demographics and clinical characteristics in both groups. Same general treatment (terlipressin and somatostatin, plus antibiotics and esomeprazole in both groups). |
|
| Interventions | Experimental: 0.5 mL cyanoacrylate plus 1.3 mL lipidiol. 23‐gauge needle. Intravariceal injection. Control: 1 mL cyanoacrylate plus 1.8 mL lipiodol. 23‐gauge needle. Intravariceal injection. Concurrent oesophageal varices: endoscopic band ligation 3 to 4 weeks after intervention. Number of session to eradicate: experimental: ≤ 4 injections. Control: ≤ 4 injections. Follow‐up endoscopy: every 3 to 4 weeks by same method until obliteration. Follow‐up post‐obliteration: every 3 months. Treatment of re‐bleeding: same as first session. |
|
| Outcomes | Control of active bleeding. Treatment failure. Re‐bleeding. Mortality. Complications. |
|
| Notes | All adverse effects were informed. All types of gastric varices. We attempted to contact the authors (23 July 2013), with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by computer‐allocated random digits. "Patients who fulfilled the inclusion criteria were randomised by using consecutively numbered envelopes that contained the treatment assignment, which were generated by a system using computer‐allocated random digits", p. 669. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered envelopes. "Patients who fulfilled the inclusion criteria were randomised by using consecutively numbered envelopes that contained the treatment assignment, which were generated by a system using computer‐allocated random digits", p. 669. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. Methods of blinding personnel were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained nurses and physicians blinded to group assignment conducted the assessments. "Well‐trained nurses and physicians blinded to group assignment conducted the assessments", p. 670. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with 2 losses in experimental group and 3 losses in control group at late stage. "The results were analysed based on intent‐to‐treat analysis" and see figure Figure 1 in the original publication, p. 670. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes (arresting of active bleeding, re‐bleeding, complications and mortality were measured. Description of outcomes in methods match those in results, pp. 671 and 672. |
| Other bias | Unclear risk | Not enough data to assess other bias. |
Lo 2001.
| Methods | N‐butyl‐2‐cyanoacrylate vs. band ligation for active bleeding from gastric varices of all types. July 1996 to December 1999. Generation of allocation sequence: table of random numbers; concealment of allocation sequence, sealed opaque envelopes. Blinding: participants not blinded. Randomisation done by assistant. Intention‐to‐treat: yes. Interim analysis: 1 after 3 years that reached significance. Follow‐up period: 14 months in cyanoacrylate, 9 months in band ligation. Time to treatment: endoscopy within 3 hours. |
|
| Participants | Republic of China. Single‐centre randomised clinical trial. 60 participants, randomised into 29 and 31 in each group. Active and recent bleeding from all types of gastric varices diagnosed by endoscopy. Cirrhosis of the liver (biopsy, clinical, laboratory, imaging). Treatment made 3 hours after admission. Similar demographics and clinical characteristics in both groups. Same general treatment (blood, frozen plasma, fluids, antibiotics and lactulose) in both groups. |
|
| Interventions | Group A: banding ligation 29 participants. Pneumatic ligation device, over tube 1 to 4 bands. 11 active bleeding and 18 recent bleeding. Group B: cyanoacrylate 31 participants. 0.5 mL cyanoacrylate, 1.5 lipiodol. 2 to 4 mL. At bleeding point. 15 active bleeding and 16 recent bleeding. Concurrent oesophageal varices for both groups: endoscopic band ligation immediately after, same session. Follow‐up endoscopy: 3 to 4 week until obliteration. Follow‐up after obliteration: 6 months. Treatment of re‐bleeding: same intervention as original group. |
|
| Outcomes | Initial haemostasis (> 72 hours). Re‐bleeding (> 72 hours). Complications. Mortality. Treatment failure. |
|
| Notes | Mixed participants with acute and past history of bleeding. All adverse effects were informed. All types of gastric varices. We attempted to contact the authors (23 July 2013), with no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. "Eligible patients were randomised into 2 groups, using opaque sealed envelopes numbered according to a table of random numbers", p. 1060. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. "Eligible patients were randomised into 2 groups, using opaque sealed envelopes numbered according to a table of random numbers", p. 1060. Randomisation made by assistant. "Randomisation was performed by an assistant, and endoscopic treatment was administered at once", p. 1060. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. Methods of blinding personnel were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. "Statistical analyses of both the groups were based on the “intention‐to‐treat” principle", p. 1061. After 3 years, interim analysis reached significant differences with the enrolled participants. Loss to follow‐up: 1 in each group. "The mean follow‐up period was 14 months in the cyanoacrylate group and 9 months in the endoscopic band ligation group. One patient in each group was lost to follow‐up", p. 1061. |
| Selective reporting (reporting bias) | Low risk | All primary (initial haemostasis) and secondary (re‐bleeding) outcomes were measured. Description of outcomes in methods match up to those in results. |
| Other bias | Unclear risk | Not enough data to assess other bias. |
Sarin 2002.
| Methods | N‐butyl‐2‐cyanoacrylate vs. absolute alcohol for active or recent bleeding from isolated (IGV1 or GOV2) gastric varices. 1995 to 1998. Generation of allocation sequence: table of random numbers; concealment of allocation sequence: not described. Blinding: participants and personnel not blinded. Intention‐to‐treat: no. Interim analysis: none. Follow‐up period: 14 months. |
|
| Participants | India. Single‐centre randomised clinical trial. 37 participants, 17 in alcohol group, 20 in cyanoacrylate group. Active or acute bleeding from IGV1 or GOV2 only gastric varices probed by endoscopy. Portal hypertension. Treatment made after admission. Similar demographics and clinical characteristics in both groups. Same general treatment: vasoactive drugs (somatostatin or octreotide 48 to 120 hours after admission). |
|
| Interventions | Experimental: cyanoacrylate 0.5 mL plus lipiodol 0.7 mL. 21‐gauge needle. 1.2 to 4.6 mL. Control: absolute alcohol group. 21‐gauge needle. 2 to 9 paravariceal injections and 1 to 3 intravariceal. 0.5 to 1.0 mL each. Concomitant oesophageal varices: only isolated varices were treated. Oesophageal was non‐existent or small. There was no treatment for them. Number of sessions to eradicate (mean ± SD): cyanoacrylate: 2.0 ± 1.6. Alcohol: 4.7 ± 3.2. Follow‐up endoscopy: every week until obliteration. Follow‐up post‐obliteration: every 3 to 6 weeks. Treatment for re‐bleeding: emergency endoscopy, same method. 2 failures: emergency rescue surgery. |
|
| Outcomes | Control active bleeding. Variceal obliteration. Re‐bleeding. Mortality. Failure of treatment. Complications. |
|
| Notes | Mixed acute and past bleeding. Only isolated varix was chosen (GOV2 and IGV1 were considered Isolated varices). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers after initial endoscopy "Patients were randomised using a table of random numbers immediately at the time of the initial endoscopy", pp 1011. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. Methods of blinding personnel were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses of follow‐up are described. No intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All primary (success controlling bleeding,obliteration and re‐bleeding) and secondary (time for obliteration, recurrence and bleeding related mortality) outcomes were described. Description of outcomes in methods match up to those in results, pp 1012, tables 1 and 3, pp 1012 to 1013. |
| Other bias | Unclear risk | Only isolated varix. Mixed acute and past bleeding. |
Tan 2006.
| Methods | N‐butyl‐2‐cyanoacrylate vs. band ligation for active or recent bleeding from gastric varices of all types. July 1996 to June 2002. Generation of allocation sequence: computer‐allocated random digit numbers; concealment of allocation sequence, sealed opaque envelopes. Blinding: participants and personnel not blinded. Nurses and physicians blinded to treatment for assessment. Intention‐to‐treat: yes. Modified intention‐to‐treat analysis. Interim analysis: none. Follow‐up period: 6 months. |
|
| Participants | Country: Taiwan. Single‐centre randomised clinical trial. 97 participants, randomised in 49 and 48 in each group. Mixed between acute and active bleeding from all types of gastric varices. Diagnosed by endoscopy. Cirrhosis of the liver (biopsy, clinical, laboratory, imaging). Hepatocellular carcinoma; cytohistological, liver biopsy, 2 imaging plus serum level of alfa fetoprotein > 400 ng/mL. Treatment made < 24 hours after admission. Similar demographics and clinical characteristics in both groups. Same general treatment: vasoactive drugs (terlipressin or somatostatin before diagnosis and proton pump inhibitor post intervention). |
|
| Interventions | Experimental: 49 participants. 0.5 mL cyanoacrylate, 0.5 mL lipiodol. No more than 6 shots. 15 active bleeding and 33 acute bleeding. Control: endoscopic band ligation. 48 participants. Pneumatic ligation device, no more than 10 bands in each session. Bleeding point first. 15 active bleeding and 33 acute bleeding. Concurrent oesophageal varices: endoscopic band ligation immediately after, same session. Number of sessions to eradicate (mean ± SD): cyanoacrylate 1.5 ± 0.7. Banding ligation 1.8 ± 1.4. Follow‐up endoscopy: 3 months, if unremarkable 6 months. Every 6 months after obliteration or death. Treatment of re‐bleeding: same intervention as original group. |
|
| Outcomes | Control of active bleeding. Re‐bleeding. Mortality. Complications. Treatment failure. |
|
| Notes | Mixed between acute and active. Hepatocellular carcinoma included. 4 participants switched from endoscopic band ligation to cyanoacrylate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐allocated random digit numbers. "Consecutively numbered envelopes that contained the treatment assignments, which were generated by a system using computer‐allocated random digit numbers", p. 691. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered envelopes. "Patients who fulfilled the inclusion criteria were immediately randomised into the two treatment groups using consecutively numbered envelopes", p. 691. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. Methods of blinding personnel were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses and physicians blinded to treatment for assessment. "Well‐trained nurses and physicians who were blinded to group assignment conducted the assessments", p. 691. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified intention‐to‐treat analysis (all randomised participants with inclusion criteria and at least 1 time treatment). "Because the study was performed on an emergency basis, enrolment error was inevitable. Therefore, the results were based on modified intention‐to‐treat analysis", p. 692. If switched from groups counted in their original group. Determination of exclusion criteria was made after endoscopy. |
| Selective reporting (reporting bias) | Low risk | All outcomes (control of active bleeding, re‐bleeding, and mortality) were measured (see Figure 1 in the original publication ‐ p. 693). |
| Other bias | Unclear risk | Mixed between acute and active bleeding. 4 participants switched from endoscopic band ligation to cyanoacrylate. "These four patients undergoing GVL [gastric varices ligation] were switched to Histocryl injection because rubber bands could not be deployed on the GV [gastric varices] when re‐bleeding occurred", p. 694. |
Zheng 2012.
| Methods | N‐butyl‐2‐cyanoacrylate vs. band ligation for active bleeding from gastric varices. Abstract only. Generation of allocation sequence: unclear; concealment of allocation sequence, unclear. Blinding: participants and personnel: unclear. Intention‐to‐treat: unclear. Interim analysis: unclear. Follow‐up period: no data. |
|
| Participants | Republic of China. Single‐centre randomised trial. 58 adults, bleeding actively from gastric varices. Type of gastric varices: no data. Cirrhosis of the liver: no data. Hepatocellular carcinoma: no data. Demographics and clinical characteristics in both groups: no data. Same general treatment for both groups: somatostatin and protein pump inhibitor before intervention. |
|
| Interventions | Experimental: cyanoacrylate 0.5 mL plus lipiodol 0.5 mL, injected intravariceally. Control: endoscopic band ligation, no data. |
|
| Outcomes | Bleeding control rate. Re‐bleeding rate (at 2 years). Complication rate. Survival. |
|
| Notes | Only abstract available. Full paper was not available. We wrote e‐mails to Bin Wu, MD, PhD, Professor and Chief, Department of Gastroenterology, The Third Affiliated Hospital of Sun Yat‐Sen University, Guangzhou (19 February 2013), and to the organisation of the VL: Conference: Asian Pacific Digestive Week 2012 Bangkok Thailand were the abstract was presented to try to contact to the authors but we received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial. No details available (abstract). |
| Allocation concealment (selection bias) | Unclear risk | No details available (abstract). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details available (abstract). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details available (abstract). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details available (abstract). |
| Selective reporting (reporting bias) | Unclear risk | No details available (abstract). |
| Other bias | Unclear risk | Not possible to judge (abstract). |
EVL: endoscopic variceal ligation; GOV1: type I gastric varices; GOV2: type II gastric varices; IGV1, isolated gastric varices.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akahoshi 2002 | Design: retrospective case series. |
| Bazeed 2013 | Randomised trial of cyanoacrylate vs. ethanolamine in gastric varices. Conference abstract. Not published article. Author contacted October 2014. Several emails were exchanged to gather further information. Randomisation by envelopes, without random sequence generation. Unbalanced results of randomisation: 36 to cyanoacrylate, 69 to ethanolamine without explanation. 23 participants randomised to ethanolamine were treated with cyanoacrylate within 1 week. Total time of follow‐up was 1 week. |
| Huang 2000 | Not a clinical trial but a case series with a long follow‐up. |
| Kim 2006 | Not a clinical trial but an 86‐participant case series. |
| Ljubicic 2011 | Randomised clinical trial of N‐butyl‐2‐cyanoacrylate for oesophageal and not gastric varices. |
| Maluf‐Filho 2001 | Mechanisms of action, indications, technique, and results of N‐butyl‐2‐cyanoacrylate endoscopic injection in the treatment of oesophageal varices, and not gastric varices. |
| Maluf‐Filho 2008 | Not a clinical trial, but a 48‐participant case series. |
| Mishra 2010 | Different objectives: secondary prophylaxis. All the acute bleeding was treated with the same cyanoacrylate. |
| Mishra 2011 | Different objectives: primary prophylaxis. |
| Ogawa 1999 | It is not a clinical trial, a 38‐participant case series of cyanoacrylate or ethanolamine. |
| Oho 1995 | Not randomised clinical trial. |
| Santos 2011 | Different objectives. Oesophageal varices, not gastric varices. |
| Shiha 2010 | Randomised trial of cyanoacrylate vs band ligation in gastric varices. Conference abstract. No published article available. Several attempts to contact authors between October and December 2014 but we received no replies. Results were expressed by significance, no actual numbers available. Percentages only available for 1 result (active bleeding). |
| Sugimoto 2007 | Not a clinical trial but a small case series. |
| Thakeb 1995 | Only 12% of the treated varices were gastric, with oesophageal varices being the remaining 88%. |
Differences between protocol and review
Selection of studies
There were heterogeneous definitions across trials regarding time‐to‐measure outcomes. We originally planned to analyse outcomes according to the Baveno criteria (de Franchis 2010), but this was not possible because not all the needed data were available.
Primary outcomes
All‐cause mortality at maximum follow‐up. Only two trials included in this review reported all‐cause mortality and, therefore, it was not possible to assess this outcome. All of the included trials included bleeding‐related mortality and, thus, we included this outcome (bleeding‐related mortality: number of participants who died from uncontrolled variceal bleeding). However, this outcome may be biased, and all‐cause mortality ought to be reported in all future trials and will be incorporated in future updates of this review.
Failure of intervention: it was not possible to assess the five‐day outcome. The rationale for this outcome was the proposed standardisation by the Baveno consensus meetings (de Franchis 2010) and proposed by other Cochrane systematic reviews (Guo 2009; D'Amico 2010), but the majority of trials reported at one‐, three‐, or seven‐day outcomes.
Re‐bleeding: it was not possible to assess this 42‐day outcome as none of the included trials in this review reported this outcome. The rationale for this outcome was the proposed standardisation by the Baveno consensus meeting (de Franchis 2010) and proposed by other Cochrane systematic reviews (Guo 2009; D'Amico 2010). Four trials used 24 hours for definition of re‐bleeding, one used 72 hours, and one used a variable time concept (bleeding before next endoscopy session).
Adverse events: adverse events analysis had to be adjusted depending on the data found in each trial. For each comparison, the reported adverse effects are measured.
Secondary outcomes
One‐day treatment failure: this outcome has the same definition of control of bleeding, and therefore the name was changed.
Number of transfusions: not all the trials included number of transfusions, and it was not possible to calculate.
Quality of life: none of the trials included quality of life.
Transjugular intrahepatic portosystemic shunt (TIPS) or surgery: number of participants that underwent TIPS or surgery. Only one trial included this outcome.
Reported outcomes, not included in the protocol
One outcome not directly considered in the protocol was the arresting/control of active bleeding; this outcome was found in all the trials and therefore, we reported this outcome.
Use of different doses of cyanoacrylate was not considered in the protocol. As different doses of cyanoacrylate were assessed in one trial, we reported this comparison.
Differences in methods
We performed trial sequential analysis for each outcome only in the cyanoacrylate versus band ligation comparison.
We did not present a funnel plot for publication bias because there was not a sufficient number of trials to construct it.
Contributions of authors
Eddy Rios: conception of the idea, design of the review, analysis and interpretation of results, writing the manuscript. Pamela Serón: design of the review, analysis and interpretation of results. Javier P Gisbert: design of the review, analysis and interpretation of results. Xavier Bonfill: design of the review, analysis, and interpretation of results.
All authors agreed to the publication of the review.
Sources of support
Internal sources
-
La Frontera University. Department of Internal Medicine, Temuco, Chile.
Time protection for preparation of the review. Helping for some fees of the PhD course
External sources
No external support was provided, Other.
Declarations of interest
None known.
New
References
References to studies included in this review
El Amin 2010 {published data only}
- Amin H, Abdel Baky L, Sayed Z, Abdel Mohsen E, Eid K, Fouad Y, et al. A randomized trial of endoscopic variceal ligation versus cyanoacrylate injection for treatment of bleeding junctional varices. Tropical Gastroenterology 2010;31(4):279‐84. [PubMed] [Google Scholar]
Hou 2009 {published data only}
- Hou MC, Lin HC, Lee HS, Liao WC, Lee FY, Lee SD. A randomized trial of endoscopic cyanoacrylate injection for acute gastric variceal bleeding: 0.5 mL versus 1.0 mL. Gastrointestinal Endoscopy 2009;70(4):668‐75. [DOI] [PubMed] [Google Scholar]
Lo 2001 {published data only}
- Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology 2001;33(5):1060‐4. [DOI] [PubMed] [Google Scholar]
Sarin 2002 {published data only}
- Sarin SK, Jain AK, Jain M, Gupta R. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. American Journal of Gastroenterology 2002;97(4):1010‐5. [DOI] [PubMed] [Google Scholar]
Tan 2006 {published data only}
- Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N‐butyl‐2‐cyanoacrylate injection versus band ligation. Hepatology 2006;43(4):690‐7. [DOI] [PubMed] [Google Scholar]
Zheng 2012 {published data only}
- Zheng F, Lin X, Tao L. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N‐butyl‐2‐cyanoacrylate injection versus band ligation. Journal of Gastroenterology and Hepatology 2012;27(Suppl 5):113. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akahoshi 2002 {published data only}
- Akahoshi T, Hashizume M, Shimabukuro R, Tanoue K, Tomikawa M, Okita K, et al. Long‐term results of endoscopic histoacryl injection sclerotherapy for gastric variceal bleeding: a 10‐year experience. Surgery 2002;131:S176‐81. [DOI] [PubMed] [Google Scholar]
Bazeed 2013 {published data only}
- Bazeed SEE, El‐Khyate HR, Swiffy YM, Nafeh HM. Results of endoscopic sclerotherapy with N‐butyl‐2‐cyanoacrylate and ethanolamine oleate in treatment of bleeding gastric varices. Hepatology International 2013;7(Suppl 1):S508. [Google Scholar]
Huang 2000 {published data only}
- Huang YH, Yeh HZ, Chen GH, Chang CS, Wu CY, Poon SK, et al. Endoscopic treatment of bleeding gastric varices by N‐butyl‐2‐cyanoacrylate (Histoacryl) injection: long‐term efficacy and safety. Gastrointestinal Endoscopy 2000;52(2):160‐7. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim JW, Baik SK, Kim KH, Kim HJ, Jo KW, Hong JH, et al. Effect of endoscopic sclerotherapy using N‐butyl‐2‐cyanoacrylate in patients with gastric variceal bleeding. Korean Journal of Hepatology 2006;12(3):394‐403. [PubMed] [Google Scholar]
Ljubicic 2011 {published data only}
- Ljubicic N, Biscanin A, Nikolic M, Supanc V, Hrabar D, Pavic T, et al. A randomized‐controlled trial of endoscopic treatment of acute esophageal variceal hemorrhage: N‐butyl‐2‐cyanoacrylate injection vs. variceal ligation. Hepato‐Gastroenterology 2011;58(106):438‐43. [PubMed] [Google Scholar]
Maluf‐Filho 2001 {published data only}
- Maluf‐Filho F, Sakai P, Ishioka S, Matuguma SE. Endoscopic sclerosis versus cyanoacrylate endoscopic injection for the first episode of variceal bleeding: a prospective, controlled, and randomized study in Child‐Pugh class C patients. Endoscopy 2001;33(5):421‐7. [DOI] [PubMed] [Google Scholar]
Maluf‐Filho 2008 {published data only}
- Marques P, Maluf‐Filho F, Kumar A, Matuguma SE, Sakai P, Ishioka S. Long‐term outcomes of acute gastric variceal bleeding in 48 patients following treatment with cyanoacrylate. Digestive Diseases and Sciences 2008;53(2):544‐50. [DOI] [PubMed] [Google Scholar]
Mishra 2010 {published data only}
- Mishra SR, Sharma BC, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta‐blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut 2010;59(6):729‐35. [DOI] [PubMed] [Google Scholar]
Mishra 2011 {published data only}
Ogawa 1999 {published data only}
- Ogawa K, Ishikawa S, Naritaka Y, Shimakawa T, Wagatsuma Y, Katsube A, et al. Clinical evaluation of endoscopic injection sclerotherapy using N‐butyl‐2‐cyanoacrylate for gastric variceal bleeding. Journal of Gastroenterology and Hepatology 1999;14(3):245‐50. [DOI] [PubMed] [Google Scholar]
Oho 1995 {published data only}
- Oho K, Iwao T, Sumino M, Toyonaga A, Tanikawa K. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: a nonrandomized study. Endoscopy 1995;27(5):349‐54. [DOI] [PubMed] [Google Scholar]
Santos 2011 {published data only}
Shiha 2010 {published data only}
- Shiha G, Khalil E, Elfakhry A. Endoscopic band ligation compared to cyanoacrylate injection for management of gastric varices: a randomized controlled study. Hepatology International 2010;4(1):264‐5. [Google Scholar]
Sugimoto 2007 {published data only}
- Sugimoto N, Watanabe K, Watanabe K, Ogata S, Shimoda R, Sakata H, et al. Endoscopic hemostasis for bleeding gastric varices treated by combination of variceal ligation and sclerotherapy with N‐butyl‐2‐cyanoacrylate. Journal of Gastroenterology 2007;42(7):528‐32. [DOI] [PubMed] [Google Scholar]
Thakeb 1995 {published data only}
Additional references
Alexander 2006
- Alexander S, Korman MG, Sievert W. Cyanoacrylate in the treatment of gastric varices complicated by multiple pulmonary emboli. Internal Medicine Journal 2006;36(7):462‐64. [DOI] [PubMed] [Google Scholar]
Arakaki 2003
- Arakaki Y, Murakami K, Takahashi K, Sato R, Okimoto T, Ishitobi H, et al. Clinical evaluation of combined endoscopic variceal ligation and sclerotherapy of gastric varices in liver cirrhosis. Endoscopy 2003;35(11):940‐5. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Belletrutti 2008
- Belletrutti PJ, Romagnuolo J, Hilsden RJ, Chen F, Kaplan B, Love J, et al. Endoscopic management of gastric varices: efficacy and outcomes of gluing with N‐butyl‐2‐cyanoacrylate in a North American patient population. Canadian Journal of Gastroenterology 2008;22(11):931‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonilha 2011
- Bonilha DQ, Correia LM, Monaghan M, Lenz L, Santos M, Libera ED. Prospective study of bacteremia rate after elective band ligation and sclerotherapy with cyanoacrylate for esophageal varices in patients with advanced liver disease. Arquivos De Gastroenterologia 2011;48(4):248‐51. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Cheng 2007
- Cheng LF, Wang ZQ, Li CZ, Cai FC, Huang QY, Linghu EQ, et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years' experience of 635 cases. Chinese Medical Journal 2007;120(23):2081‐5. [PubMed] [Google Scholar]
Chun 1995
- Chun HJ, Hyun JH. A new method of endoscopic variceal ligation‐injection sclerotherapy (EVLIS) for gastric varices. Korean Journal of Internal Medicine 1995;14:730‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipolletta 1998
- Cipolletta L, Bianco MA, Rotondano G, Piscopo R, Prisco A, Garofano ML. Emergency endoscopic ligation of actively bleeding gastric varices with a detachable snare. Gastrointestinal Endoscopy 1998;47(5):400‐3. [DOI] [PubMed] [Google Scholar]
D'Amico 2010
- D'Amico G, Pagliaro L, Pietrosi G, Tarantino I. Emergency sclerotherapy versus vasoactive drugs for bleeding oesophageal varices in cirrhotic patients. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD002233.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Franchis 2010
- Franchis R, On behalf of the Baveno V Faculty. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. Journal of Hepatology 2010;53(4):762‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evrard 2003
- Evrard S, Dumonceau JM, Delhaye M, Golstein P, Deviere J, Moine O. Endoscopic histoacryl obliteration versus propranolol in the prevention of esophagogastric variceal rebleeding: a randomized trial. Endoscopy 2003;35:729‐35. [DOI] [PubMed] [Google Scholar]
Garcia‐Pagán 2013
- Garcia‐Pagán JC, Barrufet M, Cardenas A, Escorsell À. Management of Gastric Varices. Clinical Gastroenterology and Hepatology 2013;12(6):919‐28. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2007
- Garcia‐Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46(3):922‐38. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2008
- Garcia‐Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding ‐ unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single‐topic conference. Hepatology 2008;47(5):1764‐72. [DOI] [PubMed] [Google Scholar]
Gimson 1991
- Gimson AE, Westaby D, Williams R. Endoscopic sclerotherapy in the management of gastric variceal haemorrhage. Journal of Hepatology 1991;13:274‐8. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud LL, Krag A. Banding ligation versus beta‐blockers for primary prevention in oesophageal varices in adults. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004544.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2015
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary. About Cochrane (Cochrane Review Groups (CRGs)) 2015, Issue 2. Art. No.: LIVER.
Grace 1997
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. American Journal of Gastroenterology 1997;92(7):1081‐91. [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Grade Working Group 2004‐2007, 2008.
Greig 1990
- Greig JD, Garden OJ, Anderson JR, Carter DC. Management of gastric variceal haemorrhage. British Journal of Surgery 1990;77:297‐9. [DOI] [PubMed] [Google Scholar]
Guo 2009
- Guo Z, Wu Z, Wang Y. Antacids for preventing oesophagogastric variceal bleeding and rebleeding in cirrhotic patients. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005443.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. Rating quality of evidence and strength of recommendations: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7653):1106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Harada 1997
- Harada T, Yoshida T, Shigemitsu T, Takeo Y, Tada M, Okita K. Therapeutic results of endoscopic variceal ligation for acute bleeding of oesophageal and gastric varices. Journal of Gastroenterology and Hepatology 1997;12:331‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman D. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997. [Google Scholar]
Irani 2011
- Irani S, Kowdley K, Kozarek R. Gastric varices: an updated review of management. Journal of Clinical Gastroenterology 2011;45(2):133‐48. [DOI] [PubMed] [Google Scholar]
Kitano 1989
- Kitano S, Hashizume M, Yamaga H, Wada H, Iso Y, Iwanaga T, et al. Human thrombin plus 5 per cent ethanolamine oleate injected to sclerose oesophageal varices: a prospective randomized trial. British Journal of Surgery 1989;76:715‐8. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Korula 1991
- Korula J, Chin K, Ko Y, Yamada S. Demonstration of two distinct subsets of gastric varices. Observations during a seven‐year study of endoscopic sclerotherapy. Digestive Diseases and Sciences 1991;36(3):303‐9. [DOI] [PubMed] [Google Scholar]
Laine 1995
- Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta‐analysis. Annals of Internal Medicine 1995;123(4):280‐7. [DOI] [PubMed] [Google Scholar]
Lee 2002
- Lee MS, Cho JY, Cheon YK, Ryu CB, Moon JH, Cho YD, et al. Use of detachable snares and elastic bands for endoscopic control of bleeding from large gastric varices. Gastrointestinal Endoscopy 2002;56(1):83‐8. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
McCormick 1994
- McCormick PA, Dick R, Panagou EB, Chin JKT, Greenslade L, Burroughs AK. Emergency transjugular intrahepatic portasystemic stent shunting as salvage treatment for uncontrolled variceal bleeding. British Journal of Surgery 1994;81:1324‐7. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Rengstorff 2004
- Rengstorff DS, Binmoeller KF. A pilot study of 2‐octyl cyanoacrylate injection for treatment of gastric fundal varices in humans. Gastrointestinal Endoscopy 2004;59:553‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosch 1998
- Rosch W, Rexroth G. UCTN: pulmonary, cerebral and coronary emboli during bucrylate injection of bleeding fundic varices. Endoscopy 1998;30:S89‐90. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Sarin 1992
- Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long‐term follow‐up study in 568 portal hypertension patients. Hepatology 1992;16:1343‐9. [DOI] [PubMed] [Google Scholar]
Sarin 1997
- Sarin SK. Long‐term follow‐up of gastric variceal sclerotherapy: an eleven‐year experience. Gastrointestinal Endoscopy 1997;46:8‐14. [DOI] [PubMed] [Google Scholar]
Sarin 2001
- Sarin SK, Jain AK, Jain M, Gupta R. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. American Journal of Gastroenterology 2002;97:1010‐5. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schuman 1987
- Schuman BM, Beckman JW, Tedesco FJ, Griffin JW Jr, Assad RT. Complications of endoscopic injection sclerotherapy: a review. American Journal of Gastroenterology 1987;82(9):823‐30. [PubMed] [Google Scholar]
Sharara 2001
- Sharara AI, Rockey DC. Gastroesophageal hemorrhage. New England Journal of Medicine 2001;345:669‐81. [DOI] [PubMed] [Google Scholar]
Shiha 1999
- Shiha G, El‐Sayed SS. Gastric variceal ligation: a new technique. Gastrointestinal Endoscopy 1999;49:437‐41. [DOI] [PubMed] [Google Scholar]
Soehendra 1986
- Soehendra N, Nam VC, Grimm H, Kempeneers I. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy 1986;18:25‐6. [DOI] [PubMed] [Google Scholar]
Takeuchi 1996
- Takeuchi M, Nakai Y, Syu A, Okamoto E, Fujimoto J. Endoscopic ligation of gastric varices. Lancet 1996;348:1038. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 27 January 2015).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Turler 2001
- Turler A, Wolff M, Dorlars D. Embolic and septic complications after sclerotherapy of fundic varices with cyanoacrylate. Gastrointestinal Endoscopy 2001;53:228‐30. [DOI] [PubMed] [Google Scholar]
Upadhyay 2005
- Upadhyay AP, Ananthasivan R, Radhakrishnan S, Zubaidi G. Cortical blindness and acute myocardial infarction following injection of bleeding gastric varices with cyanoacrylate glue. Endoscopy 2005;37(10):1034. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2002
- Wu CY, Yeh HZ, Chen GH. Pharmacologic efficacy in gastric variceal re bleeding and survival: including multivariate analysis. Journal of Clinical Gastroenterology 2002;35:127‐32. [DOI] [PubMed] [Google Scholar]
Yang 2002
- Yang WL, Tripathi D, Therapondos G, Todd A, Hayes PC. Endoscopic use of human thrombin in bleeding gastric varices. American Journal of Gastroenterology 2002;97:1381‐5. [DOI] [PubMed] [Google Scholar]
Yoshida 1999
- Yoshida T, Harada T, Shigemitsu T, Takeo Y, Miyazaki S, Okita K. Endoscopic management of gastric varices using a detachable snare and simultaneous endoscopic sclerotherapy and O‐ring ligation. Journal of Gastroenterology and Hepatology 1999;14(7):730‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Castellanos 2012
- Castellanos ER, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in patients with portal hypertension. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010180] [DOI] [PMC free article] [PubMed] [Google Scholar]


