Abstract
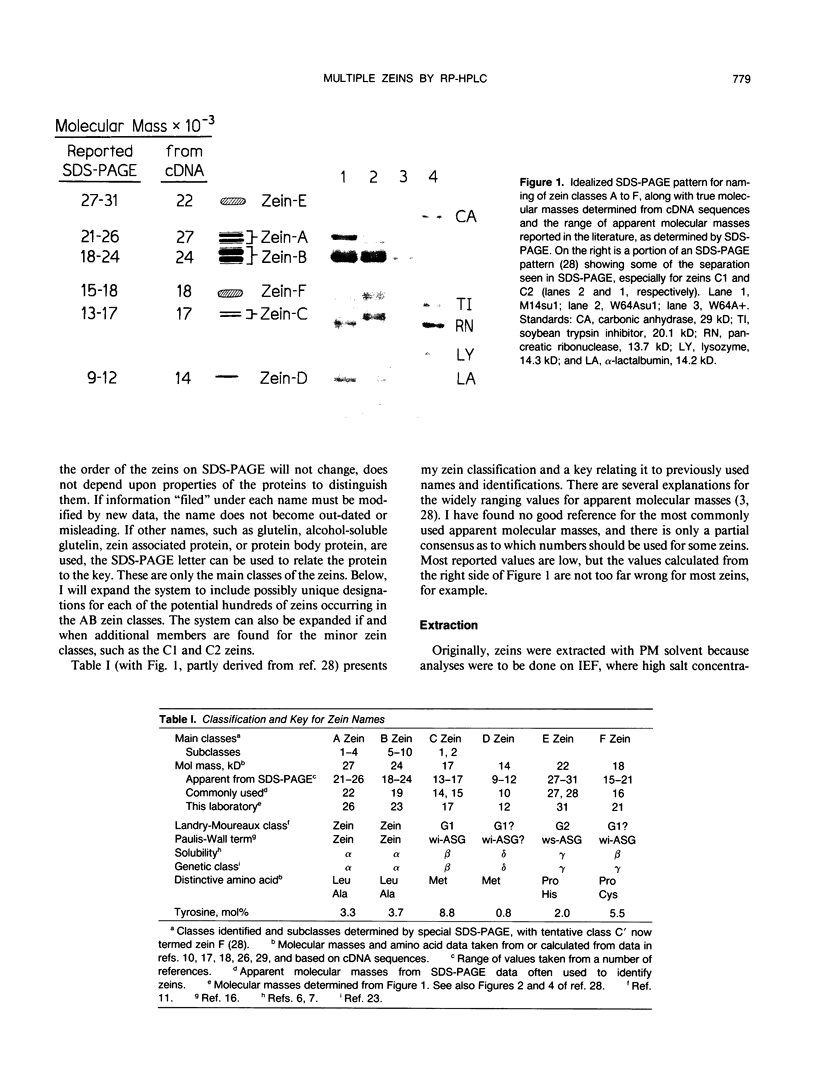
The major storage proteins of maize (Zea mays L.) endosperm are located in protein bodies, and may be separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) into two major classes and four minor classes of polypeptides. The two major classes (commonly known as zeins) have been separated previously into a large number of components by isoelectric focusing (IEF). Reversed-phase high performance liquid chromatography (HPLC) further separated the major classes into additional components, and gave distinctive peaks for each minor zein class. Some IEF bands produced two or more HPLC fractions, while some HPLC fractions produced two or more IEF bands. Apparently identical IEF bands from different inbreds may appear in different fractions after HPLC. Thus the total number of zeins revealed by separations based on apparent size (SDS-PAGE), net charge (IEF), and hydrophobicity (HPLC) is very large. Different laboratories have developed diverse nomenclatures which cause much confusion. A key is presented to provide a flexible and expandable nomenclature for this complex group of proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck M. A., Olah T. A., Weitzmann C. J., Cooperman B. S. Protein estimation by the product of integrated peak area and flow rate. Anal Biochem. 1989 Nov 1;182(2):295–299. doi: 10.1016/0003-2697(89)90597-6. [DOI] [PubMed] [Google Scholar]
- Conroy J. M., Esen A. An enzyme-linked immunosorbent assay for zein and other proteins using unconventional solvents for antigen adsorption. Anal Biochem. 1984 Feb;137(1):182–187. doi: 10.1016/0003-2697(84)90368-3. [DOI] [PubMed] [Google Scholar]
- Esen A. Separation of alcohol-soluble proteins (zeins) from maize into three fractions by differential solubility. Plant Physiol. 1986 Mar;80(3):623–627. doi: 10.1104/pp.80.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirihara J. A., Petri J. B., Messing J. Isolation and sequence of a gene encoding a methionine-rich 10-kDa zein protein from maize. Gene. 1988 Nov 30;71(2):359–370. doi: 10.1016/0378-1119(88)90053-4. [DOI] [PubMed] [Google Scholar]
- Landry J., Moureaux T. Hétérogénéité des glutélines du grain de mais: extraction sélective et composition en acides amines des trois fractions isolées. Bull Soc Chim Biol (Paris) 1970;52(10):1021–1037. [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Argos P., Naravana S. V., Larkins B. A. Sequence analysis and characterization of a maize gene encoding a high-sulfur zein protein of Mr 15,000. J Biol Chem. 1986 May 15;261(14):6279–6284. [PubMed] [Google Scholar]
- Prat S., Pérez-Grau L., Puigdomènech P. Multiple variability in the sequence of a family of maize endosperm proteins. Gene. 1987;52(1):41–49. doi: 10.1016/0378-1119(87)90393-3. [DOI] [PubMed] [Google Scholar]
- Shewry P. R., Tatham A. S. The prolamin storage proteins of cereal seeds: structure and evolution. Biochem J. 1990 Apr 1;267(1):1–12. doi: 10.1042/bj2670001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Larkins B. A. Structural elements regulating zein gene expression. Bioessays. 1989 Apr;10(4):108–113. doi: 10.1002/bies.950100404. [DOI] [PubMed] [Google Scholar]
- Wallace J. C., Lopes M. A., Paiva E., Larkins B. A. New Methods for Extraction and Quantitation of Zeins Reveal a High Content of gamma-Zein in Modified opaque-2 Maize. Plant Physiol. 1990 Jan;92(1):191–196. doi: 10.1104/pp.92.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Esen A. Primary structure of a proline-rich zein and its cDNA. Plant Physiol. 1986 May;81(1):70–74. doi: 10.1104/pp.81.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Mapping of zein polypeptides after isoelectric focusing on agarose gels. Biochem Genet. 1985 Feb;23(1-2):115–124. doi: 10.1007/BF00499117. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Serial analysis of zein by isoelectric focusing and sodium dodecyl sulfate gel electrophoresis. Plant Physiol. 1986 Sep;82(1):196–202. doi: 10.1104/pp.82.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]