ABSTRACT
Introduction
Ureteroenteric stricture (UES) is the leading cause of renal function deterioration after radical cystectomy (RC) and urinary diversion (UD). The aim of the present review is to summarize studies that discussed the risk factors associated with UES development. Identifying the responsible factors is of importance to help surgeons to modify their treatment or follow-up strategies to reduce this serious complication.
Materials and Methods
A comprehensive search of the literature using the PubMed database was conducted. The target of the search was only studies that primarily aimed to identify risk factors of UES after RC and UD. References of searched papers were also checked for potential inclusion.
Results
The search originally yielded a total of 1357 articles, of which only 15 met our inclusion criteria, comprising 13, 481 patients. All the studies were observational, and retrospective published between 2013 and 2022. The natural history of UES and the reported risk factors varied widely across the studies. In 13 studies, a significant association between some risk factors and UES development was demonstrated. High body mass index (BMI) was the most frequently reported stricture risk factor, followed by perioperative urinary tract infection (UTI), robotic-assisted radical cystectomy (RARC), occurrence of post-operative Clavian grade ≥ 3 complications and urinary leakage. Otherwise, many other risk factors were reported only once.
Conclusion
The literature is still lacking well-designed prospective studies investigating predisposing factors of UES. The available data suggest that the high BMI, RARC and complicated postoperative course are the main risk factors for stricture formation.
KEYWORDS: Bladder cancer, radical cystectomy, urinary diversion, ureteroenteric stricture
Introduction
Despite major advances in health care and the availability of well-trained surgeons around the world, radical cystectomy (RC) remains one of the most risky urological procedures, with a high early complication rate of 25% to 64% and a mortality risk of up to 5.7%. In addition, up to 50% of patients still develop complications years later, mainly related to urinary diversion (UD) [1–5].
The most serious long-term consequence of UD is renal function deterioration, which affects 20–35% of patients [6,7]. Many factors are related to renal impairment including patient’s age, chronic hypertension, diabetes, baseline renal function and diversion-related factors. The group from Bern reported a deterioration of renal function after 10 years in 36% of patients with ileal conduit and 21% of patients with neobladder. They found that urinary tract obstruction at any level is an independent predictor of renal function deterioration [7]. Gilbert et al reviewed data of 1,565 patients who underwent different forms of diversion, Kaplan–Meier analysis at 5 years showed 16% incidence of renal impairment/failure. An ureteroenteric stricture (UES) occurred in 13%, and it was the leading cause of renal function changes [8]. Eisenberg et al analyzed changes in renal function in 1.631 patients, after 10 years follow-up; they found a decrease in renal function in most patients. In the multivariate analysis, risk factors contributing to renal function decline were patient age, preoperative renal function, chronic hypertension, postoperative hydronephrosis, pyelonephritis and the most important factor was UES (HR 1.6, p < 0.0001) [9].
To date, there is no consensus in the literature on factors associated with the development of UES, despite the presence of many large studies. This can be attributed to the retrospective nature of these studies or the study design itself; some studies were either primarily based on assessing the influence of one factor on the development of the stricture, e.g. the type of ureteroileal anastomosis or preoperative radiotherapy (RT), or they did not include all possible contributing pre-operative and postoperative factors [10–26]
We tried in this systematic review to define the reported risk factors of UES in the literature, we focused only on studies that identified these factors through multivariate analysis including many possible contributing factors.
Materials and methods
Search strategy
A comprehensive search of the PubMed database has been conducted to identify studies that address the risk factors associated with the development of UES. The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [27]. The computer search was conducted in December 2022 to find relevant studies that were published between 1966 and 2022. The search was performed using different combinations of the following MeSH terms: ‘cancer, bladder’, ‘cystectomy’, ‘cystectomy/adverse effects’, ‘urinary diversion’, ‘urinary diversion/adverse effects’, ‘ureter’, ‘ureteral obstruction’, ‘anastomosis, surgical/adverse effects’ and ‘anastomosis, surgical’.
The titles were screened to identify the relevant articles. Results were deduplicated using EndNote program. For the relevant titles, the abstracts and then the articles were inspected for our inclusion criteria. The list of references in the finally selected papers were also evaluated for potentially relevant studies. We included only papers that investigated the risk factors of UES after RC and UD using multivariate analysis across many possible contributing factors. Articles that studied the effect of one variable, e.g. the technique of anastomosis without adjusting it with the other possible contributing factors were excluded. Only papers that were written in English were included.
Data extraction
The following information were extracted from each eligible study: publication details (title, first author and publication year), number of the patients, operative parameters (approach, type of diversion and technique for ureteroenteric anastomosis), the median follow-up, natural history of UES (incidence, laterality of UES and mean time to diagnosis) and the independent predictors of UES development in the multivariate analysis, which are considered the main outcome of the review.
Results
The PRISMA flowchart is shown in Figure 1. The search using the previously mentioned mesh phrases yielded 1357 articles in total. After the exclusion of duplicated and non-relevant ones based on the titles, 45 were selected for possible inclusion. The abstracts of these studies were reviewed, which results in exclusion of another 15 abstracts. After reviewing the manuscripts of the selected abstracts, only 12 articles were found to fit our inclusion criteria. Another three articles were found to be eligible through reviewing the references in the previously red manuscripts. Finally, 15 were included in the analysis.
Figure 1.
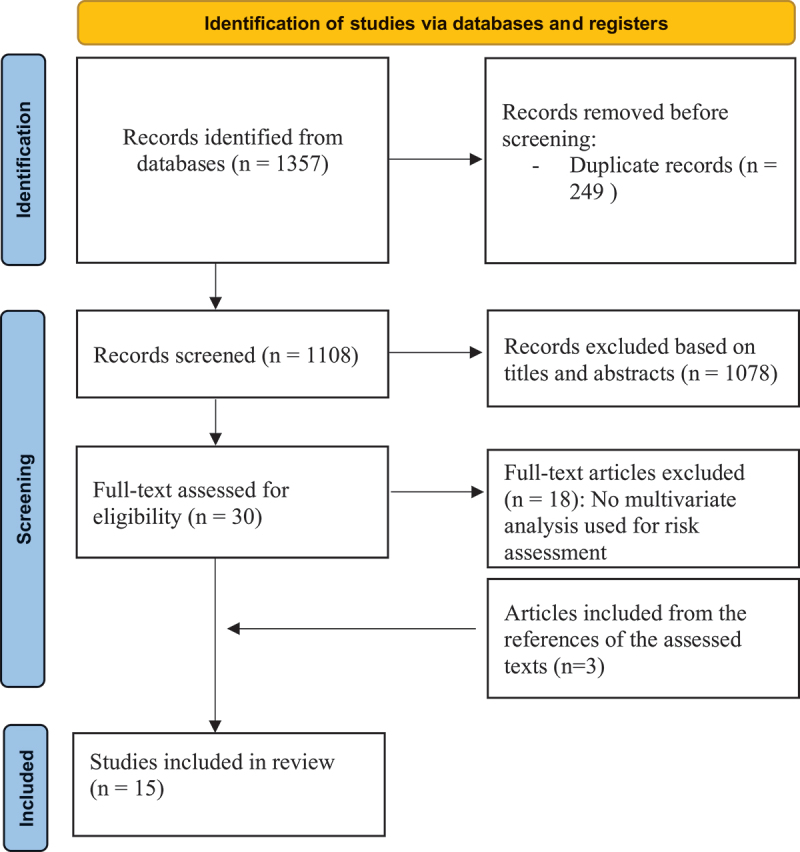
PRISMA flow diagram.
Table 1 provides detailed information on the study characteristics and the results of multivariate analysis [12–26]. The included studies were observational, retrospective and published between 2013 and 2022. Of the 15 articles, 6 were open radical cystectomy (ORC) series, 7 contained both open and robotic-assisted (RARC) and 2 were RARC only. The sample size in the studies varied between 135 and 2,888 and included a total of 13, 481 patients. The type of ureteroenteric anastomosis was mentioned in 12 articles, Bricker was the predominant choice (n = 7), followed by Wallace (n = 1) and both techniques were used in four studies. The median follow-up differed widely between the studies, ranging from 8.2 months to 12.4 years. There was a wide discrepancy in the stricture rates among the studies (2.6–17%). The median time to stricture diagnosis was similar across the studies lying between 3 and 10 months, only one study reported a later stricture diagnosis at the median time of 15.8 months [13]. Left-sided ureteral stricture was the most predominant (42.2–66%), followed by right-sided stricture (9.5–44.7%) and bilateral involvement (5–42%). Patient presentation was reported in only six articles, 70–98% of patients were presented with symptoms in four studies, while in two studies most patients were asymptomatic and were diagnosed during routine follow-up.
Table 1.
Studies discussed risk factors associated with ureteroenteric stricture development.
| Authors, year | Patients No. | Type of diversion | Technique of ureteroenteric anastomosis | UES Incidence |
Median Follow-up |
Median time to diagnosis | Affected side | Presentation | Risk factors for stricture |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al. 2022 [22] | 180 -ORC:75 -RARC:105 |
IC and ONB | Bricker and Wallace | 12.2% | NR | 11 mo. | Left: 45.4% Right: 40.9% Bilateral: 13.6% |
NR | Blood transfusion (HR 0.144, 95% CI: 0.046–0.451; P = 0.001) Extracorporeal diversion (HR 3.39, 95%CI: 1.24–9.28, P = 0.017) Postoperative UTI (HR 3.62, 95% CI: 1.4–9.3,P = 0.007) |
| Krafft et al. 2021 [23] |
135 | IC, ONB and CCD | Bricker and Wallace | 15.5% | 14 mo. | 3 mo. | Left: 47.6% Right: 9.5% Bilateral: 42% |
NR | Preoperative chemotherapy (OR 9.7, 95%CI:2–46.2; P = 0.004) Clavien ≥3 complication (OR 4, 95%CI: 1.36–11.82, P = 0.012) |
| Reesink et al. 2021 [24] | 279 -ORC:192 -RARC (ICUD):87 |
IC and ONB | Bricker | 17% -ORC: 13% -RARC:25.3% |
50 mo. | 3 mo. | Left: 48.9% Right: 21.3% Bilateral: 29.8% |
Symptomatic (40.4%) | RARC (HR 2.36, 95% CI: 1.32–4.2; P = 0.004) |
| Faraj et al. 2021 [25] |
573 -ORC:337 -RARC (ICUD):39 -RARC (ECUD):197 |
IC and ONB | Bricker and Wallace | 8.2% -ORC:8% -RARC (ICUD):1.6% -RARC (ECUD):9.6% |
ORC:55 mo. RARC (ICUD):71 mo. RARC (ECUD):70 mo. |
5 mo. | Left: 51.1% Right: 44.7% Bilateral: 4.3% |
Symptomatic (98%) | BMI (HR 1.07, 95% CI: 1.02–1.13; P = 0.003). |
| Ericson et al. 2020 [26] |
968 -ORC:279 -RARC (ICUD):307 -RARC (ECUD):382 |
IC, ONB and CCD | Bricker | 11.3% -ORC:9.3% -RARC (ICUD):13% -RARC (ECUD):11.3% |
ORC:22 mo. RARC (ICUD):16.3 mo. RARC (ECUD):10.6 mo. |
4.67 mo. | Left: 50.5% Right: 31.2% Bilateral: 17.4% |
NR | RARC (ICUD) (HR 1.66, 95% CI: 1–2.74,p = 0.05) BMI (HR 1.05, 95% CI: 1.02–1.09; P < 0.01). |
| Yang et al, 2020 [12] |
2.285 | IC and ONB | Bricker and Wallace | 8% | 10.7 years | 7 mo. | Left: 53% Right: 28% Bilateral: 18% |
Symptomatic (77%) | BMI (OR 1.06, 95% CI: 1.02–1.09;P = 0.0009). Clavien ≥3 complication (OR 2.85, 95%CI: 1.90–4.28; P < 0.0001) |
| Benson et al, 2020 [13] | 418 | ONB | NR | 8.9% | Mean: 57 mo. | Mean time: 15.8 mo. | Left: 65% Right: 27% Bilateral: 14% |
NR | Perioperative UTI (HR 2.4, 95% CI 1.09–5.09, p = 0.03) Recurrent UTI (HR 5.1, 95% CI 2.4–11,p < 0.001) History of RT (HR 11.5, 95% CI 2.5–53.4,p = 0.002) |
| Goh et al, 2019 [14] |
1781 -ORC:1449 -RARC:332 |
Continent and incontinent UD | NR | ORC: 8.3% RARC: 13.9% |
At 2 years | NR | NR | NR | RARC (HR 1.64, 95%CI 1.23–2.19) Preoperative hydronephrosis (HR 1.51, 95%CI 1.17–1.94) |
| Hosseini et al, 2018 [15] | 371 (RARC +ICUD) | IC and ONB | Wallace | 6.5% | 33 mo. | 165 d. | Left: 63% Right: 29% Bilateral: 8% |
NR | Urinary leak (HR 5.17, 95% CI 1.85–14.41,p = .002) |
| Katherine et al, 2018 [16] | 2.888 | Continent and incontinent UD | NR | 4.2% | 32 mo. | NR (80% within 1st 3 years) |
Left: 53.6% Right: 40.6% Bilateral: 5.7% |
Symptomatic (76.4%) | BMI (HR 1.04, 95% CI 1.01–107, p < 0.0001) Male gender (HR 1.81, 95% CI 1.13–2.91,p = 0.014) Prior abdominal surgery (HR 3.40, 95%CI 1.84–6.28, p = 0.013) ASA III/IV (HR 1.88, 95% CI 1.2–2.9, p = 0.005) |
| Ahmed et al, 2017 [17] |
440 (RARC) | IC | Bricker | 13% | 17 mo. | 5 mo. | Left: 45% Right: 29% Bilateral: 25% |
Symptomatic (25%) | BMI (OR 1.07, 95% CI 1.01–1.13, p = 0.02) ICUD (OR 3.28, 95% CI 1.41–7.61, p = 0.006) UTI (OR 2.68, 95% CI 1.31–5.49, p = 0.007) Urinary leak (OR 3.85, 95% CI 1.05–14.1,p = 0.04) Length of the right resected ureter (OR 0.66, 95% CI 0.50–0.88, p = 0.004) ≥ 30 d eGFR (OR 0.85, 95%CI 0.74–0.98,p = 0.03) |
| Shah et al, 2015 [18] |
1964 | IC, ONB and CCD | Bricker | 2.6% | 12.4 years | 10 mo. | Left: 66% Right: 29% Bilateral: 5% |
Symptomatic (70%) |
No independent predictor for stricture |
| Richards, 2014 [19] | 463 -ORC: 439 -RARC: 24 |
IC and ONB | Bricker | 12.5% | 459 d. | Right ureter: 235 d. Left ureter: 232 d. |
Left: 56.9% Right: 24.1% Bilateral: 19% |
NR | Clavien ≥3 complication (HR2.11,1.01–4.40) Urine leak (HR 3.37, 1.08–10.46) |
| Anderson et al, 2013 [20] | 478 -ORC:375 -RARC:103 |
IC, ONB and CCD | Bricker | ORC: 8.5% RARC: 12.6% |
8.2 mo. | 5.3 mo. | Left: 42.2% Right: 24.4% Bilateral: 33.3% |
NR | No independent predictor for stricture |
| Large et al, 2013 [21] |
258 | IC and ONB | Bricker | 16.6% | -Running sututres gp: 351 d. -Interrupted sututresgp: 497 d. |
-Running sututres gp: 289 d. -Interrupted sututres gp.: 213 days |
Left: 46.5% Right: 34.8% Bilateral: 18.6% |
NR | Postoperative UTI (HR 2.4, 95% CI 1.2–5.1, p = 0.02) Running technique (HR 1.9, 95% CI 1.0–3.7,p = 0.05) |
IC= Ileal conduit; ONB=Orthotopic neobladder; BMI=Body mass index; ORC=open radical cystectomy; RARC=Robotic-assisted radical cystectomy; UD=Urinary diversion; ICUD=Intracorporeal urinary diversion; UTI=Urinary tract infection; RT=Radiotherapy; NR=Not reported.
Of 15 articles, a significant relationship between many factors and UES was demonstrated in 13 studies, on the other hand, no correlation between patient or disease factors specific and the development of UES could be found in two articles. The most frequently mentioned stricture risk factor was BMI that was reported in five articles, followed by postoperative perioperative urinary tract infection (UTI) in four articles. The occurrence of major postoperative complications (Clavien-Dindo ≥3), urinary leakage and RARC each of which was reported three times. In two studies intracorporeal UD (ICUD) with associated with higher stricture risk adjusted to extracorporeal UD (ECUD). Otherwise, the link between many other factors and UES was observed only one time: these factors were either related to patient characteristics including the male gender, ASA score III-IV, prior abdominal surgery, history of RT, history of chemotherapy, preoperative hydronephrosis, postoperative renal function and node positive disease or related to the surgery including (ECUD), the use running sutures in creating the ureteroileal anastomosis the length of resected ureter, and blood transfusion (Table 1).
Discussion
Demographic and clinicopathological factors
The patient demographic and clinical features are important determinants for the perioperative outcome of RC and UD [28,29]. These factors are static and cannot be changed. However, their identification is important to characterize high-risk patients who should be treated in high-volume centers with experienced surgeons. Increasing patient age and comorbidities were associated with early postoperative complications in many studies, in addition, their negative influence on the long-term renal function is known [9,29]. None of the published studies showed an association between age and UES development; nevertheless, the poor physical condition of the patient, represented by an increase in the ASA (American Society of Anesthesiologists) score, was associated with two times stricture risk in one study [16]. In the same study, Katherine et al. found a link between male gender and UES, however, this association may be influenced by the high proportion of men in the study, which represents 75% of their cohort [16].
It is obvious that the high BMI is a main risk factor for UES among the patient characteristics. Five studies in our review reported the associated hazardous effect [12,16,17,25,26]. Moreover, some studies that did not observe this association either consisted of a relatively small sample size [15,21] or did not include the BMI in the analysis at all [14]. The left ureter is usually brought to the right side under the sigmoid mesentery before the ureteroenteric anastomosis; therefore, enough ureteral length is required, which is difficult to achieve in obese patients due to high abdominal obesity. Consequently, the left ureter may be exposed to extensive dissection or tension during anastomosis, factors which increase the incidence of UES. This explains also the well-known predominance of left-sided stricture. In addition to previous surgical problems, the healing process in obese patients is not optimal and impaired by associated morbidities (e.g. diabetes mellitus) or some mediators that detach from the mesenteric fat tissue [30].
Other factors that increase surgical difficulty and predispose to intra- and posoperative complications include previous exposure to major abdominal surgery and RT. In the largest series in our review: the risk of developing a stricture within 10 years was 1.9% in patients without prior abdominal surgery compared to 9.3% in patients with prior surgery [16]. Prior abdominal surgery is associated with adhesions, which in turn distorts the tissue planes and consequently predisposes to high incidence of perioperative complications like anastomotic leak and abdominal infections, factors which increase the risk of inflammatory stricture. Also, the distortion of the tissue planes increases the possibility of ureteral devascularization during dissection and subsequently the incidence of ischemic stricture. Radiation induces tissue ischemia and fibrosis, which could affect the healing process of the ureteral anastomosis. This theory seemed logical, but the data in the literature are still contradictory. Bensen et al found that preoperative RT is associated nearly with 11 times higher risk for stricture formation [13]; however, only 2% of the patients received the preoperative therapy, which is small proportion that could affects the results. Yang et al, examined 2,285 patients, with 12.5% of patients receiving RT, which is considered an appropriate number; in the univariate analysis, RT was not a risk factor (OR 1.25, CI: 0.80–1.87, p = 0.35) [12]. Despite the lack of clear evidence from the literature, surgeons should be careful and try to select a viable ileal and ureteral segment to be used for diversion outside the irradiation field or to use directly a transverse colonic segment for diversion.
Patients undergoing UD can present with unilateral or bilateral hydronephrosis due to tumor obstruction or a defunctionalized bladder. Preoperative hydronephrosis is an independent predictor of UES in one study [14]. This could be explained by the pathological changes that occur in the ureteral wall following long-standing obstruction and the associated toxic effect of recurrent infection. These changes could impair ureteral elasticity and the healing process. Hautmann et al published an important report that is not included in our review, as the hydronephrosis was not adjusted to the other possible confounding factors. The authors reviewed the data of 953 patients with neobladder. They reported 19.3% stricture rate at 10 years in patients with preoperatively obstructed ureters versus only 6.4% for patients with non-dilated ureters [10].
Only one study showed a correlation between pathological features and UES development: patients with node positive disease were more likely to develop stricture. The authors explained that neovascularization associated with nodal metastasis can alter the blood supply to the ureters and lead to scarring. Another explanation, which was not investigated in the study, is that aggressive disease and lymphadenopathy increase the difficulty of ureteral dissection and the possibility of devascularization [16].
Surgery related factors
Both ORC and RARC are comparable in the literature with regard to the oncological outcome and perioperative surgical complications [31]. Whether the surgical approach influences the long-term functional outcome or not is still questionable. Some urologists suggested that RARC would be associated with higher incidence of UES. Seven studies in our review have included both open and RARC cohorts, three of them showed high stricture rate among in the robotic group [14,24,26]. This higher stricture rate in RARC may be related to the inclusion of cases operated on early in the surgeon’s experience, as the lack of tactile sense and higher magnification may increase devascularization of the ureter. For example, Goh et al. found that a high hospital volume in the adjusted analysis was protective against stricture, which means that with increasing experience of the surgeon the result would be comparable (Goh et al., 2020). A promising technique to reduce the incidence of UES is the use of indocyanine green (ICG) with near-infrared fluorescence to identify ureteral blood supply during distal ureterectomy. Ahmadi et al used ICG in 47 patients and compared them with 132 patients without ICG. The length of excised ureter was significantly higher in the ICG group than in the other group, and no UES developed in the ICG group at one year compared to 10% in the non-ICG group (p = 0.020) [32]. Long-term follow-up studies comparing both open and robotic techniques and evaluating the effects of using near-infrared fluorescence are still needed.
The best technique for ureteroenteric anastomosis has been a question in many studies over the last 30 years, currently there is consensus that antireflux anastomosis is associated with a higher incidence of UES without having a positive effect on long-term renal function [5,33]. With respect to the refluxing techniques, the only published meta-analysis showed a comparable stricture rate between Bricker (2.9%) and Wallace (1.9%) (p = 0.57) [34]. In the present review, the effect of the technique on the outcome of the anastomosis was not proved. Regardless of the technique, anastomosis can be performed either with running or interrupted sutures, this was investigated in two studies of our search: Large et al. evaluated 258 patients who underwent various diversions. The interrupted anastomosis was performed with 4–0 polyglactin sutures in 149 patients and with the same suture in 109 patients with running anastomosis. No significant difference in surgical time was found between the two groups. In the multivariate analysis, the running suture technique was associated with stricture formation [21]. In a later study with a larger number of patients and longer follow-up time, the running suture technique did not appear to affect the incidence of UES [19].
The distal end of the ureter is usually compromised during dissection and should be resected. Surgeons want also to adjust the length to avoid redundancy of the ureter or anastomosis under tension. Two studies have investigated whether the length of the resected ureter affects the outcome of anastomosis or not. Richards et al added the length of the ureter in cystectomy specimens plus the separately resected distal ureter in 463 patients of whom 12.5% developed UES. They found no significant correlation between resected length and stricture formation [19]. In contrast, the other study demonstrated the presence of this association. The study included 440 patients undergoing RARC and 13% had UES. The stricture group had a significantly shorter length of resected ureters (left side 15 vs. 20 mm, p = 0.02, and right side 15 vs. 22, p < 0.001). In multivariate analysis, the association was significant only on the right side. Finally, the authors concluded that further studies are needed as the length of resected ureter only not enough due to the variations between the patients as regard their height and the original ureteral length [17].
In most of the included studies, ureteral stents were routinely used for a period of 1 to 2 weeks postoperatively, whereas three studies did not mention whether or not diversions were stented. Therefore, the effect of stent use on the outcome of ureteroenteric anastomosis was not examined in the analysis of these studies. Few studies compared stricture rates in stented and nonstented patients, and the results were summarized in a recently published meta-analysis. There was no statistically significant difference in the rate of urinary leakage or development of strictures between the two groups. Future prospective randomized studies on this topic are still needed [35].
3 Postoperative complications
Some early postoperative complications may predispose to UES development, such as urinary or intestinal leakage, abdominal abscess and UTI. These factors can trigger an inflammatory process at the anastomotic site and predispose to scar and UES formation. The occurrence of Clavian grade complication ≥ 3 was associated with a three times higher risk of stricture formation in one study and four times in another one [12,23], while in one study it was a predictor for stricture on the left side only [19]. Katherine et al in the largest study observed also this relationship, but only in the univariate analysis [16]. Urinary leakage alone was seen to be an independent predictor for UES in three studies. The first was conducted by Richards et al. 2014 in patients undergoing ORC and UD. All anastomoses were Bricker, stented and the anastomoses were tested for water tightness. Twenty percent of patients who developed later right UES had a history of urinary leakage postoperative vs. 6.3% in those free from strictures (p = 0.01). In the adjusted analysis, urinary leak was associated with an almost triple risk of developing a right-sided stricture [19]. This significant association was demonstrated also in two robotic series with ICUD [15,17]. The authors of both studies recommended great efforts to ensure adequate watertight anastomosis to minimize the risk of postoperative urinary leakage. Early occurrence of UTI had an almost three times higher risk of stricture in four studies [13,15,17,22]. In addition, Hosseini et al. found that recurrent UTI over a long period of time carry a risk of developing a stricture twice as high as one attack of perioperative UTI, but the authors could not determine if the recurrent UTIs are possible cause of stricture or are the results of pre-existing UES [15].
Conclusion
Although risk factors of UES development have been discussed in many studies, the exact cause is still unclear. This is due to the presence of some non-measurable factors, mainly surgical and technical that could directly influence the outcome of the anastomosis. Ischemic strictures are the most common form resulting from excessive dissection or RT. Great efforts should be made to maintain the blood supply to the ureter by gentle handling, avoiding unnecessary dissections and minimizing the use of electrocautery. Obese patients are considered a high-risk group for ischemic stricture and should be operated on by experienced surgeons. Inflammatory strictures are less common and resulted mainly from a complicated postoperative course. Special attention should be paid to meticulous surgical technique to avoid urinary and intestinal leakage. Wide spatulation of the ureter, mucosal-to-mucosal suturing and watertight stented anastomosis are basic principles for minimizing the urinary leakage and stricture. Preoperative UTI in patients undergoing UD should be treated, careful follow-up, early detection and treating new episodes of UTI are mandatory in the early postoperative course.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55(1):164–174. doi: 10.1016/j.eururo.2008.07.031 [DOI] [PubMed] [Google Scholar]
- [2].Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 2009;55(1):177–185. doi: 10.1016/j.eururo.2008.07.018 [DOI] [PubMed] [Google Scholar]
- [3].Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol. 2009;182(3):914–921. doi: 10.1016/j.juro.2009.05.032 [DOI] [PubMed] [Google Scholar]
- [4].Nieuwenhuijzen JA, de Vries RR, Bex A, et al. Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol. 2008;53(4):834–844. doi: 10.1016/j.eururo.2007.09.008 [DOI] [PubMed] [Google Scholar]
- [5].Hautmann RE, de Petriconi RC, Volkmer BG.. 25 years of experience with 1,000 neobladders: long-term complications. J Urol. 2011;185(6):2207–2212. doi: 10.1016/j.juro.2011.02.006 [DOI] [PubMed] [Google Scholar]
- [6].Shimko MS, Tollefson MK, Umbreit EC, et al. Long-term complications of conduit urinary diversion. J Urol. 2011;185(2):562–567. doi: 10.1016/j.juro.2010.09.096 [DOI] [PubMed] [Google Scholar]
- [7].Jin XD, Roethlisberger S, Burkhard FC, et al. Long-term renal function after urinary diversion by ileal conduit or orthotopic ileal bladder substitution. Eur Urol. 2012;61(3):491–497. doi: 10.1016/j.eururo.2011.09.004 [DOI] [PubMed] [Google Scholar]
- [8].Gilbert SM, Lai J, Saigal CS, et al. Downstream complications following urinary diversion. J Urol. 2013;190(3):916–922. doi: 10.1016/j.juro.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eisenberg MS, Thompson RH, Frank I, et al. Long-term renal function outcomes after radical cystectomy. J Urol. 2014;191(3):619–625. doi: 10.1016/j.juro.2013.09.011 [DOI] [PubMed] [Google Scholar]
- [10].Hautmann RE, de Petriconi R, Kahlmeyer A, et al. Preoperatively dilated ureters are a specific risk factor for the development of ureteroenteric strictures after open radical cystectomy and ileal neobladder. J Urol. 2017;198(5):1098–1106. doi: 10.1016/j.juro.2017.05.069 [DOI] [PubMed] [Google Scholar]
- [11].Katkoori D, Samavedi S, Adiyat KT, et al. Is the incidence of uretero-intestinal anastomotic stricture increased in patients undergoing radical cystectomy with previous pelvic radiation? BJU Int. 2010;105(6):795–798. doi: 10.1111/j.1464-410X.2009.08835.x [DOI] [PubMed] [Google Scholar]
- [12].Yang DY, Boorjian SA, Westerman MB, et al. Persistent, long-term risk for ureteroenteric anastomotic stricture formation: the case for long term follow-up. Transl Androl Urol. 2020;9(1):142–150. doi: 10.21037/tau.2019.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benson CR, Ajay D, Barrett-Harlow BL, et al. Ureteroenteric anastomosis in orthotopic neobladder creation: do urinary tract infections impact stricture rate? World J Urol. 2021;39(4):1171–1176. doi: 10.1007/s00345-020-03266-0 [DOI] [PubMed] [Google Scholar]
- [14].Goh AC, Belarmino A, Patel NA, et al. A population-based study of ureteroenteric strictures after open and robot-assisted radical cystectomy. Urology. 2020;135:57–65. doi: 10.1016/j.urology.2019.07.054 [DOI] [PubMed] [Google Scholar]
- [15].Hosseini A, Dey L, Laurin O, et al. Ureteric stricture rates and management after robot-assisted radical cystectomy: a single-centre observational study. Scand J Urol. 2018;52(4):244–248. doi: 10.1080/21681805.2018.1465462 [DOI] [PubMed] [Google Scholar]
- [16].Katherine AA, Emily AV, Gillian S, et al. Predictors of benign uretero-enteric anastomotic strictures after radical cystectomy and urinary diversion. Urology. 2018;144:225–229. doi: 10.1016/j.urology.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahmed YE, Hussein AA, May PR, et al. Natural history, predictors and management of ureteroenteric strictures after robot assisted radical cystectomy. J Urol. 2017;198(3):567–574. doi: 10.1016/j.juro.2017.02.3339 [DOI] [PubMed] [Google Scholar]
- [18].Shah SH, Movassaghi K, Skinner D, et al. Ureteroenteric strictures after open radical cystectomy and urinary diversion: the university of Southern California experience. Urology. 2015;86(1):87–91. doi: 10.1016/j.urology.2015.03.014 [DOI] [PubMed] [Google Scholar]
- [19].Richards KA, Cohn JA, Large MC, et al. The effect of length of ureteral resection on benign ureterointestinal stricture rate in ileal conduit or ileal neobladder urinary diversion following radical cystectomy. Urol Oncol. 2015;33(2):65.e1–8. doi: 10.1016/j.urolonc.2014.05.015 [DOI] [PubMed] [Google Scholar]
- [20].Anderson CB, Morgan TM, Kappa S, et al. Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter? J Urol. 2013;189(2):541–547. doi: 10.1016/j.juro.2012.09.034 [DOI] [PubMed] [Google Scholar]
- [21].Large MC, Cohn JA, Kiriluk KJ, et al. The impact of running versus interrupted anastomosis on ureterointestinal stricture rate after radical cystectomy. J Urol. 2013;190(3):923–927. doi: 10.1016/j.juro.2013.02.091 [DOI] [PubMed] [Google Scholar]
- [22].Liu L, Chen M, Li Y, et al. Technique selection of bricker or wallace ureteroileal anastomosis in ileal conduit urinary diversion: a strategy based on patient characteristics. Ann Surg Oncol. 2014;21(8):2808. doi: 10.1245/s10434-014-3591-z [DOI] [PubMed] [Google Scholar]
- [23].Krafft U, Mahmoud O, Hess J, et al. Comparative analysis of bricker versus wallace ureteroenteric anastomosis and identification of predictors for postoperative ureteroenteric stricture. Langenbecks Arch Surg. 2022;407(3):1233–1240. doi: 10.1007/s00423-021-02413-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reesink DJ, Gerritsen SL, Kelder H, et al. Evaluation of ureteroenteric anastomotic strictures after the introduction of robot-assisted radical cystectomy with intracorporeal urinary diversion: results from a large tertiary referral center. J Urol. 2021;205(4):1119. doi: 10.1097/JU.0000000000001518 [DOI] [PubMed] [Google Scholar]
- [25].Faraj KS, Rose KM, Navaratnam AK, et al. Effect of intracorporeal urinary diversion on the incidence of benign ureteroenteric stricture after cystectomy. Int J Urol. 2021;28(5):593–597. doi: 10.1111/iju.14521 [DOI] [PubMed] [Google Scholar]
- [26].Ericson KJ, Thomas LJ, Zhang JH, et al. Uretero-enteric anastomotic stricture following radical cystectomy: a comparison of open, robotic extracorporeal, and robotic intracorporeal approaches. Urology. 2020;144:130–135. doi: 10.1016/j.urology.2020.06.047 [DOI] [PubMed] [Google Scholar]
- [27].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boström PJ, Laato M, Nurmi M. Risk factors for mortality and morbidity related to radical cystectomy. BJU Int. 2009;103(2):191–196. doi: 10.1111/j.1464-410X.2008.07889.x [DOI] [PubMed] [Google Scholar]
- [29].Hollenbeck BK, Miller DC, Taub D, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174(4 Pt 1):1231–1237. discussion 1237. doi: 10.1097/01.ju.0000173923.35338.99 [DOI] [PubMed] [Google Scholar]
- [30].Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue–emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2(6):273–280. doi: 10.1038/ncpgasthep0186 [DOI] [PubMed] [Google Scholar]
- [31].Rai BP, Bondad J, Vasdev N, et al. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst Rev. 20194:4. doi: 10.1002/14651858.CD011903.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ahmadi N, Ashrafi AN, Hartman N, et al. Use of indocyanine green to minimise uretero-enteric strictures after robotic radical cystectomy. BJU Int. 2019;124(2):302–307. doi: 10.1111/bju.14733 [DOI] [PubMed] [Google Scholar]
- [33].Harraz AM, Mosbah A, Abdel-Latif M, et al. Impact of the type of ureteroileal anastomosis on renal function measured by diuretic scintigraphy: long-term results of a prospective randomized study. BJU Int. 2014;114(2):202–209. doi: 10.1111/bju.12511 [DOI] [PubMed] [Google Scholar]
- [34].Davis NF, Burke JP, McDermott T, et al. Bricker versus wallace anastomosis: A meta-analysis of ureteroenteric stricture rates after ileal conduit urinary diversion. Can Urol Assoc J. 2015;9(5–6):E284–90. doi: 10.5489/cuaj.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Peng YL, Ning K, Wu ZS, et al. Ureteral stents cannot decrease the incidence of ureteroileal anastomotic stricture and leakage: A systematic review and meta-analysis. Int J Surg. 2021;93:106058. doi: 10.1016/j.ijsu.2021.106058 [DOI] [PubMed] [Google Scholar]


