Abstract
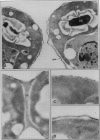
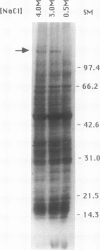
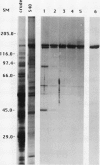
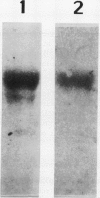
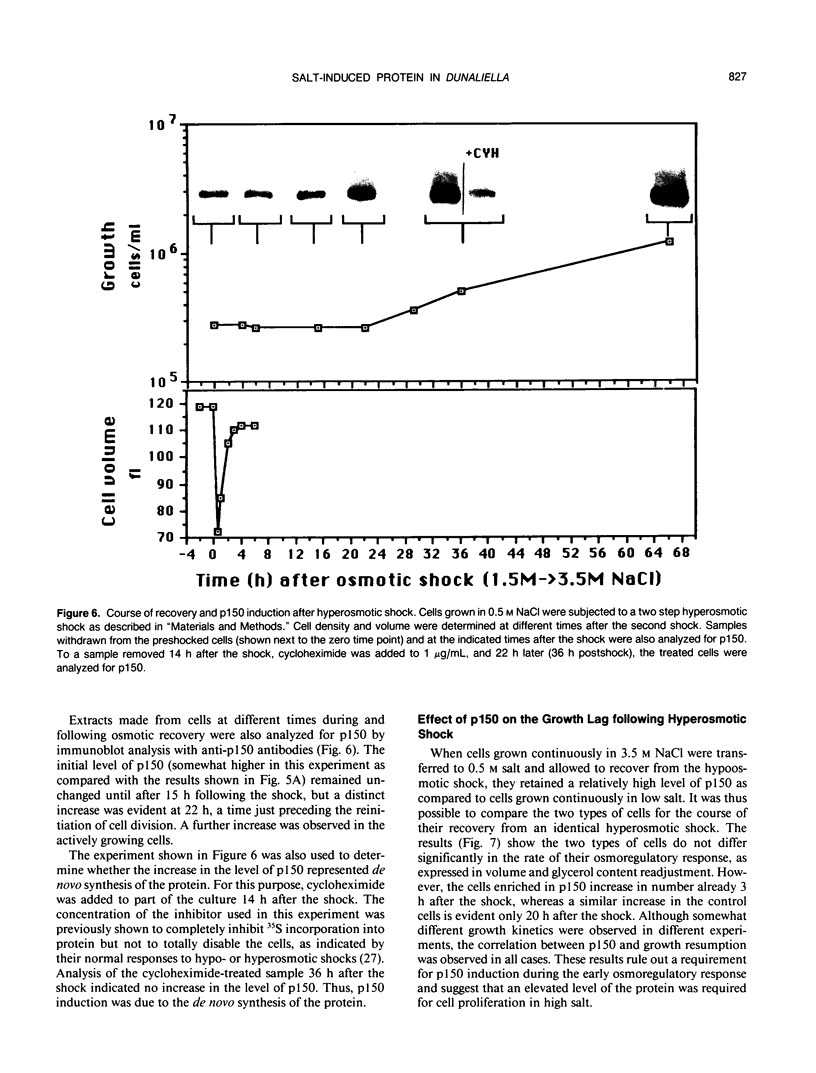
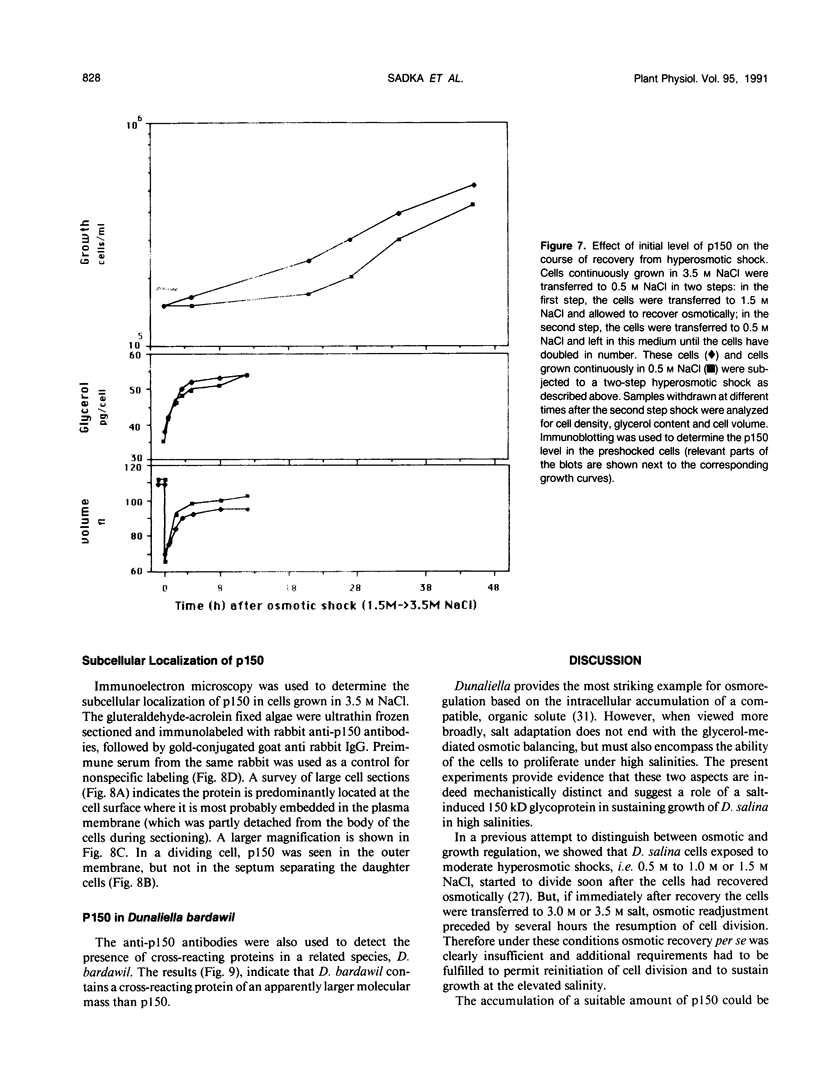
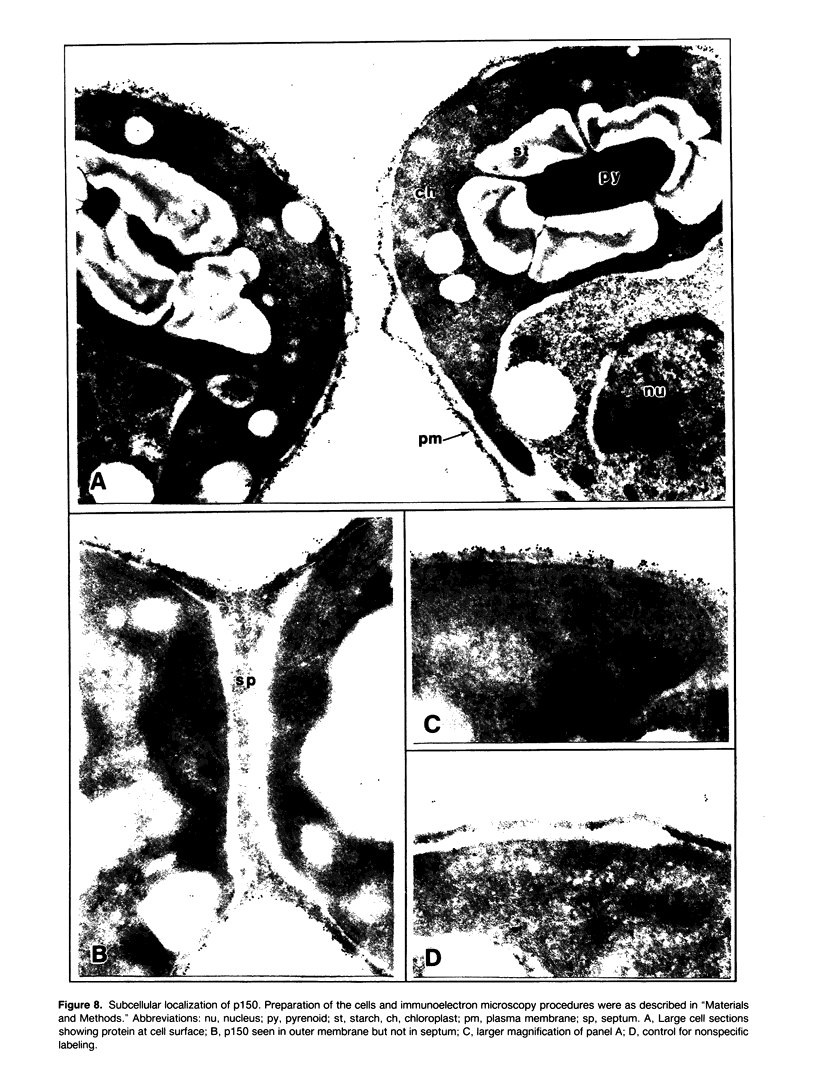
Dunaliella salina is an extremely halotolerant, unicellular, green alga lacking a rigid cell wall. Osmotic adaptation to high salinities is based on the accumulation of glycerol. To uncover other functions responsible for halotolerance, protein profiles of algae continuously grown in different salinities were compared. A 150 kilodalton protein (p 150) increased in amount with salt concentration. Furthermore, when the cells were subjected to drastic hyperosmotic shocks, p150 started to rise long after completion of the osmotic response but coincident with reinitiation of cell proliferation. Cells with an initially higher level of p150 resumed growth faster than cells with a lower level of the protein. Addition of cycloheximide early after hyperosmotic shock prevented the rise in p150, indicating this rise was due to de novo synthesis of the protein. These observations suggest that p150 is a saltinduced protein required for proliferation of the cells in saline media. p150 was purified to homogeneity and found to be a detergent-soluble glycoprotein. Polyclonal antibodies against p150 recognized a single protein component in D. salina crude extracts. A high Mr cross-reacting protein was also observed in another Dunaliella strain, D. bardawil. Immunoelectron microscopy localized p150 to the cell surface.
Full text
PDF


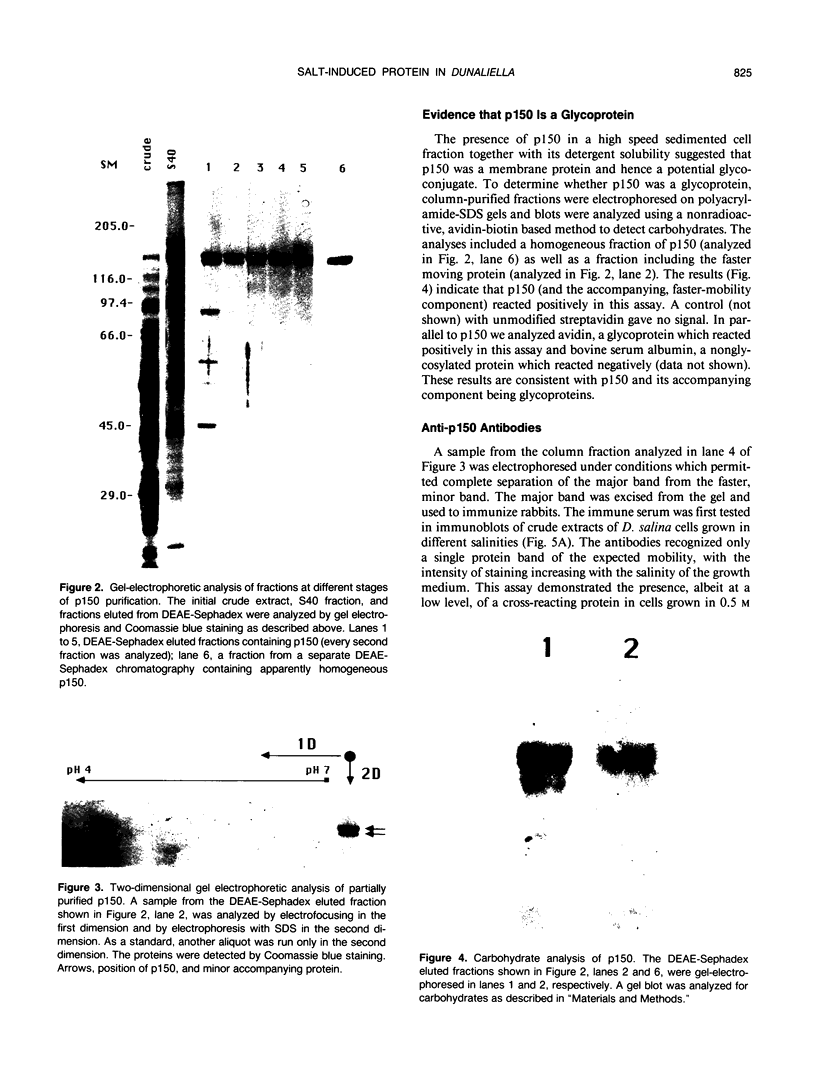
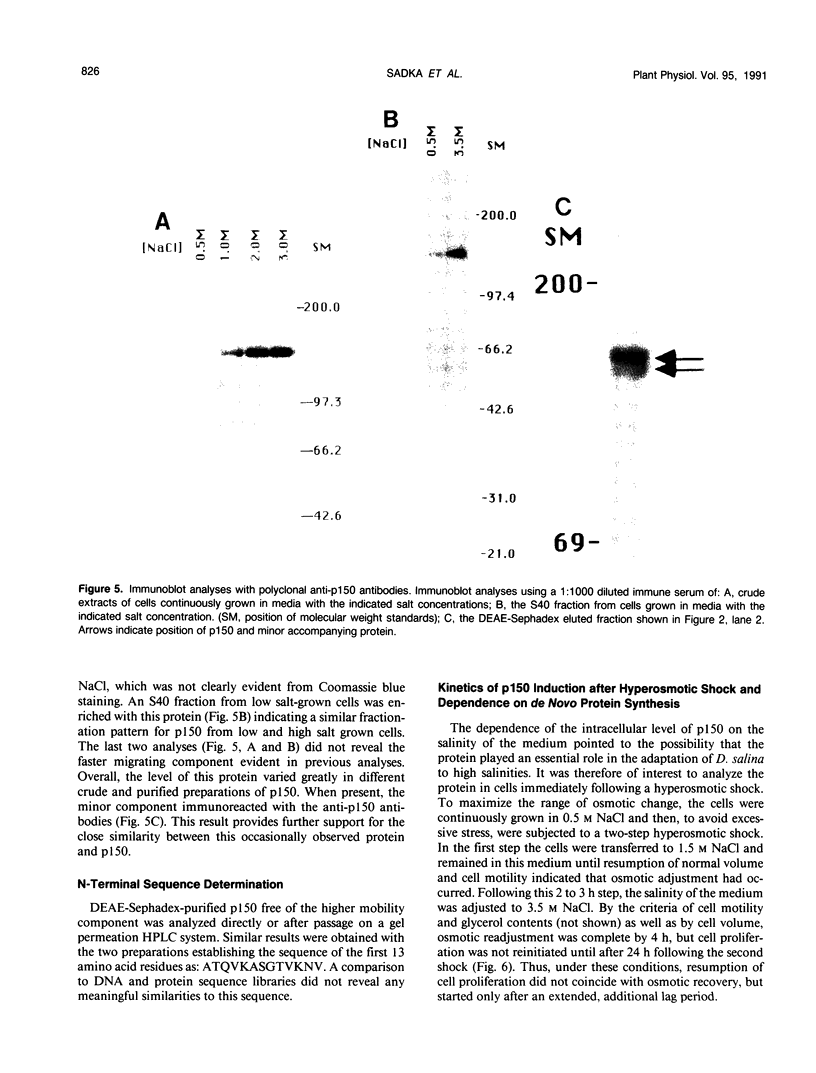


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Ben-Hur H., Wilchek M. Enzyme-based detection of glycoproteins on blot transfers using avidin-biotin technology. Anal Biochem. 1987 Feb 15;161(1):123–131. doi: 10.1016/0003-2697(87)90661-0. [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol. 1983;37:95–119. doi: 10.1146/annurev.mi.37.100183.000523. [DOI] [PubMed] [Google Scholar]
- Berman J., Gershoni J. M., Zamir A. Expression of nitrogen fixation genes in foreign hosts. Assembly of nitrogenase Fe protein in Escherichia coli and in yeast. J Biol Chem. 1985 May 10;260(9):5240–5243. [PubMed] [Google Scholar]
- Borowitzka L. J., Kessly D. S., Brown A. D. The salt relations of Dunaliella. Further observations on glycerol production and its regulation. Arch Microbiol. 1977 May 13;113(1-2):131–138. doi: 10.1007/BF00428592. [DOI] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Alfinito S. H. Proteins Produced during Salt Stress in Tobacco Cell Culture. Plant Physiol. 1984 Mar;74(3):506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K., Dupont F. M. The effects of salt stress on polypeptides in membrane fractions from barley roots. Plant Physiol. 1988 Dec;88(4):1263–1273. doi: 10.1104/pp.88.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. The effects of salt on the pattern of protein synthesis in barley roots. Plant Physiol. 1987 Mar;83(3):517–524. doi: 10.1104/pp.83.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen P. L., Collins J. H. Tryptic and chymotryptic cleavage sites in sequence of alpha-subunit of (Na+ + K+)-ATPase from outer medulla of mammalian kidney. Biochim Biophys Acta. 1986 Sep 11;860(3):570–576. doi: 10.1016/0005-2736(86)90555-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazard D., Tal N., Rubinstein M., Khen M., Lancet D., Zupko K. Identification and biochemical analysis of novel olfactory-specific cytochrome P-450IIA and UDP-glucuronosyl transferase. Biochemistry. 1990 Aug 14;29(32):7433–7440. doi: 10.1021/bi00484a012. [DOI] [PubMed] [Google Scholar]
- Lers A., Biener Y., Zamir A. Photoinduction of Massive beta-Carotene Accumulation by the Alga Dunaliella bardawil: Kinetics and Dependence on Gene Activation. Plant Physiol. 1990 Jun;93(2):389–395. doi: 10.1104/pp.93.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowski C. B., Olson S. W., Piepenbrock M., Schmitt J. M., Bohnert H. J. Time Course of mRNA Induction Elicited by Salt Stress in the Common Ice Plant (Mesembryanthemum crystallinum). Plant Physiol. 1989 Mar;89(3):811–816. doi: 10.1104/pp.89.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ramagopal S. Differential mRNA transcription during salinity stress in barley. Proc Natl Acad Sci U S A. 1987 Jan;84(1):94–98. doi: 10.1073/pnas.84.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978 Jun;63(3):287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland R. H. Mechanisms of halotolerance in microorganisms. Crit Rev Microbiol. 1987;14(4):311–356. doi: 10.3109/10408418709104443. [DOI] [PubMed] [Google Scholar]
- von Gromoff E. D., Treier U., Beck C. F. Three light-inducible heat shock genes of Chlamydomonas reinhardtii. Mol Cell Biol. 1989 Sep;9(9):3911–3918. doi: 10.1128/mcb.9.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]