Abstract
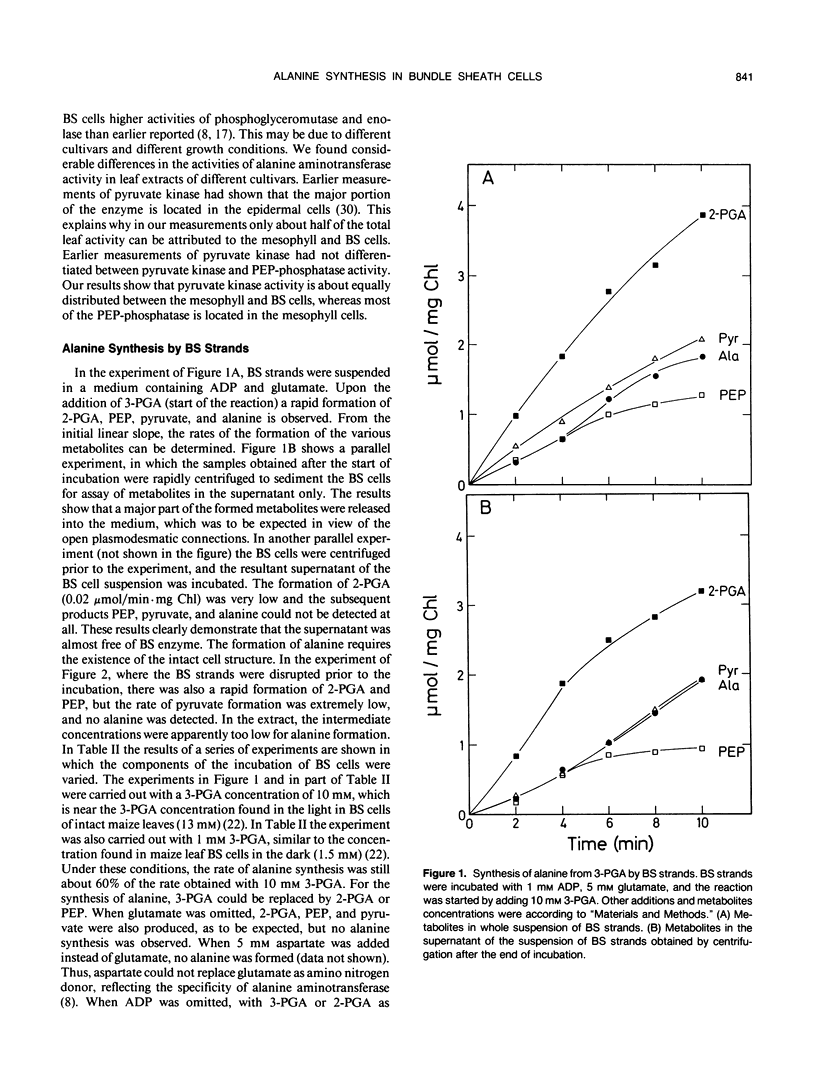
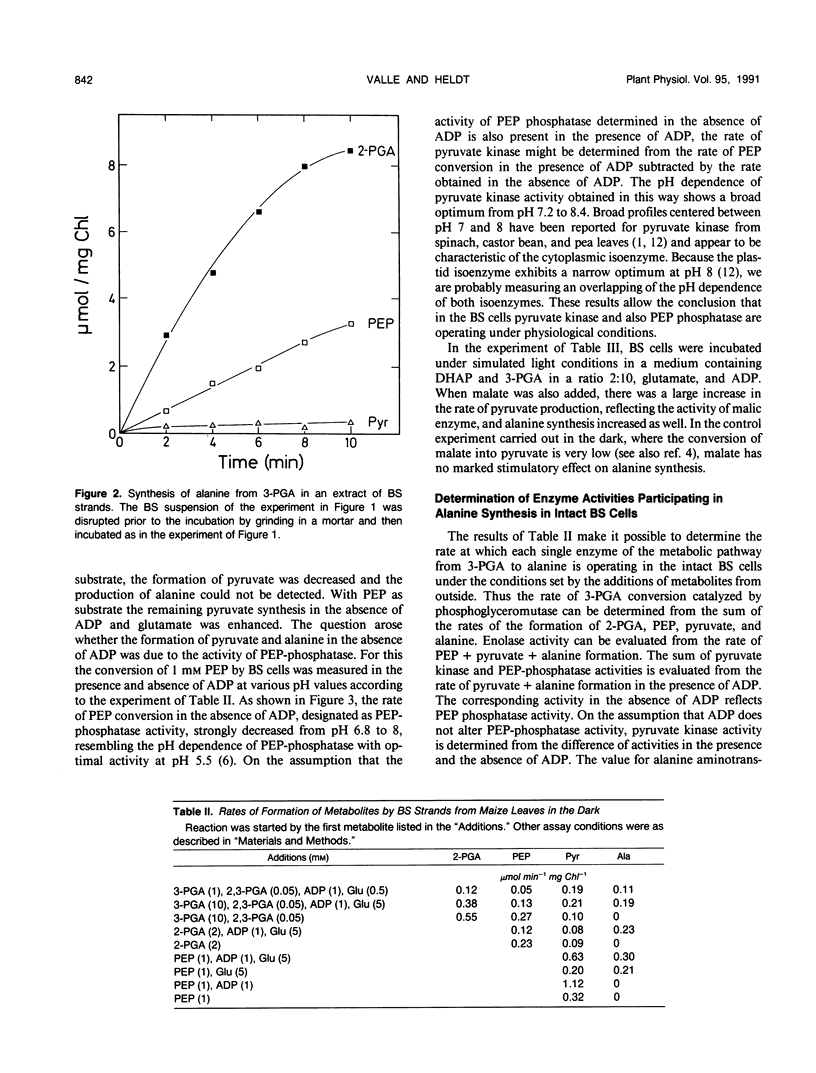
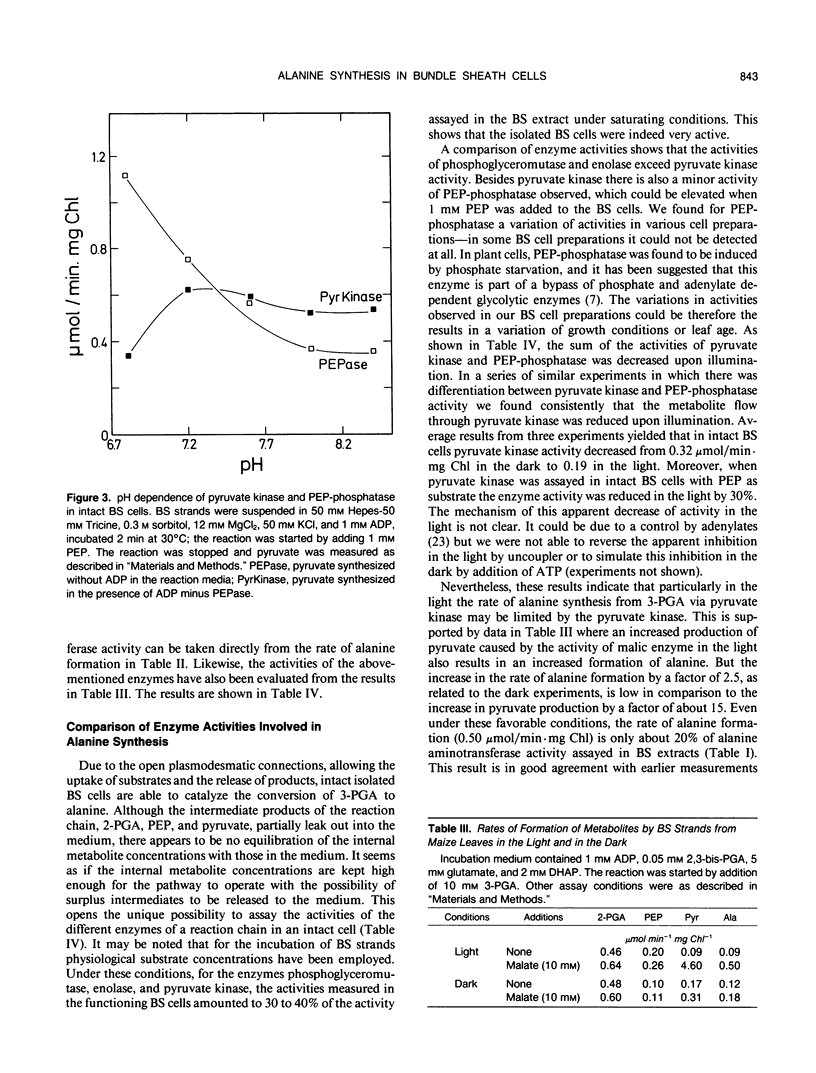
Because in the phloem sap of maize (Zea mays L.) leaves a quarter of the total amino nitrogen can be found as alanine, the capacity of a de novo synthesis of alanine from 3-phosphoglycerate (3-PGA) was studied with isolated bundle sheath (BS) strands of maize. Inasmuch as these cells have retained their plasmodesmatic openings, it was possible to study the formation of alanine from 3-PGA when glutamate and ADP were being added. Alanine synthesis required the existence of the intact cell structure. From the formation of the intermediates, partially released to the medium, the activities of the enzymes of the reaction chain from 3-PGA to alanine could be measured in the intact cells. The results show that in the BS cells the rate of alanine production from pyruvate (0.5 micromole/minute per milligram BS chlorophyll) is more than sufficient to produce one-fourth of the assimilated nitrogen as alanine. As the activity of pyruvate kinase in intact bundle sheath cells in the light was found to be only 0.2 micromole/minute per milligram BS chlorophyll, it is concluded that in the light part of the conversion of 3-PGA to pyruvate may not occur via pyruvate kinase reaction, but via phosphoeno/pyruvate carboxylase, NADP-malate dehydrogenase, and NADP-malic enzyme in the mesophyll and BS cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 plants: photosynthetic activities of isolated bundle sheath cells from Urochloa panicoides. Arch Biochem Biophys. 1988 Jan;260(1):177–186. doi: 10.1016/0003-9861(88)90439-0. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Hatch M. D. Aspartate stimulation of malate decarboxylation in Zea mays bundle sheath cells: possible role in regulation of C4 photosynthesis. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1274–1280. doi: 10.1016/0006-291x(79)90254-7. [DOI] [PubMed] [Google Scholar]
- Duff S. M., Lefebvre D. D., Plaxton W. C. Purification and Characterization of a Phosphoenolpyruvate Phosphatase from Brassica nigra Suspension Cells. Plant Physiol. 1989 Jun;90(2):734–741. doi: 10.1104/pp.90.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff S. M., Moorhead G. B., Lefebvre D. D., Plaxton W. C. Phosphate Starvation Inducible ;Bypasses' of Adenylate and Phosphate Dependent Glycolytic Enzymes in Brassica nigra Suspension Cells. Plant Physiol. 1989 Aug;90(4):1275–1278. doi: 10.1104/pp.90.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Activity, location, and role of asparate aminotransferase and alanine aminotransferase isoenzymes in leaves with C4 pathway photosynthesis. Arch Biochem Biophys. 1973 May;156(1):195–206. doi: 10.1016/0003-9861(73)90357-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. Separation and properties of leaf aspartate aminotransferase and alanine aminotransferase isoenzymes operative in the C4 pathway of photosynthesis. Arch Biochem Biophys. 1973 May;156(1):207–214. doi: 10.1016/0003-9861(73)90358-5. [DOI] [PubMed] [Google Scholar]
- Ireland R. J., Deluca V., Dennis D. T. Isoenzymes of pyruvate kinase in etioplasts and chloroplasts. Plant Physiol. 1979 May;63(5):903–907. doi: 10.1104/pp.63.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Bruno P. L. NADP-malate dehydrogenase from leaves of Zea mays: purification and physical, chemical, and kinetic properties. Arch Biochem Biophys. 1988 Feb 1;260(2):674–695. doi: 10.1016/0003-9861(88)90497-3. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H. Changes in Levels of Intermediates of the C(4) Cycle and Reductive Pentose Phosphate Pathway under Various Light Intensities in Maize Leaves. Plant Physiol. 1987 Jun;84(2):549–554. doi: 10.1104/pp.84.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H., Burnell J. N., Woodrow I. E., Heldt H. W., Hatch M. D. Metabolite diffusion into bundle sheath cells from c(4) plants: relation to c(4) photosynthesis and plasmodesmatal function. Plant Physiol. 1988 Nov;88(3):815–822. doi: 10.1104/pp.88.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wurtele E. S., Nikolau B. J. Enzymes of Glucose Oxidation in Leaf Tissues : The Distribution of the Enzymes of Glycolysis and the Oxidative Pentose Phosphate Pathway between Epidermal and Mesophyll Tissues of C(3)-Plants and Epidermal, Mesophyll, and Bundle Sheath Tissues of C(4)-Plants. Plant Physiol. 1986 Oct;82(2):503–510. doi: 10.1104/pp.82.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]


