Abstract
Background:
Despite being one of the safest, most effective treatments for severe mood disorders, the therapeutic mechanisms of electroconvulsive therapy remain unknown. Electroconvulsive seizure (ECS) induces rapid, high-level expression of immediate early genes (IEGs) and brain-derived neurotrophic factor (BDNF), in addition to stimulation of neurogenesis and dendritic remodeling of dentate gyrus (DG) neurons. We have previously shown that this upregulation of BDNF fails to occur in the hippocampus of mice lacking the IEG Egr3. Since BDNF influences neurogenesis and dendritic remodeling, we hypothesized that Egr3−/− mice will exhibit deficits in neurogenesis and dendritic remodeling in response to ECS.
Objective:
To test this hypothesis, we examined dendritic remodeling and cellular proliferation in the DG of Egr3−/− and wild-type mice following repeated ECS.
Methods:
Mice received 10 daily ECSs. Dendritic morphology was examined in Golgi-Cox-stained tissue and cellular proliferation was analyzed through bromodeoxyuridine (BrdU) immunohistochemistry and confocal imaging.
Results:
Serial ECS in mice results in dendritic remodeling, increased spine density, and cellular proliferation in the DG. Loss of Egr3 alters the dendritic remodeling induced by serial ECS but does not change the number of dendritic spines or cellular proliferation consequences of ECS.
Conclusion:
Egr3 influences the dendritic remodeling induced by ECS but is not required for ECS-induced proliferation of hippocampal DG cells.
Keywords: Early growth response 3, Immediate early gene, Electroconvulsive seizure, Electroconvulsive therapy, Repeated ECS, Neurogenesis
1. Introduction
Electroconvulsive therapy (ECT) remains one of the most effective treatments for severe mood and psychotic disorders. However, the neurobiological mechanisms responsible for its therapeutic benefits remain unclear. Electroconvulsive seizure (ECS) has been used to investigate these potential mechanisms in rodents. ECS causes global neuronal depolarization, which leads to the upregulation of immediate early gene expression [1–5], increased levels of growth factors, including brain-derived neurotrophic factor (Bdnf) [6–9], remodeling of dendritic architecture [10–13], and the proliferation of adult-generated neurons (neurogenesis) [7,14–18]. One of the immediate early genes (IEGs) activated by ECS is early growth response 3 (Egr3), a gene that has been implicated in risk for neuropsychiatric illnesses including bipolar disorder [19,20] and schizophrenia [21–24].
Mice lacking functional Egr3 have deficits in hippocampal long-term depression, a form of synaptic plasticity, and abnormalities in social, stress-response, and spatial navigation behaviors and memory [25,26]. As a transcription factor, EGR3 regulates downstream genes that also influence synaptic plasticity, including activity regulated cytoskeleton associated protein (Arc) [25,27–30], and EGR3 is required to maintain the prolonged expression of Arc induced in hippocampal dentate gyrus (DG) granule cells by exposure to a novel environment [31]. We have recently shown that Egr3 is required for the induction of Bdnf in DG granule cells of mice following ECS [32]. This finding is of particular interest as BDNF serum levels positively correlate with symptom remission in patients treated with ECT [33].
In animal models, BDNF mediates hippocampal dendritic remodeling and neurogenesis [34,35], neurobiological processes correlated with anti-depressive behaviors in rodents [36]. Because our prior work showed that Egr3 is required for the hippocampal induction of Bdnf expression following ECS [32], we hypothesized that dendritic remodeling and neurogenesis would likewise require Egr3. Moreover, since Egr3 is maximally induced in DG granule cells following ECS, we sought to determine the role of Egr3 following ECS on dendritic structure of DG granule cells as well as on the first step of neurogenesis, the proliferation of DG granule cells.
For treatment of severe mood disorders and mixed mood and psychotic disorders, ECT is administered three times a week to patients, which is eventually tapered to one treatment per 3–4 weeks [37]. Following this regimen, serum concentrations of BDNF are significantly increased [38]. The induction of an ECS in animals provides a model of this treatment and allows for the investigation of candidate therapeutic mechanisms of action. However, a limited number of studies have investigated the effects of serial ECS in mice, and few have examined both dendritic morphology and cellular proliferation [13,39–42].
Here we investigated the requirement of the immediate early gene Egr3, a transcription factor acting both upstream and downstream of BDNF, for dendritic remodeling and cellular proliferation in the hippocampal formation following repeated ECS. The dorsal hippocampus largely mediates the acquisition of visuospatial tasks [43] while the ventral hippocampus encodes non-spatial representations, and plays an important role in motivation as well as emotional processing [44–46]. Since the dorsal and ventral regions of the hippocampal formation perform different functions, we analyzed these regional domains independently to determine whether ECS and Egr3 affect these regions differently.
2. Materials and methods
2.1. Mice
C57BL/6 male wildtype (WT) and Egr3−/− littermate mice [47] were group housed with ad libitum access to food and water on a 14:10 light cycle. Age matched experimental pairs of WT and Egr3−/− mice were designated at the time of weaning and underwent all procedures in parallel. Pairs assigned to ECS or no ECS conditions were age matched. The age of mice was approximately 2 months old for dendritic morphology studies and 9–12 months for cellular proliferation studies. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Arizona.
2.2. Electroconvulsive stimulation
ECS was administered via ocular electrodes in anesthetized mice (2% isoflurane) once daily for 10 consecutive days using a Ugo Basile instrument (Varese, Italy); settings: pulse frequency 260 Hz, pulse width 0.3 ms, duration 100 ms, and current 80 mA. ECS was performed before mice fully recovered from anesthesia. Under these conditions, animals displayed behaviors such as lip smacking, arching, stiffening, and running but did not display tonic-clonic movements following delivery of current. Seizure effectiveness was validated by antibody staining for the IEG Arc (1:1000 rabbit anti-ARC antibody, Synaptic Systems, Cat. # 156 003), which was similar between anesthetized WT animals displaying these behaviors and un-anesthetized WT animals exhibiting tonic-clonic seizure. Sham control animals underwent the identical procedure without current administration.
2.3. Golgi-Cox staining/sectioning
Eleven days following the final ECS, mice were sacrificed under isoflurane anesthesia and perfused with PBS. Hemisected brains were stained with Golgi-Cox solution as previously described [48]. Briefly, hemisected brains were submerged in Golgi-Cox solution (5 vol parts (VP) solution A (5% potassium dichromate in distilled H2O), 5 VP solution B (5% mercuric chloride in distilled H2O), 4 VP solution C (5% potassium chromate in distilled H2O) and 10 VP H2O). The Golgi-Cox solution was prepared 14 days prior to use and dark-stored. Solution was refreshed after 2 days of staining. Tissue was transferred to 30% sucrose prior to vibratome sectioning (200 μm) using 6% chilled sucrose, mounted on subbed, gelatinized slides, and incubated in a humidified chamber for up to 4 days prior to Kodak development. Tissue was rinsed with H2O for 1 min, treated with 30% ammonium hydroxide for 30 min in the dark, rinsed again in H2O for 1 min, and was developed with Kodak fix solution for 30 min in the dark. Tissue was then rinsed in distilled H2O for 1 min, followed by a series of alcohol dehydrations (1 min in 50% EtOH, 1 min in 70% EtOH, 1 min in 95% EtOH, and 3 × 5 min in 100% EtOH). Slides were then treated with (1:1:1 chloroform, xylene, EtOH) solution for 15 min, and were immediately cover-slipped with Permount mounting media. Slides were stored in a dry and dark environment for approximately 2 months prior to data analysis.
2.4. Dendrite analyses
Identified cells were imaged in a z-stack throughout the thickness of the section at ~10μm increments using a brightfield microscope equipped with a digital camera (AmScope, Irvine, CA). Using ImageJ (NIH), the image stack was used for both Sholl and branch order analyses. Eight fully impregnated cells were selected from the DG suprapyramidal (DGsp) and infrapyramidal (DGip) blades per animal, for a total of sixteen cells per animal. Each of these cells was imaged in a z-stack throughout the thickness of the section using a bright-field microscope (Leica Microsystems, Concord, ON) equipped with a digital camera (Pixelink, Ottawa, ON). DG granule cells were randomly selected along the longitudinal axis, from the dorsal (Bregma −1.34 mm to −2.35 mm) and ventral (Bregma −2.7 mm to −3.4 mm) hippocampus and granule cell layer. The basis of being identifiable as granule cells by morphology and having dendritic trees that were fully impregnated with the Golgi-Cox solution [49].
Changes in the dendritic arbor were assessed using two measures – the Sholl technique [50], and branch order analysis [51]. Briefly, the Sholl technique involves quantifying the number of intersections between the dendrites of a cell and concentric circles centered at the soma and placed at 20 μm intervals using ImageJ (NIH, Bethesda, MD) or Metamorph (Molecular Devices, Sunnyvale, CA) software. Branching order was also calculated for all cells as previously described [48]. This involved assigning a branch order of one to the primary dendrites originating from the soma. Any dendritic processes originating from that dendrite are counted as second order branches, and each subsequent bifurcation is assigned a progressively higher branch order [51].
These two analyses provide complementary measures of dendritic complexity. Sholl analysis is sensitive to changes in the total amount of dendritic material, and is considered to provide an estimate of the total complement of synapses contained on a cell. Branch order analysis, on the other hand, provides a measure of the complexity of dendritic computation that may be occurring. Moreover, these two measures may change independently – if the branches of a dendritic arbor each grow in length without forming new branches, for instance, this is expected to result in increasing the number of intersections without altering branch order.
Dendritic spine density was analyzed at 100x under oil immersion. In each section, the inner, middle, and outer molecular layers were defined by dividing the length of the molecular layer into three equal parts. The total number of dendritic spines in each division of the molecular layer were determined as well as the collective number of spines in all layers.
2.5. Bromodeoxyuridine (BrdU) staining
Mice received two injections of BrdU (100 mg/kg), 24 h apart, beginning 24 h after the final ECS. Mice were returned to their home cage between procedures and sacrificed by isoflurane overdose 24 h after the second BrdU injection. Tissue was flash frozen in −40 °C methylbutane. Brains from four conditions (WT no ECS, WT serial ECS, Egr3−/− no ECS and Egr3−/− serial ECS) were arranged in a brain mold and sectioned (20 μm) and mounted together, to control for slide-slide variation. Tissue was stored at −80 °C until immunofluorescent staining.
Prior to staining, tissue was brought to −20 °C overnight and was placed at room temperature for 1 h. Tissue was then fixed in 2% paraformaldehyde for 5 min at 4 °C. Tissue was rinsed in tris buffer saline solution (TBS) 2 × 10 min, followed by protein delipidation with a one-to-one mixture of acetone-methanol. Tissue was rinsed again in TBS solution 2 × 10 min prior to blocking. Tissue was incubated with the primary-antibody, rabbit polyclonal α-Prox1 (Millipore rabbit polyclonal α-Prox1, Sigma AB575), a marker of dentate granule cells, diluted in 1:1000 TSA blocking buffer. Tissue was cover-slipped and left in a humid chamber overnight at 4 °C. The following day, coverslips were removed, and tissue was washed in TBS prepared with Tween-20 for 10 min, followed by 2 × 10-min washes in TBS. Anti-rabbit horseradish peroxidase (Cat. 12–348, Sigma) diluted 1:500 in blocking buffer was applied to each slide. Super glow green (1:75) was applied to the tissue for 20 min. Tissue was then washed 3 × 10 min in TBS and quenched with 1% hydrogen peroxide in TBS for 15 min. Tissue was washed again in TBS for 10 min and was taken through a series of stringency washes (2x saline sodium citrate (SSC) (2 × 10 min), one-to-one ratio of formamide:2x SSC (2 h at 65 °C), 2x SSC (10 min)). Tissue was incubated in 2 N HCl for 30 min at 37 °C followed by rinsing in borate buffer (5 min at room temperature) and 8 × 5-min washes in TBS. Anti-BrdU antibody with peroxidase fragments (Cat. 11585860001, Roche) was diluted 1:100 in blocking buffer and was applied to the tissue for 2 h at room temperature. Tissue was washed 3 × 10 min in TBS prior to the application of the super glow blue (1:100) (15-min incubation). Tissue was washed again (3 × 10 min in TBS) and was cover-slipped with buffered glycerol prepared with fluorescent preservative.
Six tissue sections were analyzed per animal. The dentate gyrus DGip and DGsp blades, including the subgranular zone (SGZ), of the left and right hemispheres were imaged using a confocal microscope. The number of BrdU positive (recently divided) cell profiles were counted and represented per mm2 averaging the results from each of the 6 counted sections.
2.6. Statistical analysis
Poisson or negative binomial models with random effects were used to compare count variables between different conditions (Egr3−/− vs. wildtype and ECS stimulation vs. sham) with cell as the unit of analysis and with animal-level random effects to account for correlation among multiple cells of the same animal. All models were adjusted for blade (superior vs. inferior). In any model where effects of loss of Egr3 and ECS were both included, an interaction term was included first and, if it was not statistically significant, it was removed and the main effects of loss of Egr3 and ECS was reported; if the interaction term was significant individual subgroups were compared with Bonferroni correction for multiple comparisons. Bonferroni correction was also performed for comparisons of number of dendritic intersections at individual distance levels, of dendritic spine number at individual molecular layers, and of number of dendritic branches of each individual order. The lme4 package (version 1.1–28) of R (version 4.1.2) was used for this part of the analysis.
3. Results
Studies to assess the effects of serial ECS in mice have been limited due to the high rates of lethality from seizure in unanesthetized mice. Recent development of protocols using isoflurane anesthesia prior to ECS have overcome this problem enabling serial ECS in mice [40]. As anesthetics are commonly administered to patients prior to ECT [52], use of isoflurane prior to ECS more closely models the clinical use of ECT. Using this approach, we examined the effects of serial-ECS on dendritic remodeling and cellular proliferation to determine the role of Egr3 in these processes in mice.
We administered either ECS or sham stimulation to male mice for 10 consecutive days to determine the effect of serial ECS on dendritic remodeling in mouse DG granule cells (Fig. 1A). We analyzed the effects of chronic ECS by examining three separate characteristics of dendritic structure: dendritic intersections, dendritic branches, and dendritic spine density, in Golgi Cox-stained neurons.
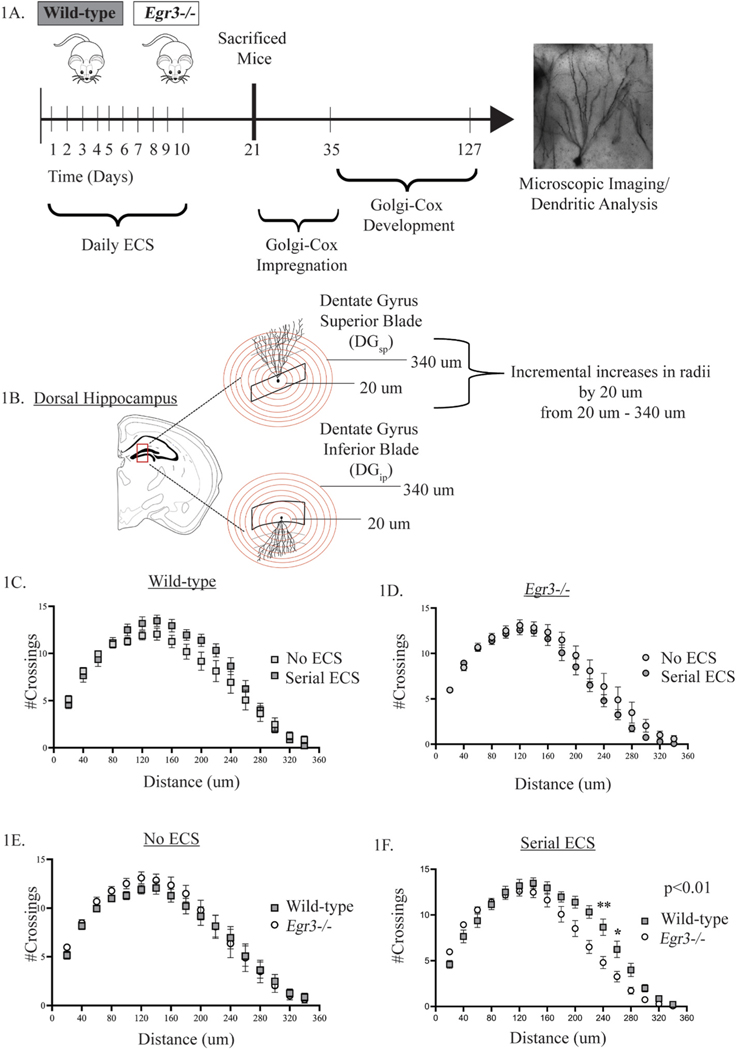
Fig. 1. Serial ECS alters dorsal hippocampal dendritic complexity differently in Egr3−/− mice.
The numbers of dendritic intersections were analyzed using Golgi-Cox staining in WT and Egr3−/− mice following 10 daily ECS (A). Sholl analysis was conducted in the dorsal hippocampus (B). Compared to sham treatment, serial ECS did not increase the number of dendritic intersections in WT (C) or Egr3−/− mice (D). Comparison of WT and Egr3−/− mice showed no difference in the numbers of dendritic intersections between genotypes at baseline (no ECS) (E). However, following ECS, fewer dendritic intersections are observed in Egr3−/− mice than WT mice. Individual comparisons at specific distances revealed significantly fewer intersections at 200 and 220 μm from the soma (F). Values represent means ± SEM. P-values for pairwise comparisons, adjusted for multiple comparisons: *p < 0.05, **p < 0.01. n =7–8.
3.1. Dendritic remodeling
3.1.1. Dendritic intersections
A previous study showed that administration of a single ECS in mice increases dendritic complexity, determined by the number of dendritic intersections, in dentate gyrus granule cells [10]. In the current study we examined whether serial ECS has a similar effect on dendritic complexity in mice, and whether this process requires Egr3. We used Sholl analysis to count the number of dendritic intersections in circumferential rings at distances ranging from 20 μm to 340 μm from the soma in the DGsp and DGip (Fig. 1B).
We first examined whether serial ECS alters the number of dendritic intersections in the dorsal hippocampus of mice. Compared with sham treated controls, serial ECS did not produce a statistically significant change in the numbers of dendritic intersections of DG granule cells in WT mice (Fig. 1C) or in Egr3−/− mice (Fig. 1D). We then examined whether Egr3 influenced dendritic complexity. We found that there was no difference in the number of dendritic intersections in Egr3−/− mice compared to WT mice under control (sham) conditions (Fig. 1E). However, following serial ECS, Egr3−/− mice had significantly fewer dendritic intersections than WT mice (Fig. 1F, p < 0.01). The greatest difference was at distances 220 μm and 240 μm from the soma (Fig. 1F, post-hoc pairwise comparisons = p < 0.01 and p < 0.05 respectively). Supplemental Figs. S1A-D presents graphs showing the total number of intersections for each condition.
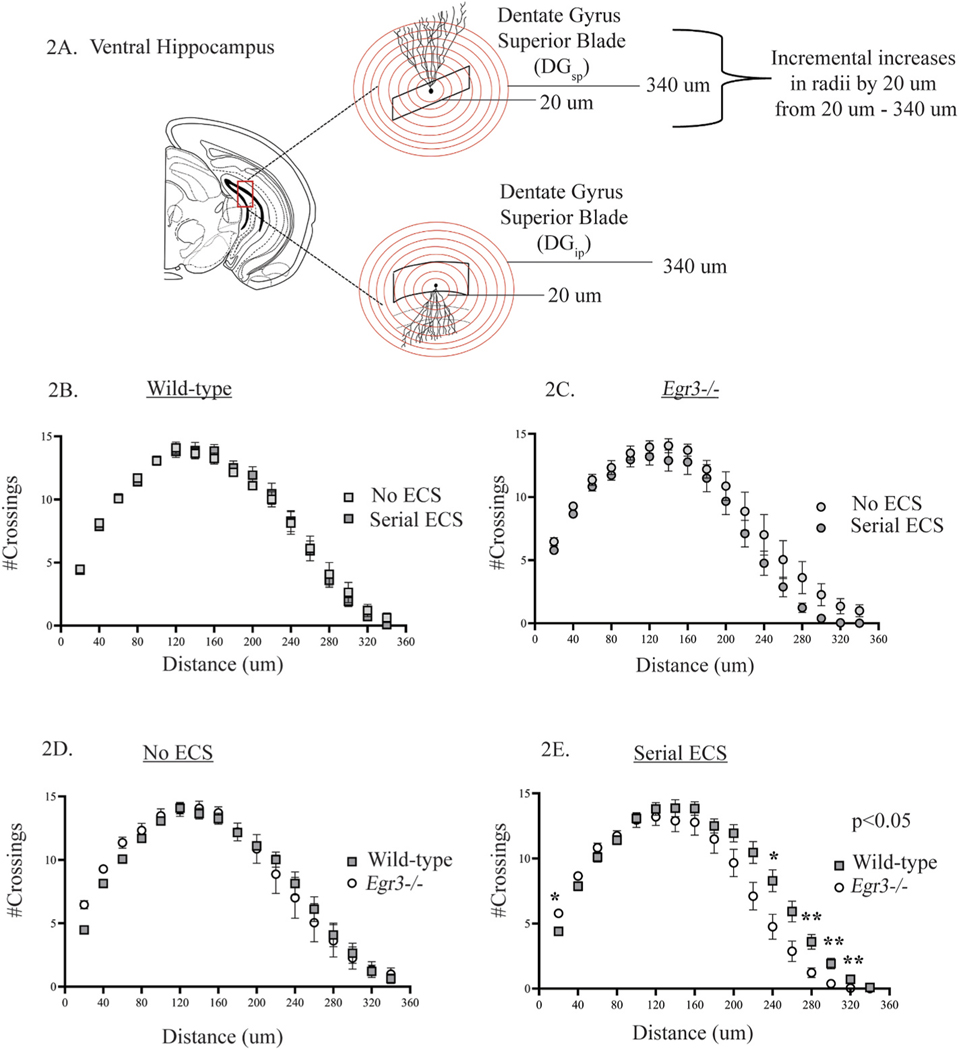
Next, we analyzed the effect of serial ECS on dendritic intersections in ventral hippocampal DG granule cells (Fig. 2A). As in the dorsal hippocampus, ECS did not cause a significant overall change in dendritic intersections in either WT (Fig. 2B), or Egr3−/− mice (Fig. 2C). However, when comparing genotypes, we found a similar effect as in the dorsal hippocampus. While the number of dendritic intersections did not differ between WT and Egr3−/− mice under unstimulated conditions (Fig. 2D), serial ECS induced significant changes in dendritic intersections between the two genotypes (Fig. 2E, p < 0.05). These differences occurred immediately adjacent to the soma, where Egr3−/− mice showed more intersections than WT mice (20 μm, p < 0.05), and at distances far from the soma, where Egr3−/− mice showed significantly fewer dendritic intersections compared to WT mice (240 μm, p < 0.05; 280 μm, p < 0.01; 300 μm, p < 0.01: 320 μm, p < 0.01, Fig. 2E). Supplemental Figs. S1E-H shows graphs of the total number of intersections for each condition in the ventral hippocampus.
Fig. 2. Serial ECS alters ventral hippocampal dendritic complexity differently in Egr3−/− mice.
Sholl analysis was conducted in the ventral hippocampus (A). Serial ECS did not change the number of dendritic intersections in the ventral hippocampus of WT (B) or Egr3−/− (C) mice. In the absence of stimulation, the number of dendritic intersections did not differ between WT and Egr3−/− mice (D). Following ECS, Egr3−/− mice show more intersections than WT mice at 20 μm from the soma, and WT mice show significantly more dendritic intersections than Egr3−/− mice at 240 μm, 280 μm, 300 μm, and 320 μm (E). Values represent means ± SEM. P-values for pairwise comparisons, adjusted for multiple comparisons: *p < 0.05, **p < 0.01. n = 7–8.
These results show that Egr3 influences the dendritic complexity of hippocampal DG granule cells in response to serial ECS. Although ECS did not result in a statistically significant effect in either WT or Egr3−/− genotypes independently, the effects of the stimulation trended toward increasing dendritic crossings in WT mice (more strongly in the dorsal hippocampus Fig. 1C than in the ventral hippocampus 2B), while causing a trend in the opposite direction in Egr3−/− mice. Together these effects resulted in the significant difference between Egr3−/− and WT mice following ECS in the number of dendritic intersections.
3.1.2. Dendritic branches
In rats, ECS enhances dendritic arborization (i.e. increases numbers of dendritic branches) of hippocampal neurons [12] and repeated ECS prevents stress-induced decreases in the number of dendritic branches [53]. To determine whether ECS alters the number of dendritic branches in mice, and whether these effects require Egr3, we counted the number of dendritic branches following serial ECS. The number of dendritic branches was counted at each individual branch order, from the primary (or first order) to the sixth branch order, in both DGip and DGsp blades of the dentate gyrus (Fig. S2A).
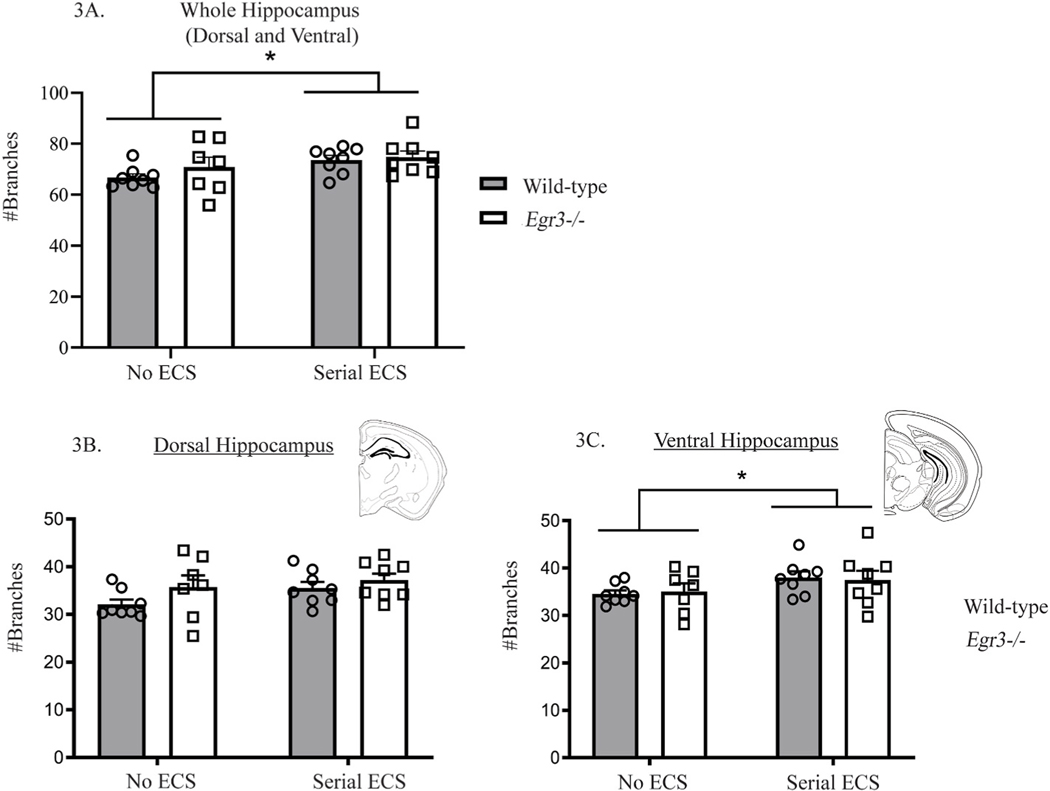
We first analyzed the total number of dendritic branches per cell across the whole hippocampal formation (dorsal plus ventral) from each group. This revealed a significant main effect of treatment, *p < 0.05 (Fig. 3A), showing that, like in rats, serial ECS increased the number of dendritic branches in DG granule cells in mice. To determine if this effect differed between hippocampal regions, we analyzed the total number of dendritic branches separately in the dorsal and ventral hippocampal formation. While the effect of serial ECS on the total number of dendritic branches in the dorsal hippocampus showed a trend effect that did not reach statistical significance (Fig. 3B, p = 0.059), we observed a significant increase in the ventral hippocampus (Fig. 3C, p < 0.05). No significant interaction between genotype and ECS was observed in the hippocampal formation as a whole, or in either dorsal or ventral regions separately.
Fig. 3. Serial ECS increases dendritic branching in mouse dentate granule cells.
Serial ECS significantly increases the number of dendritic branches in the whole hippocampus, combining the dorsal and ventral hippocampus (A). Significant increases were not observed individually in the dorsal hippocampus (B) but were observed in the ventral hippocampus (C). Values represent means ± SEM. Bars represents significant main effect of treatment with both genotypes combined. P-values adjusted for multiple comparisons: *p < 0.05, n = 7–8.
We then examined the number of branches at each branch order (first through sixth) in the dorsal and ventral hippocampal formation (Fig. S2). This analysis revealed that, although there was no main effect of serial ECS at any branch order in either region, a main effect of genotype was identified at multiple branch orders in both hippocampal regions. In the dorsal hippocampus Egr3−/− mice showed significantly more dendritic branches in the third and fourth branch order than did WT mice (Figs. S2D-E, p < 0.05), but not in the first, second, fifth, or sixth (Figs. S2B, S2C, S2F, and S2G, respectively). In the ventral hippocampus, ECS increased the numbers of dendritic branches significantly more in Egr3−/− mice at the first (Fig. S2H, p < 0.01) second (Fig. S2I, p < 0.001), and third (Fig. S2J, p < 0.05) branch orders, but not the fourth-sixth branch orders (Fig. S2K-S2M).
These results indicate that serial ECS increases the number of dendritic branches in mice in the hippocampal formation. In addition, Egr3 appears to influence where these changes occur in the dendritic tree, and loss of Egr3 resulting in more branching at specific levels of the DG granule cell dendritic arbor than in WT mice following ECS.
3.1.3. Dendritic spine density
We then analyzed the number of dendritic spines following ECS (Fig. 4A). The number of dendritic spines was quantified in both the dorsal and ventral hippocampal formation by counting in the inner, middle, and outer molecular layers, summing the counts from both the DGsp and DGip (Fig. 4B). ECS produced significant increases in the numbers of dendritic spines in both the dorsal (Fig. 4C, p <0.01) and the ventral (Fig. 4D, p < 0.01) hippocampal formation.
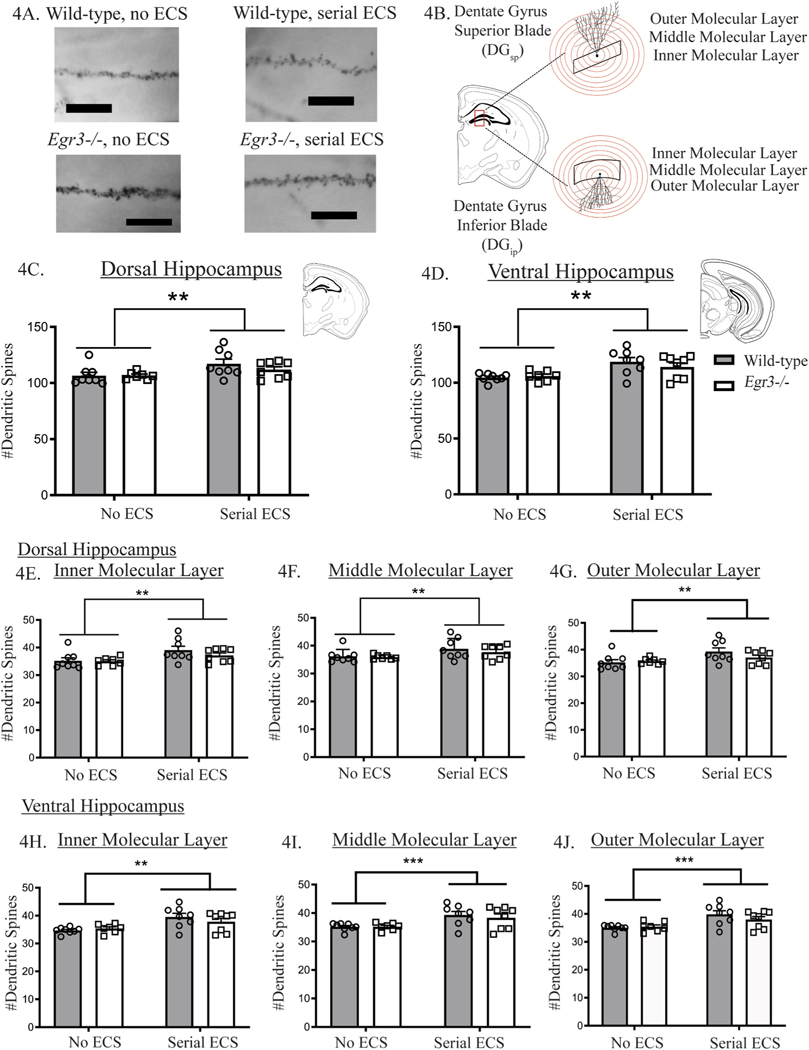
Fig. 4. Serial ECS increases dendritic spine numbers.
Representative examples of dentate granule cell dendritic spines for each condition (A). Schematic showing dentate granule cell dendrites across the inner, middle, and outer molecular layers of the hippocampus (B). ECS increases the number of dendritic spines in both the dorsal (C) and ventral hippocampus (D) and in each layer: dorsal hippocampus inner molecular layer (E), middle molecular layer (F), outer molecular layer (G), and ventral hippocampus inner molecular layer (H), middle molecular layer (I), and outer molecular layer (J). Values represent means ± SEM. Bars represents significant main effect of treatment with both genotypes combined. P-values adjusted for multiple comparisons: *p < 0.05, **p < 0.01, ***p < 0.001. n = 7–8.
In the dorsal hippocampal formation, analysis of dendritic spines by hippocampal layer revealed that serial ECS significantly increased spine numbers in each of the three layers of the dentate gyrus: inner, middle, and outer molecular layer (Fig. 4E–G, p < 0.01 for each). In the ventral hippocampal formation, ECS also increased the number of dendritic spines in each: inner molecular layer, (Fig. 4H, p < 0.01), middle molecular layer, (Fig. 4I, p < 0.001), and outer molecular layer, (Fig. 4J, p < 0.001). There was no interaction between ECS and genotype and no main effect of genotype in any of the analyses of dendritic spine density.
These findings indicate that serial-ECS increases the number of dendritic spines in both the dorsal and ventral hippocampal formation in all three molecular layers. There was no evidence that loss of Egr3 affected dendritic spine density in response to serial ECS.
3.2. Cellular proliferation
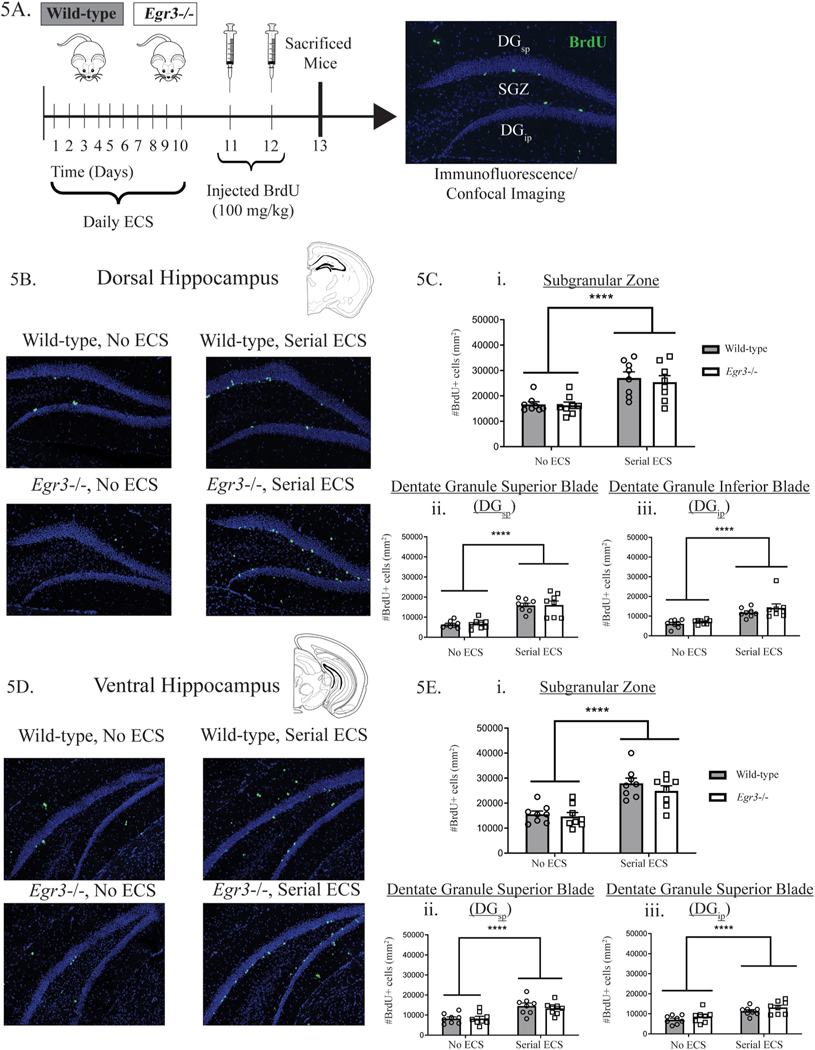
To determine whether Egr3 is necessary for the effect of serial ECS on proliferation of hippocampal cells in adult mice, we injected WT and Egr3−/− mice with BrdU for two days following ten days of daily ECS and sacrificed animals 24 h after the second injection (Fig. 5A). BrdU-labeled cells were counted to determine the average number of dividing (i.e., proliferating) cells per mm2 in the dorsal and ventral hippocampal formation.
Fig. 5. Serial ECS increases hippocampal cell proliferation.
Schematic of experimental design (A). ECS significantly increases dividing cells (BrdU-positive, green-labeled) in each subregion of the dorsal hippocampus. (B): the subgranular zone (SGZ) (Ci), dentate granule superior blade (DGsp) (Cii), and the dentate granule inferior blade (DGip) (Ciii). Similarly, ECS significantly increases BrdU-positive cells in each ventral hippocampus subregion (D): SGZ (Ei), DGsp (Eii) and DGip (Eiii). Values represent means ± SEM. Bars represents significant main effect of treatment with both genotypes combined. P-values adjusted for multiple comparisons: ****p < 0.0001, n = 8. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5 (B–C) shows that serial ECS increased the number of BrdU-positive cells in the dorsal hippocampal formation. This was seen in all three subregions of the dentate gyrus: the SGZ, the DGsp, and the DGip (Fig. 5Ci-Ciii, p < 0.0001 in each). A similar result was seen in the ventral hippocampal formation (Fig. 5D–E), where serial ECS increased the number of proliferating cells in all three subregions of the dentate gyrus: the SGZ, the DGsp, and the DGip (Fig. 5Ei-iii, p < 0.0001 in each). No significant interactions between genotype and ECS, and no main effects of genotype, were seen for any of the analyses. Table S1 shows the uniform age distribution of mice across experimental groups. There was no correlation between number of BrdU + cells and animal age (across the 9–12-month range) for cells in any hippocampal region or in any group.
We have previously reported that the number of DG granule cells does not differ in Egr3−/− mice compared to their WT littermates [32]. In the current study we used an anti-Prox1 antibody to mark DG granule cells. The average number of Prox1+ cells/section for each region of the hippocampal formation was DGsp = 5.6 × 105 cells/mm2, DGip = 5.7 × 105 cells/mm2, SGZ = 2.1 × 105 cells/mm2. Fig. S3 shows representative images of Prox1 staining.
These results show that serial ECS produced a 2-fold increase in the number of proliferating cells in the hippocampal formation of mice. These effects were seen in both the dorsal and ventral hippocampal formation. However, we did not observe differences in the number of proliferating cells between WT and Egr3−/− mice following serial ECS in either the dorsal or ventral hippocampal formation. These results indicate that serial ECS stimulates the proliferation of DG granule cells in mice, and that this effect does not appear to require Egr3.
4. Discussion
In this study, we aimed to address whether the immediate early gene Egr3, which we have previously shown to be required for the induction of Bdnf following ECS [32], plays a role in cellular proliferation and dendritic remodeling induced by repeated ECS. In addition, our results from WT mice contribute to the small, but growing, literature on the effects of repeated ECS in mice. We found that ten days of once daily ECS modified the dendritic structure and synaptic spine density of hippocampal DG granule cells and increased cellular proliferation. The ECS-induced morphological changes were disrupted in the absence of Egr3, while the synaptic spine changes and hippocampal cellular proliferation were unaffected by loss of Egr3.
4.1. Dendritic changes
In rats, chronic restraint stress produces depressive-like behaviors and induces dendritic atrophy and reduced branching of hippocampal CA3 neurons [54]. The administration of ECS prevents dendritic retraction in CA3 pyramidal neurons following chronic restraint stress [53], enhances the numbers of hippocampal synapses [55], and increases spine density in mature granule cells [11]. Recently, studies conducted in mice have shown changes in dendritic structure following repeated ECS treatments including increases in the dendritic length of immature granule cells [42], as well as the number of dendritic intersections [56]. Because Egr3 plays an important role in hippocampal synaptic plasticity [25], we hypothesized that loss of Egr3 would alter ECS-induced changes in dendritic structure.
We assessed three aspects of dendritic changes following 10 daily administrations of ECS in WT and Egr3−/− mice: dendritic intersections, dendritic branching, and dendritic spine density. Our results show that repeated ECS increases the number of dendritic branches, as well as dendritic spine density, of granule cells in the mouse hippocampal formation. While ECS did not result in an overall increase in the number of dendritic intersections, ECS produced the opposite effect on Egr3−/− mice than on WT mice. At points most distal to the soma, the number of dendritic intersections increased in WT mice following ECS but decreased in Egr3−/− mice. Thus, after the course of ECS, WT mice showed significantly more dendritic intersections than Egr3−/− mice, indicating a role for Egr3 in ECS-induced dendritic remodeling.
The effect of ECS on dendritic spines occurred to the same degree in both WT and Egr3−/− mice, suggesting that this change in response to ECS does not require Egr3. The number of dendritic branches also did not differ between WT and Egr3−/− mice. However, Egr3 does appear to influence where in the dendritic tree new branching occurs. While these differences were subtle, even slight differences in dendritic branching can alter the ability of synaptic inputs to generate, propagate, and time action potentials, thereby affecting the ability of the neuron to fire in response to stimuli [57]. These findings demonstrate that in mice, serial ECS leads to synaptic plasticity of granule cells and suggests that Egr3 influences their dendritic complexity.
Where branching occurs in the dendritic tree has important implications for inputs to the DG. The dendrites of DG granule cells can be divided into 3 equal sections, the inner (IML), middle (MML), and outer (OML) molecular layers, each reflecting different input. The IML (the third closest to the soma) contains predominantly commissural and associational fibers, while the middle (MML) and outer (OML) thirds receive input from the medial and lateral perforant path, respectively. Moreover, these inputs carry different kinds of information, with the MML input, for instance, carrying much more spatial information relative to the OML (e.g. Ref. [58]). Because of this unique lamination, knowing the location of changes caused by ECS within the molecular layer may provide insight into the mechanism underlying the unique physiological responses of these cells during behavior.
Following ECS, Egr3−/− mice increase dendritic material (intersections) more in IML, indicating greater intra-hippocampal connections relative to WT mice. Conversely, Egr3−/− mice show a reduction in dendritic material post-stimulation exclusively in the distal third of the dendritic arbor. This suggests that the reorganization of the tri-synaptic circuitry that occurs following stimulation takes a different trajectory in these 2 groups of animals, with the hippocampus of WT mice increasing their input from the lateral perforant path, while Egr3−/− mice increase their intra-hippocampal connectivity. The observation of increased dendritic material in the OML is consistent with recent data, anatomical and physiological studies indicate that adult-born neurons are preferentially innervated by the lateral perforant path [59,60], and is particularly intriguing given observations that serotonin preferentially modulates plasticity in the lateral perforant path [61]. These data suggest that the balance of information coming in from cortical inputs is shifted in Egr3−/− mice, although it remains difficult to predict how these differences may affect behavior.
Our findings of ECS-induced dendritic remodeling are consistent with prior studies in rats and add to the limited number of studies that have examined the effects of repeated ECS on dendritic structure in mice [13,39,41,62]. The earliest report we identified examining the effects of chronic ECS in mice investigated the requirement for BDNF. In this study, Vaidya and colleagues found that 5–10 daily ECS treatments increased hippocampal DG cell sprouting, which was significantly reduced in BDNF +/− mice [39]. Although BDNF was required for this effect of ECS, exogenous administration of BDNF was not sufficient to induce sprouting [39]. This result is consistent with our findings that Egr3−/− mice, which we have shown fail to upregulate hippocampal BDNF expression following ECS [32], have reduced dendritic complexity compared to WT mice following repeated ECS.
In more recent studies, Chang and colleagues found that 5 daily ECS increased the number of dendritic intersections in the dentate gyrus molecular layer in WT mice, but not in mice lacking another immediate early gene, Narp [41]. In contrast to Egr3, Narp is not required for ECS-induced Bdnf expression [41]. Maynard and colleagues examined the effects of 7 ECS, delivered three times per week, on dendritic structure of neurons in the mouse cortex. They found that repeated ECS prevented the synaptic changes caused by glucocorticoid administration, which they attributed to stabilization of existing synaptic spines, rather than reversal of cortical spine loss [13].
4.2. Cellular proliferation
We also investigated the effect of repeated ECS on cellular proliferation in the hippocampal dentate gyrus. The addition of newborn dentate granule neurons in the adult hippocampus was originally shown to occur in the mammalian brain in the 1960s [63]. Both intrinsic factors, including neurotrophic factors [34,64] and transcriptional regulators [65,66] have been shown to influence proliferation, as well as the differentiation and integration of these cells into the hippocampal network. Evidence of adult neurogenesis has been found in the DG of the human hippocampus [67–69]. These studies have shown that the addition of newborn neurons in the DG occurs throughout life.
However, a study published by Sorrells and colleagues raised questions about the phenomenon. They showed that the number of proliferating progenitors and young neurons in the DG decline rapidly in the first year of life, with negligible levels found in post-mortem brains by 7 and 13 years of age [70]. Following that publication, Boldrini and colleagues provided evidence for neurogenesis showing thousands of immature neurons in the DG, with the numbers of proliferating neural progenitors, immature and mature granule neurons remaining unchanged in human individuals ranging from 14 to 79 years of age [69]. Additional studies showed an extensive number of neural progenitors, neuroblasts, and immature neurons in healthy individuals ranging in distinct maturation stages, from the fourth to ninth decade of life [71,72]. Discrepancies in the findings published by Sorrels and colleagues may be attributed to variation in histological methods, with differences being introduced in the time the tissue was fixed following patient death, the fixative and preservation of the tissue, and cell quantification techniques regarding the tissue section thickness and the numbers of sections quantified, which can introduce significant variations. Additionally, pathological conditions may have introduced drastic differences in these findings as patients suffering from a severe illness may have been treated with medication or faced chronic systemic toxic conditions that inhibited neurogenesis. Thus, although there remains controversy surrounding human adult neurogenesis, a number of studies have provided strong evidence of the addition of newborn neurons in the DG throughout life in healthy older individuals.
Approximately one-third of the neurons residing within the hippocampus are subject to exchange, with approximately 700 new neurons being added daily. In patients diagnosed with major depressive disorder (MDD), fewer, and smaller, DG neurons were found in the hippocampus suggesting that proliferation of these cells may confer resilience against MDD [73]. Moreover, antidepressant medications used to treat MDD have been shown to increase human hippocampal neural progenitor cells [74].
Studies performed in rats have shown that repeated ECS induces a 2-fold increase in proliferating cells in the hippocampus [14–16,75]. We found that serial ECS caused a 2-fold increase in the numbers of BrdU-positive cells in the SGZ as well as in the DGsp and DGip of the hippocampal formation. The effect was present in both the dorsal and ventral hippocampus and was similar in both WT and Egr3−/− mice, indicating that Egr3 is not required for the proliferation of hippocampal cells induced by repeated ECS. However, it is possible that Egr3 may play a role in survival of newborn neurons, which should be investigated in future studies.
Studies have shown that adult-generated neurons can be differentiated based on their location throughout the depth of the DG granule cell layer (i.e., from the inner subgranular surface of the cell layer to the outer surface nearest the molecular layer) in both rats (e.g. Ref. [76]) and mice (e.g. Ref. [77]). This makes it tempting to attempt to make similar distinctions to further categorize Golgi-based data (which lacks BrdU labeling) into older and more-recently generated cell populations. We hesitate to make this distinction in our data because it would not be possible to “birthdate” adult-generated cells based on location alone. Although data indicate that the majority (approx. 50–80%, depending on the timing of BrdU injections) of adult-generated cells remain within the inner (closest to the SGZ) third of the granule cell layer, this region contains granule cells of a wide range of ages, from 1 day to at least 11 months old [77]. This is problematic, as differentiating adult-generated cells born post-stimulation from those born prior to stimulation would be a key basis for comparison in the current study. This limits the value of separating these cell populations based on their location within the granule cell layer.
Our results add to the recent studies showing that repeated ECS increases proliferation of neuronal precursors in the dentate gyrus of mice [40–42,78]. Schloesser and colleagues found that repeated ECS (7 ECS over 15 days) increased hippocampal cellular proliferation in mice. Using a genetic method to ablate these newly-born cells, they found that the neurogenesis was essential for the ECS-induced amelioration of mood and anxiety-like behaviors in mice exposed to corticosterone, a model of stress-related depression [40]. Ueno and colleagues examined the role of repeated ECS (11 every-other-day ECS over 3 weeks) in distinct phases of neurogenesis. They found that ECS during early (differentiation) phase of neurogenesis enhanced survival and neuronal differentiation, while repeated ECS during later maturation phase suppressed expression of mature neuronal markers [42]. In their study examining the role of Narp on the neurobiological and behavioral effects of repeated ECS, Chang and colleagues found that Narp is required for the antidepressant-like effects, but not the proliferation of DG cells, in response to five daily ECS treatments [41]. Kobayashi and Segi-Nishida examined the effect of adrenocorticotropic hormone (ACTH) administration on the cellular, molecular, and behavioral effects of ECS, and found that 11 daily ECS upregulates Bdnf expression and cellular proliferation in mice. While chronic ACTH prevented the increase in Bdnf expression, the treatment did not affect the ECS-induced cellular proliferation [78].
In each of these studies, the intervention, whether ACTH or a genetic disruption (of BDNF or another immediate early gene, Narp), was not sufficient to abrogate the pro-proliferative effect of repeated ECS on hippocampal DG cells. Thus, our finding that Egr3 is not required for the serial ECS-induced hippocampal cellular proliferation is consistent with the findings of other genes, including Bdnf, and is also consistent with our prior finding that ECS-induced Bdnf expression is absent in Egr3−/− mice [32].
Because the dorsal and ventral hippocampus are involved in different functions, we examined the effects of repeated ECS in both of these regions. The dorsal hippocampus largely mediates the encoding of spatial representations [43] while the ventral hippocampus encodes non-spatial representations, and plays an important role in motivation as well as emotional processing [44–46]. Differences in neurogenesis along the dorsal-ventral, or septo-temporal, axis of the hippocampus have likewise been reported, with increased numbers of proliferating cells identified in the dorsal hippocampus following exposure to a spatial-learning task [79]. In addition, the suprapyramidal and infrapyramidal blades of the dentate gyrus have shown differences that contribute uniquely to hippocampal function and behavior. As these blades demonstrate differential functions in response to activity, and as the dorsal and ventral hippocampus play disparate roles, we examined the responses of these subdivisions individually following multiple ECS.
For most of the measures we examined, we found identical results in both regions. However, while ECS increased dendritic branching in the whole hippocampus (Fig. 3A), post-hoc analyses examining each region independently showed a significant increase in the ventral hippocampus, and only a trend effect in the dorsal hippocampus (p = 0.059). While it is possible that this indicates a regional effect of serial ECS, the trend makes it difficult to conclude that DG granule cells in the dorsal hippocampus are less sensitive to the dendritic structural effects of ECS than cells in ventral hippocampus.
4.3. Implications
Egr3−/− mice are a conventional knockout line and thus lack expression of the gene throughout pre- and post-natal development [47]. Although no major neuroanatomical abnormalities have been identified in the central nervous system of the mice, we and others have shown that they have deficits in synaptic plasticity that could share a common mechanism with the altered neurobiological response to serial ECS [25,26]. These deficits in synaptic plasticity, and our current findings of changes in dendritic remodeling in response to ECS, demonstrate that loss of Egr3 alters the way that neurons respond to activity and therefore likely also the response to environmental stimuli. These responses may underlie the memory deficits and behavioral abnormalities we have reported in Egr3−/− mice [25,26,80].
These findings have implications for mental illnesses that are characterized by deficits in cognition and memory, including depression and psychotic disorders. Indeed, recent post-mortem transcriptomic studies in brains from schizophrenia and bipolar disorder subjects identified a module of activity-dependent IEGs that was associated with medication response [81]. Their findings supported studies suggesting that IEGs modulation by antipsychotic may underlie the therapeutic efficacy of these medications [82]. Expression of IEGs, including Egr3, is also induced by psychedelic drugs that produce rapid and long-lasting antidepressant effects [83,84]. Intriguingly, ECS maximally activates expression of IEGs. As suggested by the rodent studies discussed above, the high-level stimulation provided by ECS appears able to overcome the neurobiological and behavioral deficits caused by specific genetic disruptions, paralleling the effectiveness of this therapy for the most severe cases of mood and psychotic disorders in humans.
In summary, these results show that serial ECS causes dendritic remodeling and cellular proliferation in mice, and that Egr3, an activity-induced IEG transcription factor, influences the dendritic structural changes but is not required for ECS-induced cellular proliferation, consistent with reports of other genes investigated to date. These processes may contribute the clinical effectiveness of ECT, which has been shown to cause reorganization and plasticity in neural networks in the human brain [85–87]. In addition, these results suggest that Egr3 may also mediate neuronal remodeling in response to environmental experience, potentially influencing learning, memory, and cognition. This is consistent with known requirements of Egr3 in hippocampal synaptic plasticity and memory behaviors [25,26].
Supplementary Material
Acknowledgments
We are grateful to A. Aden, R. Khoshaba, R. Chung and K. Beck for assistance with ECS. Funding: Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R21MH113154 (to ALG & DFM) and Award Number R01MH097803 (to ALG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- Arc
activity regulated cytoskeleton associated protein
- ACTH
adrenocorticotropic hormone
- Bdnf
brain-derived neurotrophic factor
- BrdU
Bromodeoxyuridine
- DG
dentate gyrus
- ECS
Electroconvulsive seizure
- ECT
Electroconvulsive therapy
- Egr3
early growth response 3
- IEG
immediate early gene
- ip
infrapyramidal
- MDD
major depressive disorder
- Prox1
prospero homeobox 1
- SGZ
subgranular zone
- sp
suprapyramidal
- WT
wildtype
Footnotes
CRediT authorship contribution statement
K.T. Meyers: Lead, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. C.C. Damphousse: Investigation, Formal analysis, Writing – review & editing. A.B. Ozols: Investigation, Formal analysis, Writing – review & editing. J.M. Campbell: Investigation. J.M. Newbern: Supervision, Writing – review & editing. C. Hu: Formal analysis, Writing – review & editing. D.F. Marrone: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – review & editing. A.L. Gallitano: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2023.04.022.
References
- [1].Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Andreasson KI, et al. Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learn Mem 1994;1(2):140–52. [PubMed] [Google Scholar]
- [2].Jung HY, Kang UG, Joo YH, Cho SC, Jeon SH, Park JB, et al. Electroconvulsive shock does not induce c-fos and junB, but TIS1 and TIS8/zif-268, in neonatal rat hippocampus. Brain Res Dev Brain Res 1998;108(1–2):303–6. [DOI] [PubMed] [Google Scholar]
- [3].O’Donovan KJ, Wilkens EP, Baraban JM. Sequential expression of Egr-1 and Egr-3 in hippocampal granule cells following electroconvulsive stimulation. J Neurochem 1998;70(3):1241–8. [DOI] [PubMed] [Google Scholar]
- [4].Kim KA, Chakraborti T, Goldstein G, Johnston M, Bressler J. Exposure to lead elevates induction of zif268 and Arc mRNA in rats after electroconvulsive shock: the involvement of protein kinase C. J Neurosci Res 2002;69(2):268–77. [DOI] [PubMed] [Google Scholar]
- [5].Dyrvig M, Christiansen SH, Woldbye DP, Lichota J. Temporal gene expression profile after acute electroconvulsive stimulation in the rat. Gene 2014;539(1):8–14. [DOI] [PubMed] [Google Scholar]
- [6].Lindefors N, Brodin E, Metsis M. Spatiotemporal selective effects on brain-derived neurotrophic factor and trkB messenger RNA in rat hippocampus by electroconvulsive shock. Neuroscience 1995;65(3):661–70. [DOI] [PubMed] [Google Scholar]
- [7].Chen AC, Shin KH, Duman RS, Sanacora G. ECS-Induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. J ECT 2001;17(1): 27–32. [DOI] [PubMed] [Google Scholar]
- [8].Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatr 2003;54(7):703–9. [DOI] [PubMed] [Google Scholar]
- [9].O’Donovan S, Dalton V, Harkin A, McLoughlin DM. Effects of brief pulse and ultrabrief pulse electroconvulsive stimulation on rodent brain and behaviour in the corticosterone model of depression. Int J Neuropsychopharmacol 2014;17(9): 1477–86. [DOI] [PubMed] [Google Scholar]
- [10].Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 2009;323(5917):1074–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao C, Warner-Schmidt J, Duman RS, Gage FH. Electroconvulsive seizure promotes spine maturation in newborn dentate granule cells in adult rat. Dev Neurobiol 2012;72(6):937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smitha JS, Roopa R, Khaleel N, Kutty BM, Andrade C. Images in electroconvulsive therapy: electroconvulsive shocks dose-dependently increase dendritic arborization in the CA1 region of the rat hippocampus. J ECT 2014;30(3):191–2. [DOI] [PubMed] [Google Scholar]
- [13].Maynard KR, Hobbs JW, Rajpurohit SK, Martinowich K. Electroconvulsive seizures influence dendritic spine morphology and BDNF expression in a neuroendocrine model of depression. Brain Stimul 2018;11(4):856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol 2000;165(2):231–6. [DOI] [PubMed] [Google Scholar]
- [15].Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatr 2000;47(12):1043–9. [DOI] [PubMed] [Google Scholar]
- [16].Nakamura K, Ito M, Liu Y, Seki T, Suzuki T, Arai H. Effects of single and repeated electroconvulsive stimulation on hippocampal cell proliferation and spontaneous behaviors in the rat. Brain Res 2013;1491:88–97. [DOI] [PubMed] [Google Scholar]
- [17].Bolwig TG, Madsen TM. Electroconvulsive therapy in melancholia: the role of hippocampal neurogenesis. Acta Psychiatr Scand Suppl 2007;(433):130–5. [DOI] [PubMed] [Google Scholar]
- [18].Smitha JS, Roopa R, Sagar BK, Kutty BM, Andrade C. Images in electroconvulsive therapy: ECS dose-dependently increases cell proliferation in the subgranular region of the rat hippocampus. J ECT 2014;30(3):193–4. [DOI] [PubMed] [Google Scholar]
- [19].Pfaffenseller B, da Silva Magalhaes PV, De Bastiani MA, Castro MA, Gallitano AL, Kapczinski F, et al. Differential expression of transcriptional regulatory units in the prefrontal cortex of patients with bipolar disorder: potential role of early growth response gene 3. Transl Psychiatry 2016;6:e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pfaffenseller B, Kapczinski F, Gallitano AL, Klamt F. EGR3 immediate early gene and the brain-derived neurotrophic factor in bipolar disorder. Front Behav Neurosci 2018;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamada K, Gerber DJ, Iwayama Y, Ohnishi T, Ohba H, Toyota T, et al. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc Natl Acad Sci U S A 2007;104(8):2815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim SH, Song JY, Joo EJ, Lee KY, Ahn YM, Kim YS. EGR3 as a potential susceptibility gene for schizophrenia in Korea. Am J Med Genet Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 2010;153B(7):1355–60. [DOI] [PubMed] [Google Scholar]
- [23].Zhang R, Lu S, Meng L, Min Z, Tian J, Valenzuela RK, et al. Genetic evidence for the association between the early growth response 3 (EGR3) gene and schizophrenia. PLoS One 2012;7(1):e30237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huentelman MJ, Muppana L, Corneveaux JJ, Dinu V, Pruzin JJ, Reiman R, et al. Association of SNPs in EGR3 and ARC with schizophrenia supports a biological pathway for schizophrenia risk. PLoS One 2015;10(10):e0135076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, et al. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience 2007;148(3):633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, et al. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci 2007;35(1):76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol Cell Biol 2005;25(23):10286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, et al. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci U S A 2005;102(33):11894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem 2006;281(40):29431–5. [DOI] [PubMed] [Google Scholar]
- [30].Zhao X, Ozols AB, Meyers KT, Campbell J, McBride A, Marballi KK, et al. Acute sleep deprivation upregulates serotonin 2A receptors in the frontal cortex of mice via the immediate early gene Egr3. Mol Psychiatr 2022;27(3):1599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maple A, Lackie RE, Elizalde DI, Grella SL, Damphousse CC, Xa C, et al. Attenuated late-phase arc transcription in the dentate gyrus of mice lacking Egr3. Neural Plast 2017;2017:6063048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meyers KT, Marballi KK, Brunwasser SJ, Renda B, Charbel M, Marrone DF, et al. The immediate early gene Egr3 is required for hippocampal induction of Bdnf by electroconvulsive stimulation. Front Behav Neurosci 2018;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li J, Ye F, Xiao W, Tang X, Sha W, Zhang X, et al. Increased serum brain-derived neurotrophic factor levels following electroconvulsive therapy or antipsychotic treatment in patients with schizophrenia. Eur Psychiatr 2016;36:23–8. [DOI] [PubMed] [Google Scholar]
- [34].Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 2002;82(6):1367–75. [DOI] [PubMed] [Google Scholar]
- [35].Makiura Y, Suzuki F, Chevalier E, Onteniente B. Excitatory granule cells of the dentate gyrus exhibit a double inhibitory neurochemical content after intrahippocampal administration of kainate in adult mice. Exp Neurol 1999;159 (1):73–83. [DOI] [PubMed] [Google Scholar]
- [36].Yau SY, Lau BW, Tong JB, Wong R, Ching YP, Qiu G, et al. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS One 2011;6(9):e24263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sartorius A, Henn FA. [Continuation ECT]. Psychiatr Prax 2005;32(8):408–11. [DOI] [PubMed] [Google Scholar]
- [38].Vanicek T, Kranz GS, Vyssoki B, Komorowski A, Fugger G, Hoflich A, et al. Repetitive enhancement of serum BDNF subsequent to continuation ECT. Acta Psychiatr Scand 2019;140(5):426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 1999;89(1):157–66. [DOI] [PubMed] [Google Scholar]
- [40].Schloesser RJ, Orvoen S, Jimenez DV, Hardy NF, Maynard KR, Sukumar M, et al. Antidepressant-like effects of electroconvulsive seizures require adult neurogenesis in a neuroendocrine model of depression. Brain Stimul 2015;8(5):862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chang AD, Vaidya PV, Retzbach EP, Chung SJ, Kim U, Baselice K, et al. Narp mediates antidepressant-like effects of electroconvulsive seizures. Neuropsychopharmacology 2018;43(5):1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ueno M, Sugimoto M, Ohtsubo K, Sakai N, Endo A, Shikano K, et al. The effect of electroconvulsive seizure on survival, neuronal differentiation, and expression of the maturation marker in the adult mouse hippocampus. J Neurochem 2019;149 (4):488–98. [DOI] [PubMed] [Google Scholar]
- [43].Levita L, Muzzio IA. Role of the hippocampus in goal-oriented tasks requiring retrieval of spatial versus non-spatial information. Neurobiol Learn Mem 2010;93 (4):581–8. [DOI] [PubMed] [Google Scholar]
- [44].Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A 2002;99(16):10825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem 2004;81(3):172–84. [DOI] [PubMed] [Google Scholar]
- [46].Fendt M, Fanselow MS, Koch M. Lesions of the dorsal hippocampus block trace fear conditioned potentiation of startle. Behav Neurosci 2005;119(3):834–8. [DOI] [PubMed] [Google Scholar]
- [47].Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet 1998;20(1):87–91. [DOI] [PubMed] [Google Scholar]
- [48].Gallitano AL, Satvat E, Gil M, Marrone DF. Distinct dendritic morphology across the blades of the rodent dentate gyrus. Synapse 2016;70(7):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods 1998;79(1):1–4. [DOI] [PubMed] [Google Scholar]
- [50].Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 1953;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- [51].Uylings HB, Ruiz-Marcos A, van Pelt J. The metric analysis of three-dimensional dendritic tree patterns: a methodological review. J Neurosci Methods 1986;18 (1–2):127–51. [DOI] [PubMed] [Google Scholar]
- [52].Avramov MN, Husain MM, White PF. The comparative effects of methohexital, propofol, and etomidate for electroconvulsive therapy. Anesth Analg 1995;81(3): 596–602. [DOI] [PubMed] [Google Scholar]
- [53].Hageman I, Nielsen M, Wortwein G, Diemer NH, Jorgensen MB. Electroconvulsive stimulations prevent stress-induced morphological changes in the hippocampus. Stress 2008;11(4):282–9. [DOI] [PubMed] [Google Scholar]
- [54].Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002;22(15):6810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen F, Danladi J, Wegener G, Madsen TM, Nyengaard JR. Sustained ultrastructural changes in rat hippocampal formation after repeated electroconvulsive seizures. Int J Neuropsychopharmacol 2020;23(7):446–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yanpallewar SU, Barrick CA, Palko ME, Fulgenzi G, Tessarollo L. Tamalin is a critical mediator of electroconvulsive shock-induced adult neuroplasticity. J Neurosci 2012;32(7):2252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ferrante M, Migliore M, Ascoli GA. Functional impact of dendritic branch-point morphology. J Neurosci 2013;33(5):2156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci 2014;369(1635):20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 2012;3:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Woods NI, Vaaga CE, Chatzi C, Adelson JD, Collie MF, Perederiy JV, et al. Preferential targeting of lateral entorhinal inputs onto newly integrated granule cells. J Neurosci 2018;38(26):5843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nozaki K, Kubo R, Furukawa Y. Serotonin modulates the excitatory synaptic transmission in the dentate granule cells. J Neurophysiol 2016;115(6):2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bouckaert F, Sienaert P, Obbels J, Dols A, Vandenbulcke M, Stek M, et al. ECT: its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J ECT 2014;30(2):143–51. [DOI] [PubMed] [Google Scholar]
- [63].Altman J. Are new neurons formed in the brains of adult mammals? Science 1962; 135(3509):1127–8. [DOI] [PubMed] [Google Scholar]
- [64].Alshammari MA, Alshammari TK, Nenov MN, Scala F, Laezza F. Fibroblast growth factor 14 modulates the neurogenesis of granule neurons in the adult dentate gyrus. Mol Neurobiol 2016;53(10):7254–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 2004;131(12):2791–801. [DOI] [PubMed] [Google Scholar]
- [66].Gu F, Hata R, Ma YJ, Tanaka J, Mitsuda N, Kumon Y, et al. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res 2005;81(2): 163–71. [DOI] [PubMed] [Google Scholar]
- [67].Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998;4(11):1313–7. [DOI] [PubMed] [Google Scholar]
- [68].Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013;153(6): 1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018;22(4): 589–599 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018;555(7696):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al. Human hippocampal neurogenesis persists in aged adults and alzheimer’s disease patients. Cell Stem Cell 2019;24(6):974–982 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 2019;25(4):554–60. [DOI] [PubMed] [Google Scholar]
- [73].Boldrini M, Galfalvy H, Dwork AJ, Rosoklija GB, Trencevska-Ivanovska I, Pavlovski G, et al. Resilience is associated with larger dentate gyrus, while suicide decedents with major depressive disorder have fewer granule neurons. Biol Psychiatr 2019;85(10):850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatr 2012;72(7):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20(24): 9104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. J Neurobiol 2000;42(2):248–57. [PubMed] [Google Scholar]
- [77].Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 2003;130(2):391–9. [DOI] [PubMed] [Google Scholar]
- [78].Kobayashi Y, Segi-Nishida E. Search for factors contributing to resistance to the electroconvulsive seizure treatment model using adrenocorticotrophic hormone-treated mice. Pharmacol Biochem Behav 2019;186:172767. [DOI] [PubMed] [Google Scholar]
- [79].Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 2009;19(4):360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gallitano-Mendel A, Wozniak D, Pehek E, Milbrandt J. Mice lacking the immediate early gene Egr3 respond to the anti-aggressive effects of clozapine yet are relatively resistant to its sedating effects. Neuropsychopharmacology 2008;33(6):1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science 2018;362(6420). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].de Bartolomeis A, Buonaguro EF, Latte G, Rossi R, Marmo F, Iasevoli F, et al. Immediate-early genes modulation by antipsychotics: translational implications for a putative gateway to drug-induced long-term brain changes. Front Behav Neurosci 2017;11:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 2003;23(26):8836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gukasyan N, Davis AK, Barrett FS, Cosimano MP, Sepeda ND, Johnson MW, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: prospective 12-month follow-up. J Psychopharmacol 2022;36(2):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A 2014;111(3):1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Petrican R, Soderlund H, Kumar N, Daskalakis ZJ, Flint A, Levine B. Electroconvulsive therapy “corrects” the neural architecture of visuospatial memory: implications for typical cognitive-affective functioning. Neuroimage Clin 2019;23:101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Qi S, Abbott CC, Narr KL, Jiang R, Upston J, McClintock SM, et al. Electroconvulsive therapy treatment responsive multimodal brain networks. Hum Brain Mapp 2020;41(7):1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.