Abstract
“All things are poison, and nothing is without poison; the dosage alone makes it so a thing is not a poison” (Paracelsus, ~ 1538 AD). This well-known quote seems to aptly summarize the current understanding of the interaction between exercise and atrial fibrillation (AF). A host of data strongly suggests that regular exercise has a protective effect against developing AF. A small but well-conducted group of trials also demonstrates beneficial effects of exercise in the treatment of AF. Recently, however, potentially detrimental effects of large volumes of high-intensity exercise on the probability of developing AF have moved into the sports-cardiological focus. This effect is well documented for elite athletes; data regarding the general population is less clear. This review presents the current data regarding the protective, therapeutic and potentially risk-enhancing effects of exercise regarding AF. The authors demonstrate that the benefits are clear and strongly outweigh the potential disadvantages.
Key words: atrial fibrillation, exercise, prevention, treatment, risk factor, athlete
Key Points
A sedentary life-style increases the life-time risk of atrial fibrillation (AF).
Exercise is a beneficial tool in preventing atrial fibrillation. Men seem to maximize their benefit by exercising between 1.5 and 4 hours/week, while up to 6 hours/week seem safe regarding any potential risk-elevation. In men who perform very high volumes of high intensity exercise (i. e. >8 hours/week) the AF-risk may be increased. No such risk-elevation has been observed in women (see Fig. 1 (central illustration)).
Patients already diagnosed with atrial fibrillation seem to benefit from therapeutic physical activity. Based on current data, moderate-intensity exercise for 2 to 3 hours/week seems to be a sensible recommendation. High-intensity interval training may be a safe and beneficial alternative or supplement.
Exercise is beneficial as a preventive and therapeutic tool. Increasing one’s risk of atrial fibrillation through recreational exercise is extremely unlikely, while the general health benefits are well documented.
Fig. 1.
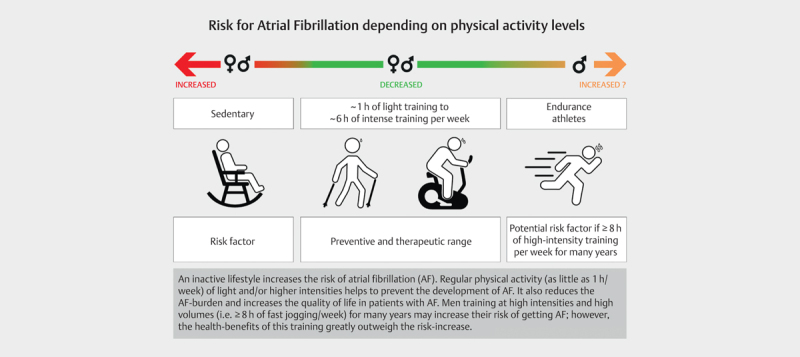
(Central illustration): Overview of the association between inactivity and different levels of activity and atrial fibrillation.
Introduction
Atrial fibrillation (AF) is the most common lasting arrhythmia worldwide. It is characterized as “a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction” 1 . While the current prevalence lies at around 3%, demographic change and a strong age-association of AF lead to rising numbers of patients. Thus, the prevalence is 10% in people >65 years old and >15% among the >80-year-olds 1 . In the coming decades, the prevalence of AF in Europe is calculated to reach ~ 25 million patients 2 3 , likely further elevated by improved detection tools (i. e. smart watches). Atrial fibrillation is one of the leading causes of hospitalization 4 5 6 . In Germany, the number of hospitalizations due to AF in 2016 was >300,000 cases, which was surpassed only by heart failure (~ 450.000 cases). Thus, AF has a highly relevant socioeconomic impact 7 8 9 .
The development of AF does not seem to be monocausal but rather the result of multifactorial processes and risk factors such as older age, arterial hypertension or inflammatory processes 1 . These coincide with the general risk factors of cardiovascular disease, but a strong elevation of the body mass index could be especially relevant 10 . Exercise may have to be listed among these risk factors, albeit only in men and only above a (very high) threshold of training volume 11 .
Atrial fibrillation can appear paroxysmally for only short durations, may terminate spontaneously or may persist for longer periods. Some patients have accepted to live with atrial fibrillation permanently 1 . Symptoms can vary greatly intra- and interindividually between a-, oligo- and highly symptomatic episodes. Symptoms can include one or more of the following: Palpitations, dyspnea, fatigue or angina pectoris. Patients in AF may be able to perform physical work or physical exercise without relevant impairment or suffer large reductions in cardiorespiratory performance 1 .
Treatment strategy depends on symptoms, age, co-morbidities and patient preference. A key element is oral anticoagulation due to the elevated risk of ischemic stroke among patients with AF 1 . The arrhythmia itself can be treated by rate control (slowing down a tachycardic arrhythmic heart beat) or rhythm control (re-establishing and maintaining sinus rhythm). The latter includes medication, cardioversion and ablation therapy (i. e. pulmonary vein isolation, etc.) 1 . More recently life-style interventions including physical activity have broadened the therapeutic strategy (see chapter Exercise in the treatment of AF ).
Exercise and Atrial fibrillation
Physiological and anatomical mechanisms of the interaction between exercise and AF
Regarding the effects of physical activity in preventing AF the modulation of classic cardiovascular risk factors is likely to play a key role. A vast host of evidence demonstrates beneficial effects of regular physical activity on many key risk factors for AF: Hypertension, obesity, diabetes mellitus, coronary artery disease, hyperlipoproteinemia 1 . Several factors are likely associated on a (patho)physiological, anatomical and structural level with the effects of exercise (or its absence) on AF. Exercise modulates inflammatory and antioxidative processes, blood-flow associated transmitter emissions (i. e. vasodilation via nitric oxide) and several metabolic processes 12 13 . These may influence the development of atrial enlargement and fibrosis and the whole complex process called atrial remodeling ; likely associated with changes in electrical conduction, generating a substrate for the occurrence and perpetuation of AF 14 . These mechanisms likely affect the preventive and therapeutic benefits of exercise. Moderate physical activity has been demonstrated to oppose and reverse atrial fibrotic changes in what has been called “reverse remodeling” 15 . While exercise acutely elevates atrial pressure and inflammatory processes during the activity, in the long-run blood-pressure and inflammation are reduced in active compared to inactive subjects. The association between professional athletic careers and an increased risk of AF may be due to the high frequency and duration of the phases of cardiac volume overload and upregulation of inflammatory processes. This may lead to atrial enlargement and fibrotic restructuring 16 17 . Also, the increased vagal activity leading to reduced resting heart rates in professional (endurance) athletes may increase the risk of ectopic beats and electric re-entry-processes 13 18 .
Exercise as prevention- and risk-factor?
While some therapeutic effects of exercise for AF have been demonstrated in prospective randomized controlled trials 19 20 21 , insights regarding its preventive effect are based on observational data 3 22 23 . This is to be expected, as randomizing large numbers of participants to “active” vs. “inactive” for many years of prospective analysis is unethical and unfeasible. However, as has been the case for classic cardiovascular risk-factors like smoking and low-density-lipoprotein(LDL)-cholesterol, large prospective observational studies allow an assessment of inactivity as a risk-factor for AF while statistically minimizing confounding. A challenge for these studies is the heterogeneous definition of “exercise,” “activity & inactivity” and “training”. Initial trials and meta-analysis only assessed the volume of physical activity without considering levels of intensity. Newer trials try to specify, but self-reported activity data remains at risk for inaccuracies. Thus, for example the often applied and repeatedly validated international physical activity questionnaire – short form (IPAQ-SF) – has been deemed “rather inaccurate” in a meta-analyses of validation studies 24 . The metabolic equivalent (MET) has been a helpful tool in more recent studies to classify activities by intensities. It also allows for easier inclusion of different exercise forms into assessments of cumulative activity by grouping activities into MET-minutes or MET-hours 23 .
By now many observational reports demonstrate that exercise has a protective effect against AF, but may also function as a risk factor. The key variables seem to be volume, intensity and sex 13 .
Moderate physical activity
In a large meta-analysis in 2016 Mohanty et al. assessed 22 cohort-, case-control- and observational studies with a total of >650,000 participants, deducing that an inactive life-style increases the life-time risk of AF 2.5-fold independent of the participants’ sex. Moderate physical activity, albeit defined very differently in this heterogenous study-collective, reduced the risk of AF ~ 9% in women and ~ 18% in men when compared to the inactive group 3 . These results are plausible considering the well documented inverse association between cardio-respiratory fitness and the incidence of atrial fibrillation 25 26 27 . In military veterans elevated fitness – often a result of regular moderate intensity training – was significantly associated with a reduced likelihood of developing AF 28 .
In 2020 Elliot et al. 23 published data from the UK Biobank in a large prospective observational analysis including >500,000 participants who were observed since 2007. Data was collected using the IPAQ-SF questionnaire, and in contrast to the heterogenous data of the meta-analysis by Mohanty et al. from 2016 3 this study could work with homogenous definitions of physical activity in a similarly large total cohort. The total amount of physical activity was documented as well as the time spent in vigorous physical activity (defined as ≥ 8 MET). The following activity-groups were assessed regarding their association with the risk of AF and compared to the inactive participants: 500, 1,000, 1,500, 2,000, 2,500, and 5,000 MET-min/week. A similar stratified analysis was conducted only for vigorous activities 23 .
The results of this study underscore the protective effect of exercise against developing AF, starting at an activity level of 500 MET-min per week (i. e. biking leisurely at 100 W for 30 minutes 3x/week). This benefit increased among men up to 1500 MET-min/week (i. e. moderate bike riding for 4 hours/week) and was without upper limit for women. Absolute risk reduction in this zone was between 5 and 10% for both sexes 23 . These findings underscore the prior documentation of the benefits of moderate activity 22 29 30 . In conclusion, moderate levels of physical activity and elevated cardio-respiratory fitness showed a protective effect in men and women.
Vigorous physical activity
Varying definitions of “vigorous” exercise reduce the comparability of different publications as well as their pooled assessment in meta-analyses. More recent studies aimed to clearly define assessed volumes and intensities of exercise. In their 2016 meta-analysis including data of 100,000 women Mohanty et al. 3 found a reduced risk of AF by 28% in women who regularly exercised vigorously, without any reduction of this benefit even with very high volumes of vigorous exercise. The data sets of the approx. 80,000 men included were highly heterogenous, but suggested a 2.5- to 3.5-fold AF-risk increase for those engaging in high volumes of vigorous physical activity 3 . The positive association between all volumes of vigorous physical activity and an AF-risk-reduction was underlined in the large study based on the UK Biobank data with the largest risk-reduction of 16% documented at 2,500 MET-min/week 23 . The isolated analysis of vigorous exercise in men showed no significant risk reduction, but at a volume of 5,000 MET-min/week (i. e. 8 hours of fast jogging/week) an observed risk-increase of 12% 23 .
Atrial fibrillation in Athletes.
An elevated life-time risk of atrial fibrillation among athletes is a well-established phenomenon 11 32 33 34 35 . Data ranges from small retrospective case-control studies to larger prospective cohort studies. Reviews and meta-analyses define between a 2.5- and 5-fold increase in AF-risk for athletes compared to the general population 32 . Most studies conclude that sports disciplines with high volumes and intensities of endurance training (i. e. marathon running, cycling, cross-country skiing, etc.) convey the highest risk-increase 34 . Importantly, >90% of data regarding AF-risk in athletes has been collected in men 33 . Athletes combine many of the well documented and suspected AF-risk factors (see Fig. 2 ). Based on the current understanding of the mechanistic link between exercise and AF (i. e. less atrial enlargement in female than male athletes; see section above titled Physiological and anatomical mechanisms of the interaction between exercise and AF ) a reduced risk among female athletes compared to male athletes seems plausible. Generally, the endurance athlete well exemplifies the tendency deduced in the large observational studies that high levels of endurance exercise may increase AF-risk in men.
Mishima et al. 29 published a large meta-analysis with the most recent evaluation of the association between exercise and incident AF. Prospective cohort studies with a minimum follow-up of 4 years were included. However, exercise levels pooled moderate and intensive activities by translating all data into MET-min/week, thus disenabling any differentiation of effects between moderate and intense forms of exercise regarding AF-risk. The authors found that meeting the World Health Organization (WHO)-recommendations 31 of exercise resulted in a 6% risk-reduction of incident AF. An analysis of the most active sub-group resulted in a risk reduction of 8%. No increase of AF-risk was documented for any assessed exercise volume, but the beneficial association between exercise and freedom from AF became less clear for training volumes above 2,000 MET-min/week.
A sub-group analysis by gender found persistent beneficial effects among women meeting the WHO-recommendations (9% risk reduction) and women exceeding them (12% risk reduction). In men, meeting WHO-recommendations reduced AF-risk by 4%, while the most active male subgroup showed no effect for AF-risk.
Fig. 2.
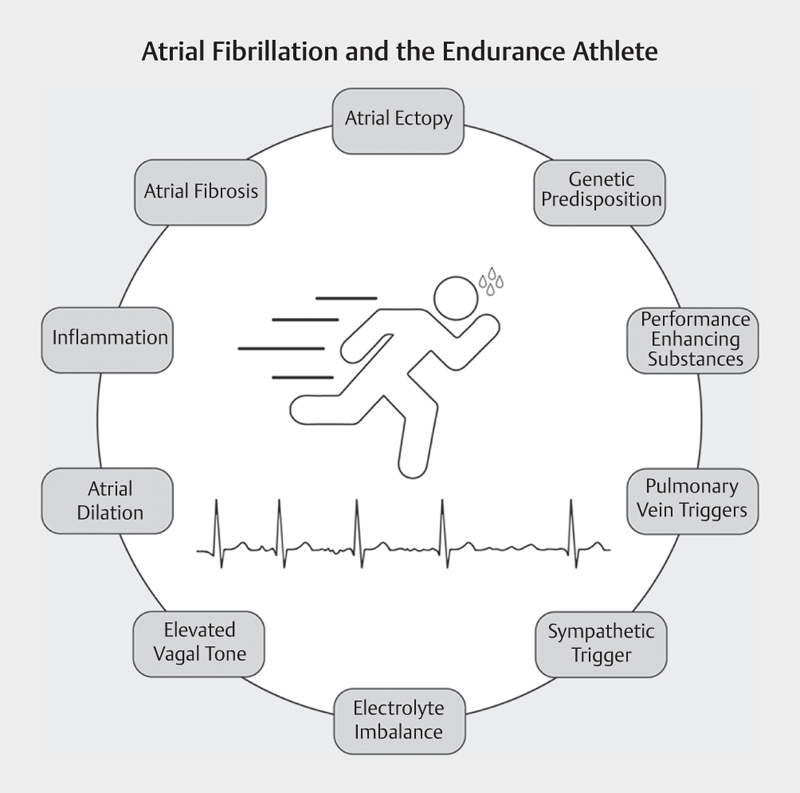
Potential risk factors of atrial fibrillation in athletes.
Exercise as therapeutic agent
While a lot of observational data has been accumulated regarding the association of inactivity and different activity modalities with the risk of AF, data regarding the therapeutic effect of exercise for patients with AF is scarce. In 2014 and 2015 initial non-randomized interventions demonstrated that life-style-adaptations including exercise regimens reduced the incidence, duration and subjective burden of AF-episodes 36 . The few prospective randomized controlled trials are small but promising: In 2016 Risom et al. 20 demonstrated in 200 AF-patients that moderate physical activity improved cardiorespiratory fitness and quality of life without producing relevant adverse events. In the same year Malmo et al. 21 showed a reduction of paroxysmal AF-load between 4 and 8% in patients undergoing high-intensity interval training twice a week compared to standard care. Most recently a trial by Elliot et al. 37 found that a six months vigorous exercise regimen among patients with paroxysmal AF halved the risk of AF-recurrence; a benefit that was sustained for another six months follow-up even after the exercise-intervention period had ended. Several reviews summarizing the available data draw the conclusion that exercise seems to be a beneficial therapy modality in the treatment of AF, but further study is needed 38 39 40 . Trials assessing safety, feasibility and exercise modalities are ongoing 12 . Current guidelines by the European Society of Cardiology (ESC) include recommendations for the practical approach to exercise in AF-patients 41 . Thus, exercise receives a IA-recommendation as a preventive measure of AF. Athletes engaging in high volumes of vigorous exercise experiencing AF should be counselled regarding the effects of the different exercise forms regarding development and recurrence of AF (IB). Patients currently in AF may participate in exercise (IIaC), ideally with heart-rate monitoring via ECG or subjective symptoms. If AF is well tolerated, exercise may be considered without any antiarrhythmic therapy in patients without structural heart disease (IIaC) 41 . Generally, a cardiological work-up is recommended, including a screening for atherosclerotic disease and for further potential causes of AF. Contact sports or those with elevated trauma risk should be avoided in anticoagulated patients. Beta-blockers as the most common form of rate control may negatively impact physical performance and are on the doping list in case of specific sports. An interventional approach using ablation is recommended (IB) for physically active AF-patients, if symptomatic and/or unwilling to undergo medical therapy due to its potentially performance limiting effects. The rate and rhythm control strategy must be tailored to the individual, but the risk of 1:1-conducted atrial flutter must be considered in patients taking class I anti-arrhythmic drugs without a beta-blocker. In these patients, a restraint from intense physical exercise is recommended for at least two days after the use of Flecainide or Propafenone; alternatively a prophylactic ablation of the cavo-tricuspid-isthmus should be considered (IIaC) 41 .
Summary and Conclusion
Atrial fibrillation is the most common lasting arrhythmia and is strongly age associated. Due to demographic changes and the high prevalence of risk factors, the number of AF-patients is rising.
While medical (rhythm and rate-control) and interventional therapies are improving continually, the focus is starting to include the potential of life style interventions, including exercise as a key component: Exercise seems to benefit in the prevention and therapy of AF, but may also play a role as a risk factor. Key factors regarding the latter are volume and intensity of training, as well as sex.
Fig. 1 summarizes the association between exercise and atrial fibrillation: While women seem to reduce their risk of AF with exercise regardless of volume and intensity, current data suggest that an optimal preventive range for men lies within 1.5 to 4 hours of moderate exercise per week. High intensity exercise for >8 hours per week may increase the life-time risk of AF in men, but this remains to be elucidated. Athletes, as a select group with very high life-time training volumes and intensities, seem to have an increased AF-risk of up to five times.
Data assessing the effects of exercise training in AF-therapy are scarce. The little available evidence is promising and strongly suggests that exercise increases cardiorespiratory fitness and reduces the burden of AF in affected patients. Further research is needed.
The mechanisms of the effects of exercise on AF-risk and therapy include direct effects on cardiac structure such as atrial size and fibrosis, as well as the influence of exercise on AF-risk factors such as arterial hypertension or diabetes. While certain volumes and intensities of exercise may slightly increase the risk of AF (in men), based on vast amounts of data underlining its cardiovascular benefits, exercise should be recommended and implemented in prevention and therapy.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Hindricks G, Potpara T, Dagres N et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS)The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Di Carlo A, Bellino L, Consoli D et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace. 2019;21:1468–1475. doi: 10.1093/europace/euz141. [DOI] [PubMed] [Google Scholar]
- 3.Mohanty S, Mohanty P, Tamaki M et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol. 2016;27:1021–1029. doi: 10.1111/jce.13023. [DOI] [PubMed] [Google Scholar]
- 4.Oldgren J, Healey J S, Ezekowitz M et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation. 2014;129:1568–1576. doi: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 5.Kannel W, Wolf P, Benjamin E et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 6.Ball J, Carrington M J, McMurray J J et al. Atrial fibrillation: Profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807–1824. doi: 10.1016/j.ijcard.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 7.Andersson T, Magnuson A, Bryngelsson I-L et al. All-cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995-2008: A Swedish nationwide long-term case-control study. Eur Heart J. 2013;34:1061–1067. doi: 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin E J, Wolf P A, D'Agostino R B et al. Impact of atrial fibrillation on the risk of death: the framingham heart study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Hart C L, Hole D J et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. The. American Journal of Medicine. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 10.Magnussen C, Niiranen T J, Ojeda F M et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the biomarcare consortium (biomarker for cardiovascular risk assessment in europe) Circulation. 2017;136:1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo N, Ramos P, Montserrat S et al. Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: A prospective case-control study. Europace. 2016;18:57–63. doi: 10.1093/europace/euv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zacher J, Dillschnitter K, Freitag N et al. Exercise training in the treatment of paroxysmal atrial fibrillation: study protocol of the Cologne ExAfib Trial. BMJ Open. 2020;10:e040054. doi: 10.1136/bmjopen-2020-040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nattel S. Physical activity and atrial fibrillation risk: It’s complicated; and sex is critical. Eur Heart J. 2020;41:1487–1489. doi: 10.1093/eurheartj/ehz906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan A Y, Zimetbaum P. Atrial Fibrillation and Atrial Fibrosis. J Cardiovasc Pharmacol. 2011;57:625–629. doi: 10.1097/FJC.0b013e3182073c78. [DOI] [PubMed] [Google Scholar]
- 15.Thomas L, Abhayaratna W P. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10:65–77. doi: 10.1016/j.jcmg.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Benito B, Gay-Jordi G, Serrano-Mollar A et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 17.Guasch E, Benito B, Qi X et al. Atrial fibrillation promotion by endurance exercise: Demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 18.Drum S N, Donath L, Dehlin C et al. Atrial Fibrillation: Should Lifelong Athletes Be Worried? Strength Cond J. 2019;42:1. [Google Scholar]
- 19.Pathak R K, Middeldorp M E, Lau D H et al. Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation: The ARREST-AF Cohort Study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Risom S S, Zwisler A-D, Rasmussen T B et al. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: Results of the randomized CopenHeartRFA trial. Am Heart J. 2016;181:120–129. doi: 10.1016/j.ahj.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Malmo V, Nes B M, Amundsen B H et al. Aerobic Interval Training Reduces the Burden of Atrial Fibrillation in the Short Term: A Randomized Trial. Circulation 2016. 2016;133:457–459. doi: 10.1161/CIRCULATIONAHA.115.018220. [DOI] [PubMed] [Google Scholar]
- 22.Elliott A D, Maatman B, Emery M S et al. The role of exercise in atrial fibrillation prevention and promotion: Finding optimal ranges for health. Heart Rhythm. 2017;14:1713–1720. doi: 10.1016/j.hrthm.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Elliott A D, Linz D, Mishima R et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: Evidence from the UK Biobank cohort. Eur Heart J. 2020;41:1479–1486. doi: 10.1093/eurheartj/ehz897. [DOI] [PubMed] [Google Scholar]
- 24.Lee P H, Macfarlane D J, Lam T H et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdicchio C, Elliott A, Mahajan R et al. Greater cardiorespiratory fitness reduces incidence of atrial fibrillation: A meta-analysis. Eur J Prev Cardiol. 2021:28. doi: 10.1093/eurjpc/zwab061.200. [DOI] [Google Scholar]
- 26.Hussain N, Gersh B J, Gonzalez Carta K et al. Impact of Cardiorespiratory Fitness on Frequency of Atrial Fibrillation, Stroke, and All-Cause Mortality. Am J Cardiol. 2018;121:41–49. doi: 10.1016/j.amjcard.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Garnvik L E, Malmo V, Janszky I et al. Physical activity, cardiorespiratory fitness, and cardiovascular outcomes in individuals with atrial fibrillation: The HUNT study. Eur Heart J. 2020;41:1467–1475. doi: 10.1093/eurheartj/ehaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faselis C, Kokkinos P, Tsimploulis A et al. Exercise capacity and atrial fibrillation risk in veterans: a cohort study. Mayo Clinic Proceedings. 2016;91:558–566. doi: 10.1016/j.mayocp.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Mishima R S, Verdicchio C V, Noubiap J J et al. Self-reported Physical Activity and Atrial Fibrillation Risk: A Systematic Review and Meta-analysis. Heart Rhythm. 2021;18:520–528. doi: 10.1016/j.hrthm.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Ricci C, Gervasi F, Gaeta M et al. Physical activity volume in relation to risk of atrial fibrillation. A non-linear meta-regression analysis. Eur J Prev Cardiol. 2018;25:857–866. doi: 10.1177/2047487318768026. [DOI] [PubMed] [Google Scholar]
- 31.Bull F C, Al-Ansari S S, Biddle S et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdulla J, Nielsen J R. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 33.Flannery M D, Kalman J M, Sanders P et al. State of the Art Review: Atrial Fibrillation in Athletes. Heart Lung Circ. 2017;26:983–989. doi: 10.1016/j.hlc.2017.05.132. [DOI] [PubMed] [Google Scholar]
- 34.Newman W, Parry-Williams G, Wiles J et al. Risk of atrial fibrillation in athletes: a systematic review and meta-analysis. Br J Sports Med. 2021;55:1233–1238. doi: 10.1136/bjsports-2021-103994. [DOI] [PubMed] [Google Scholar]
- 35.Mascia G, Perrotta L, Galanti G et al. Atrial fibrillation in athletes. Int J Sports Med. 2013;34:379–384. doi: 10.1055/s-0032-1321896. [DOI] [PubMed] [Google Scholar]
- 36.Pathak R K, Middeldorp M E, Meredith M et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (legacy) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Elliott A. An exercise and physical activity program in patients with atrial fibrillation: The ACTIVE-AF randomised controlled trial. European Society of Cardiology Yearly Congress. 2021.
- 38.Myrstad M, Malmo V, Ulimoen S R et al. Exercise in individuals with atrial fibrillation. Clin Res Cardiol. 2019;108:347–354. doi: 10.1007/s00392-018-1361-9. [DOI] [PubMed] [Google Scholar]
- 39.Reed J L, Terada T, Chirico D et al. The effects of cardiac rehabilitation in patients with atrial fibrillation: a systematic review. Can J Cardiol. 2018;34:284–295. doi: 10.1016/j.cjca.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Risom S S, Zwisler A-D, Johansen P P et al. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst Rev. 2017;2:CD011197. doi: 10.1002/14651858.CD011197.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelliccia A, Sharma S, Gati S et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa735. [DOI] [PubMed] [Google Scholar]


