Abstract
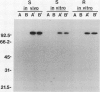
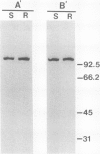
The fungus Cochliobolus victoriae causes victoria blight of oats and produces the host-specific toxin victorin. The reaction of oats to the fungus and its toxin is controlled by a single dominant gene whose product has been hypothesized to function as the site of action (receptor) of the toxin in susceptible oat genotypes. Previously, using a biologically active 125I derivative of the toxin, we identified a 100 kilodalton victorin-binding protein (VBP) which binds victorin in a ligand-specific manner and binds in vivo only in susceptible oat genotypes. However, a VBP in both the susceptible and resistant oat genotypes was identified by in vitro binding experiments. One interpretation of the lack of genotype-specific binding in vitro is that the 100 kilodalton protein detected in vitro is not the same 100 kilodalton protein detected in vivo. To clarify the relationship between the 100 kilodalton protein(s) labeled in vivo and in vitro, we developed antisera to the in vitro-labeled VBP from the susceptible genotype and demonstrated that these preparations react with the in vivo-labeled VBP from the susceptible genotype. This finding coupled with previous observations strongly suggest that the VBP observed in vivo is the same protein detected in vitro. Furthermore, the results support our previous observations which suggest that the VBPs labeled in vitro in susceptible and resistant genotypes are closely related or identical.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Macko K. A., Hodos W. Near point of accommodation in pigeons. Vision Res. 1985;25(10):1529–1530. doi: 10.1016/0042-6989(85)90232-9. [DOI] [PubMed] [Google Scholar]
- Scheffer R. P., Livingston R. S. Host-selective toxins and their role in plant diseases. Science. 1984 Jan 6;223(4631):17–21. doi: 10.1126/science.223.4631.17. [DOI] [PubMed] [Google Scholar]
- Wolpert T. J., Macko V., Acklin W., Arigoni D. Molecular Features Affecting the Biological Activity of the Host-Selective Toxins from Cochliobolus victoriae. Plant Physiol. 1988 Sep;88(1):37–41. doi: 10.1104/pp.88.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert T. J., Macko V. Specific binding of victorin to a 100-kDa protein from oats. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4092–4096. doi: 10.1073/pnas.86.11.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]