Abstract
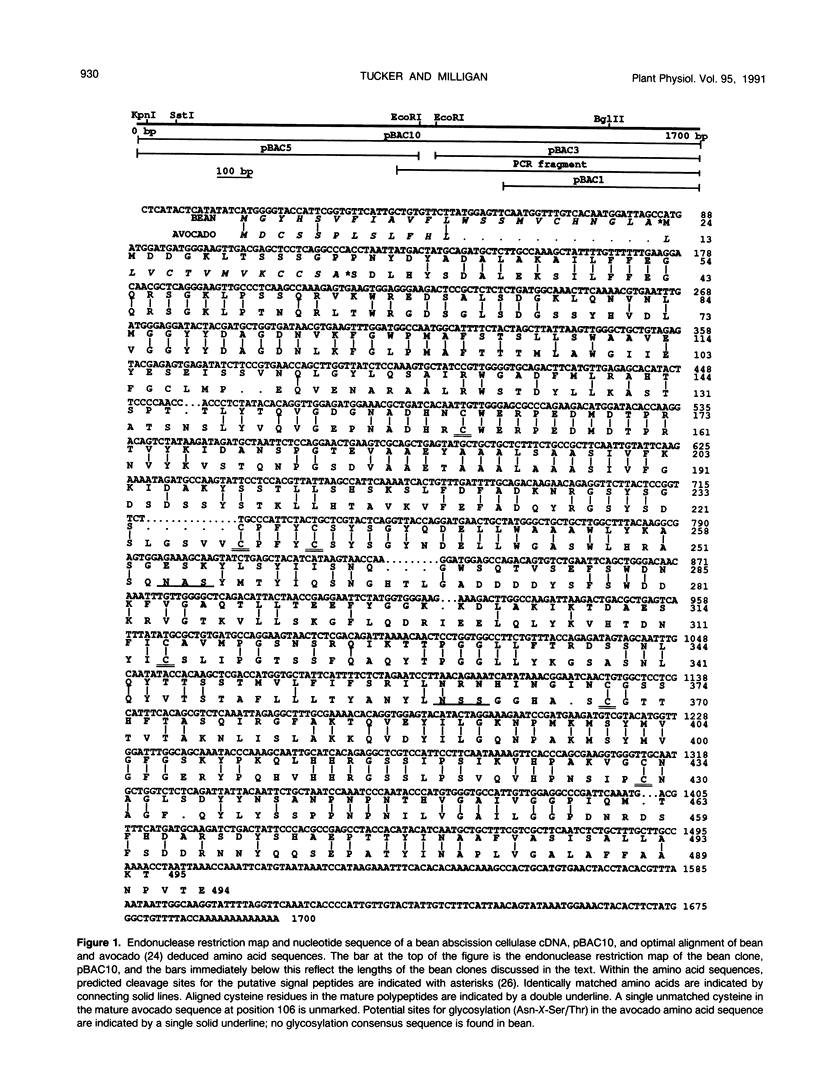
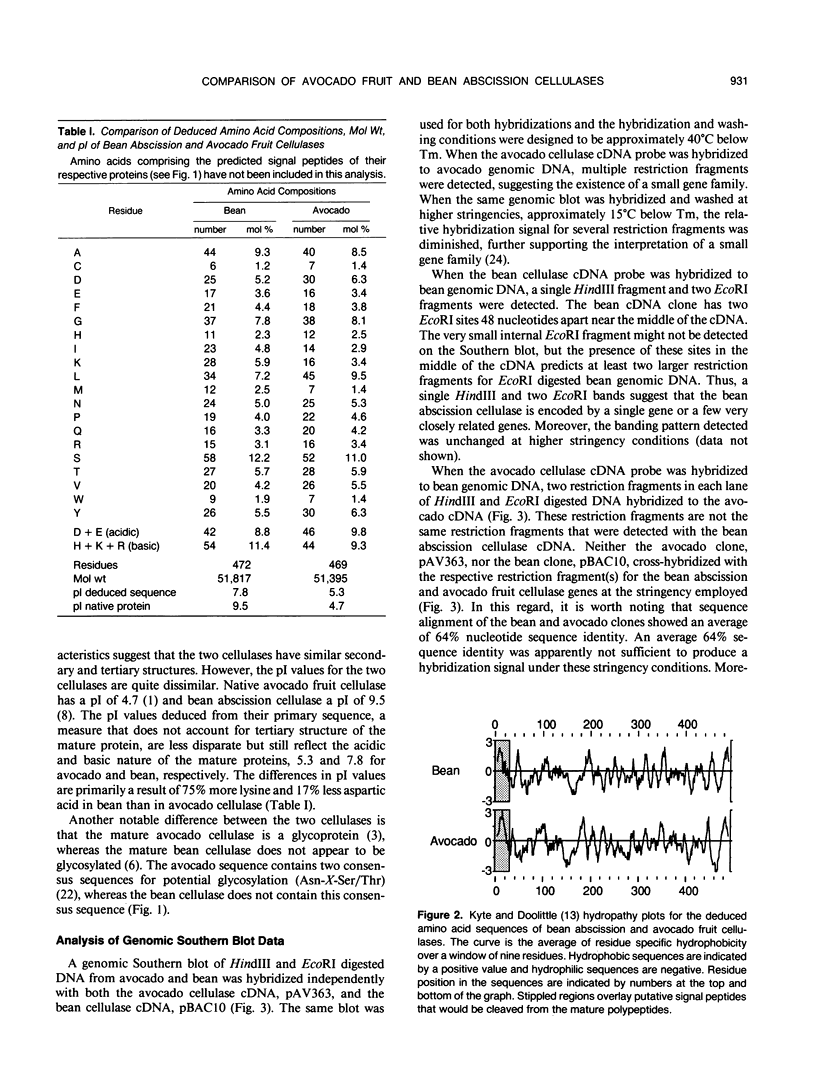
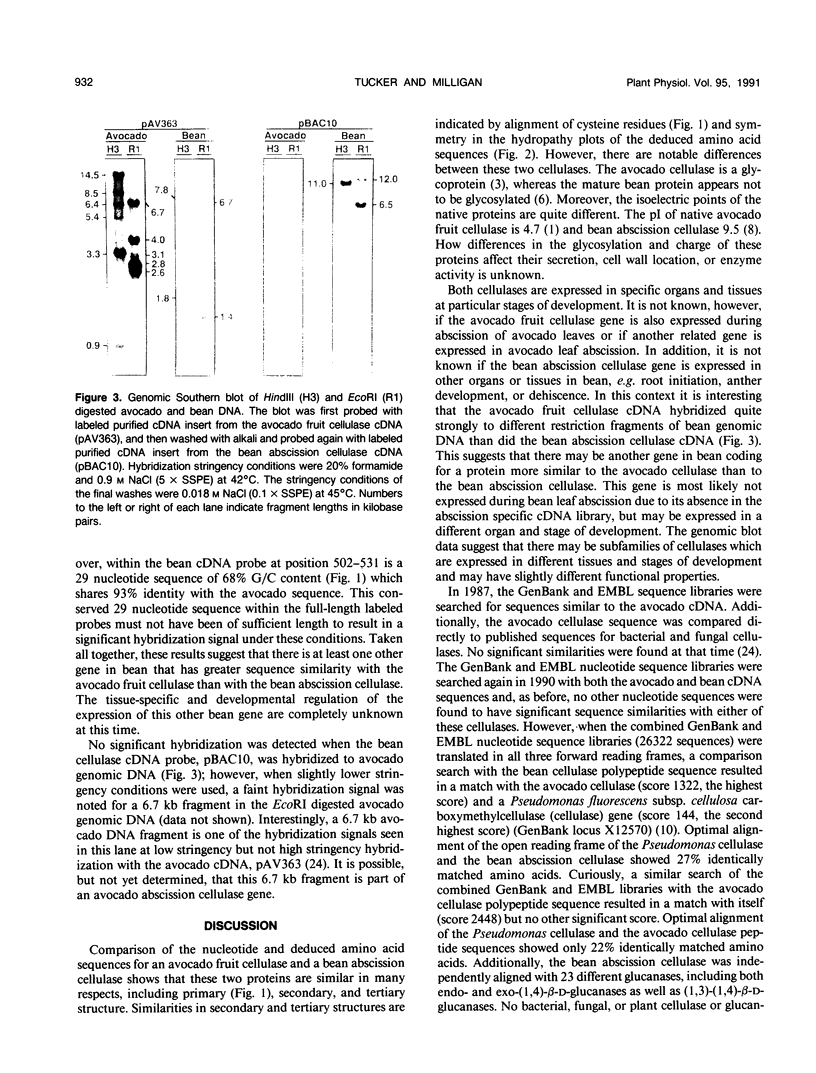
A 1700 nucleotide cDNA clone for a bean (Phaseolus vulgaris cv Red Kidney) abscission cellulase (endo-(1,4)-β-d-glucanase) has been identified and sequenced. This cDNA clone contains a 1485 nucleotide open reading frame which includes coding sequences for a putative signal peptide and mature protein. The nucleotide and deduced amino acid sequences for the bean abscission cellulase are compared to the previously reported sequences of an avocado fruit ripening cellulase. Optimal alignment of these sequences shows 64% and 50% identically matched nucleotides and amino acids, respectively. Analysis of the deduced amino acid sequences for the mature bean and avocado cellulases indicates that these two proteins share similar molecular weights, position of cysteine residues, and hydropathic character, but have very different isoelectric points and glycosylation. Genomic blot data suggest that the avocado fruit cellulase belongs to a small gene family, whereas the bean abscission cellulase appears to be encoded by a single gene or a few very closely related genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., Christoffersen R. E. Synthesis and processing of cellulase from ripening avocado fruit. Plant Physiol. 1986 Jul;81(3):830–835. doi: 10.1104/pp.81.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E., Jones W. A., Jones D. T., Woods D. R. Sequencing and expression of a cellodextrinase (ced1) gene from Butyrivibrio fibrisolvens H17c cloned in Escherichia coli. Mol Gen Genet. 1990 Sep;223(2):310–318. doi: 10.1007/BF00265068. [DOI] [PubMed] [Google Scholar]
- Byrne H., Christou N. V., Verma D. P., Maclachlan G. A. Purification and characterization of two cellulases from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1012–1018. [PubMed] [Google Scholar]
- Del Campillo E., Durbin M., Lewis L. N. Changes in Two Forms of Membrane-Associated Cellulase during Ethylene-Induced Abscission. Plant Physiol. 1988 Nov;88(3):904–909. doi: 10.1104/pp.88.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hall J., Gilbert H. J. The nucleotide sequence of a carboxymethylcellulase gene from Pseudomonas fluorescens subsp. cellulosa. Mol Gen Genet. 1988 Jul;213(1):112–117. doi: 10.1007/BF00333406. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauris S., Rücknagel K. P., Schwarz W. H., Kratzsch P., Bronnenmeier K., Staudenbauer W. L. Sequence analysis of the Clostridium stercorarium celZ gene encoding a thermoactive cellulase (Avicelase I): identification of catalytic and cellulose-binding domains. Mol Gen Genet. 1990 Sep;223(2):258–267. doi: 10.1007/BF00265062. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Linkins A. E., Lewis L. N., Palmer R. L. Hormonally Induced Changes in the Stem and Petiole Anatomy and Cellulase Enzyme Patterns in Phaseolus vulgaris L. Plant Physiol. 1973 Dec;52(6):554–560. doi: 10.1104/pp.52.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M. L., Laties G. G. Interrelationship of Gene Expression, Polysome Prevalence, and Respiration during Ripening of Ethylene and/or Cyanide-Treated Avocado Fruit. Plant Physiol. 1984 Feb;74(2):307–315. doi: 10.1104/pp.74.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M. L., Sexton R., Del Campillo E., Lewis L. N. Bean abscission cellulase : characterization of a cDNA clone and regulation of gene expression by ethylene and auxin. Plant Physiol. 1988 Dec;88(4):1257–1262. doi: 10.1104/pp.88.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]



