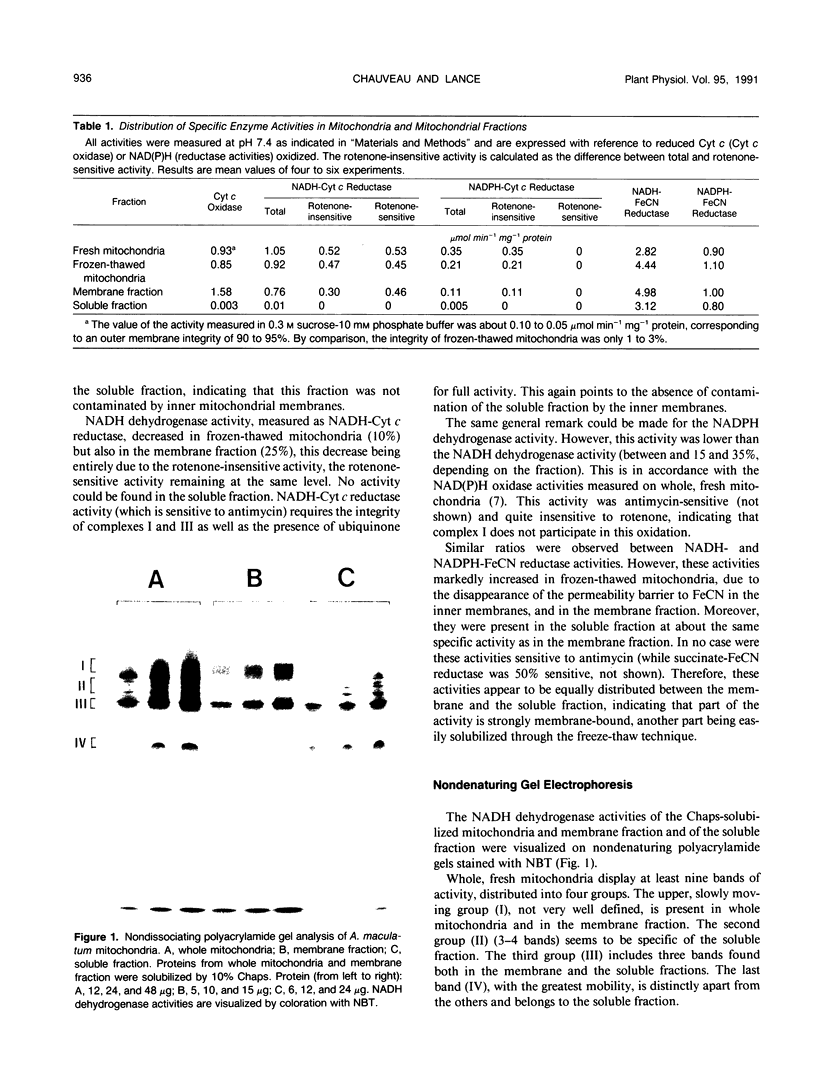
Abstract
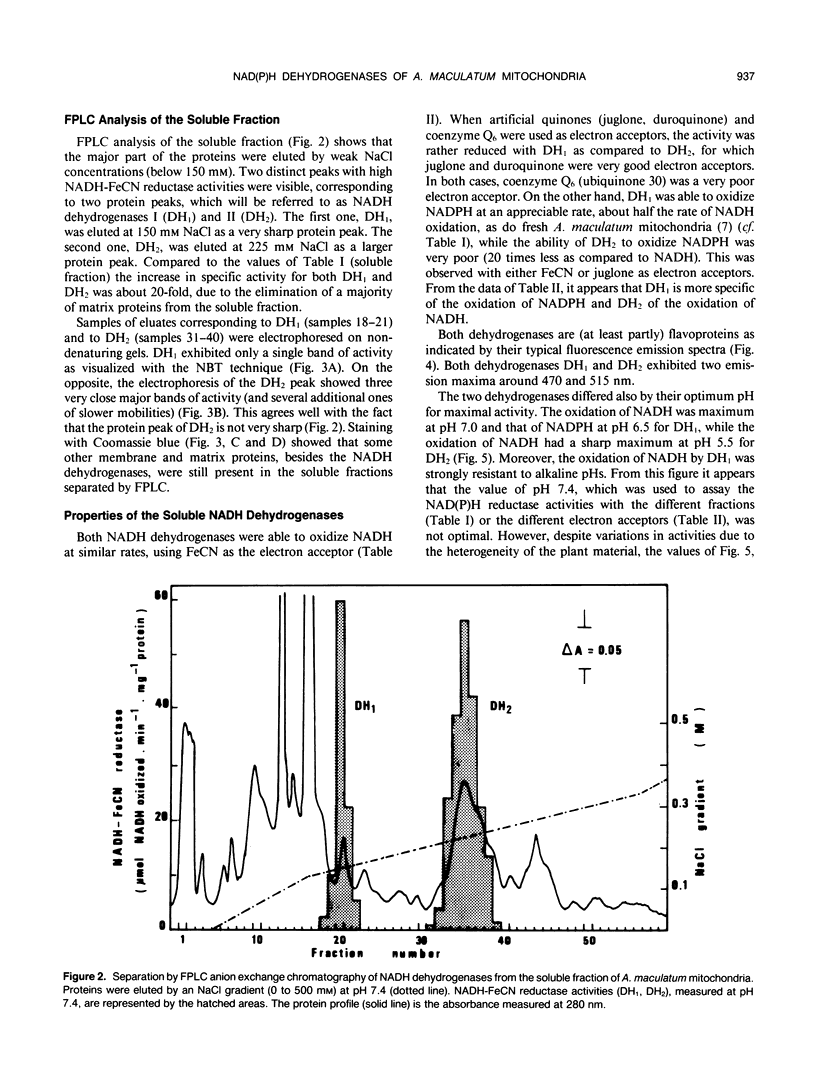
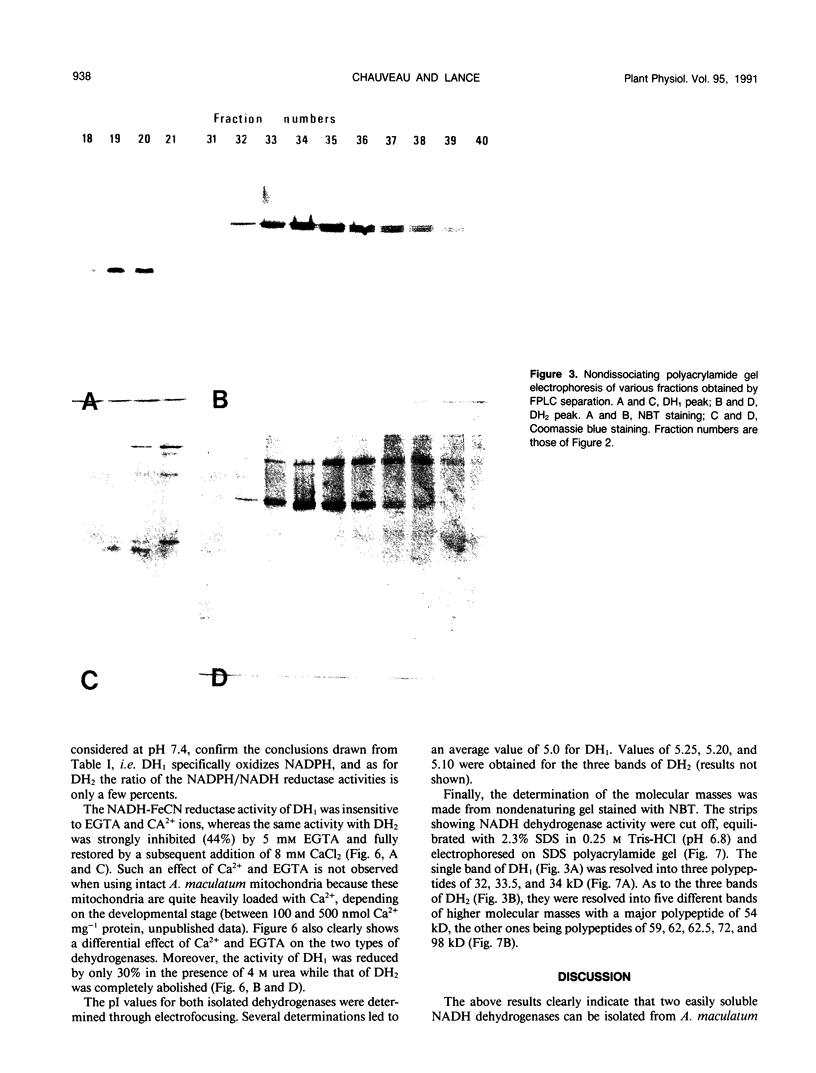
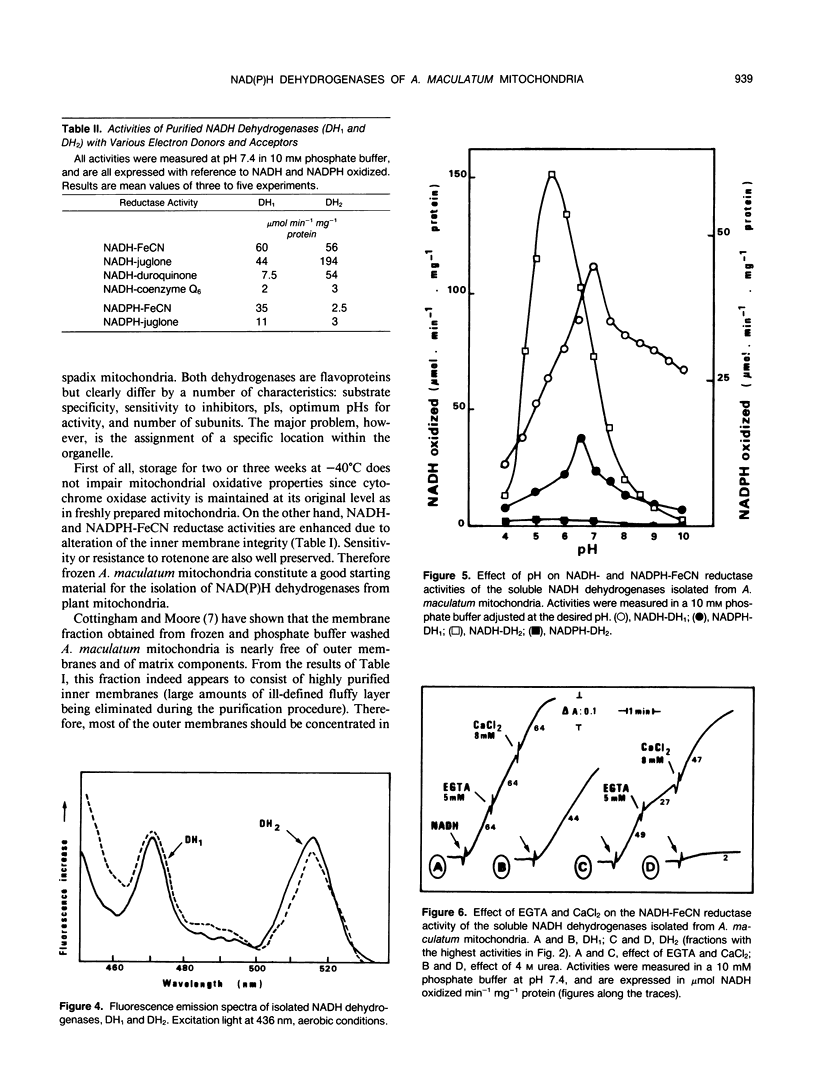
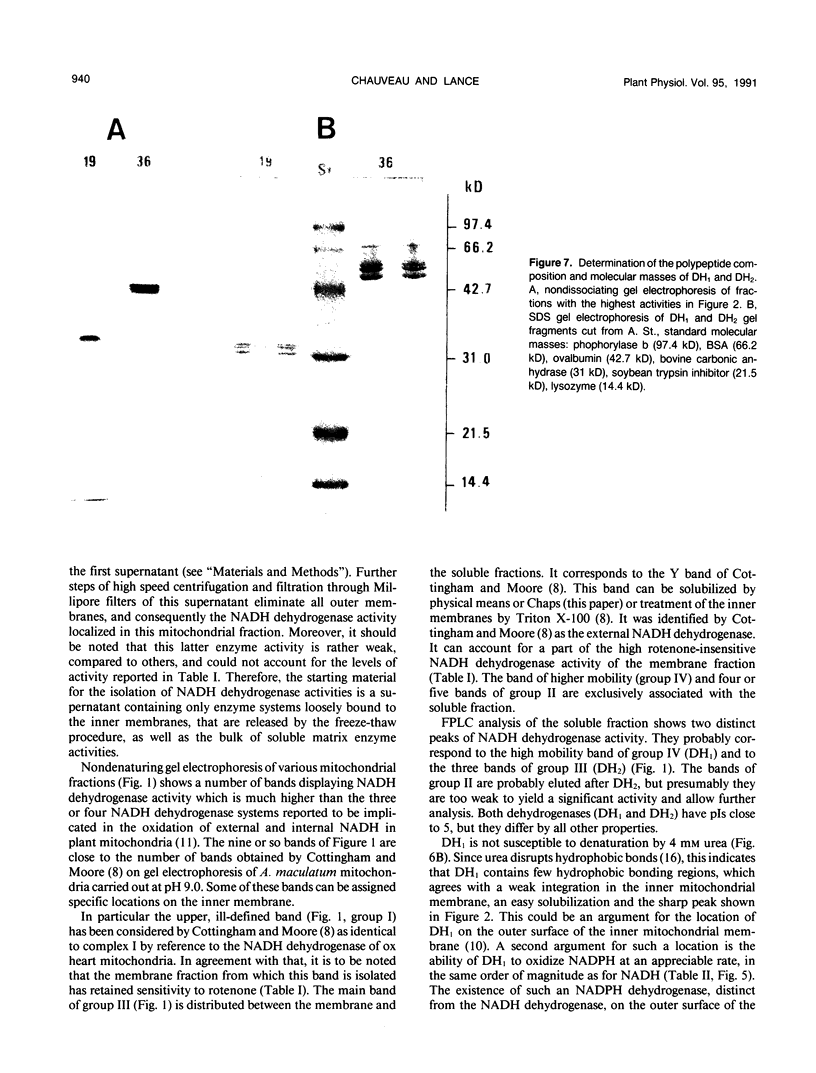
Two enzyme systems carrying out the oxidation of NAD(P)H in the presence of various electron acceptors have been isolated and partially characterized from the supernatant of frozen-thawed mitochondria from Arum maculatum spadices. The two systems contain flavoproteins and differ by their ability to oxidize NADH or NADPH, optimum pH and pI values, sensitivity to Ca2+ and EGTA, denaturation by 4 molar urea, molecular mass, and number of subunits. These properties, together with methodological considerations, are compatible with the location of these enzyme activities on the outer surface of the inner mitochondrial membrane, and support the hypothesis of the existence of two separate dehydrogenases responsible for the mitochondrial oxidation of cytosolic NADH and NADPH.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arron G. P., Edwards G. E. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by plant mitochondria. Can J Biochem. 1979 Dec;57(12):1392–1399. doi: 10.1139/o79-185. [DOI] [PubMed] [Google Scholar]
- Brunton C. J., Palmer J. M. Pathways for the oxidation of malate and reduced pyridine nucleotide by wheat mitochondria. Eur J Biochem. 1973 Nov 1;39(1):283–291. doi: 10.1111/j.1432-1033.1973.tb03125.x. [DOI] [PubMed] [Google Scholar]
- Coleman J. O.D., Palmer J. M. Role of Ca(2+) in the oxidation of exogenous NADH by plant mitochondria. FEBS Lett. 1971 Oct 1;17(2):203–208. doi: 10.1016/0014-5793(71)80148-5. [DOI] [PubMed] [Google Scholar]
- Cook N. D., Cammack R. Purification and characterization of the rotenone-insensitive NADH dehydrogenase of mitochondria from Arum maculatum. Eur J Biochem. 1984 Jun 15;141(3):573–577. doi: 10.1111/j.1432-1033.1984.tb08231.x. [DOI] [PubMed] [Google Scholar]
- Cottingham I. R., Cleeter M. W., Ragan C. I., Moore A. L. Immunological analysis of plant mitochondrial NADH dehydrogenases. Biochem J. 1986 May 15;236(1):201–207. doi: 10.1042/bj2360201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham I. R., Moore A. L. Analysis of NADH dehydrogenases from plant [mung bean (Phaseolus aureus)] mitochondrial membranes on non-denaturing polyacrylamide gels and purification of complex I by band excision. Biochem J. 1988 Aug 15;254(1):303–305. doi: 10.1042/bj2540303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham I. R., Moore A. L. Partial purification and properties of the external NADH dehydrogenase from cuckoo-pint (Arum maculatum) mitochondria. Biochem J. 1984 Nov 15;224(1):171–179. doi: 10.1042/bj2240171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Edman K., Ericson I., Møller I. M. The regulation of exogenous NAD(P)H oxidation in spinach (Spinacia oleracea) leaf mitochondria by pH and cations. Biochem J. 1985 Dec 1;232(2):471–477. doi: 10.1042/bj2320471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger B. O. Adaptation of the Bradford protein assay to membrane-bound proteins by solubilizing in glucopyranoside detergents. Anal Biochem. 1987 Apr;162(1):11–17. doi: 10.1016/0003-2697(87)90004-2. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Johnston S. P., Møller I. M., Palmer J. M. The stimulation of exogenous NADH oxidation in Jerusalem artichoke mitochondria by screening of charges on the membranes. FEBS Lett. 1979 Dec 1;108(1):28–32. doi: 10.1016/0014-5793(79)81171-0. [DOI] [PubMed] [Google Scholar]
- Kamoun P. P. Denaturation of globular proteins by urea: breakdown of hydrogen or hydrophobic bonds? Trends Biochem Sci. 1988 Nov;13(11):424–425. doi: 10.1016/0968-0004(88)90211-3. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Burke J. J. Separation Procedure and Partial Characterization of Two NAD(P)H Dehydrogenases from Cauliflower Mitochondria. Plant Physiol. 1984 Oct;76(2):436–441. doi: 10.1104/pp.76.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe D. E., Miller R. J. Oxidation of reduced nicotinamide adenine dinucleotide phosphate by isolated corn mitochondria. Plant Physiol. 1972 Mar;49(3):353–357. doi: 10.1104/pp.49.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Akerman K. E. Ca2+ stimulation of the external NADH dehydrogenase in Jerusalem artichoke (Helianthus tuberosum) mitochondria. Biochem Biophys Res Commun. 1982 Nov 30;109(2):513–517. doi: 10.1016/0006-291x(82)91751-x. [DOI] [PubMed] [Google Scholar]
- Moreau F., Romani R. Preparation of Avocado Mitochondria Using Self-Generated Percoll Density Gradients and Changes in Buoyant Density during Ripening. Plant Physiol. 1982 Nov;70(5):1380–1384. doi: 10.1104/pp.70.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Johnston S. P., Palmer J. M. A specific role for Ca2+ in the oxidation of exogenous NADH by Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1981 Feb 15;194(2):487–495. doi: 10.1042/bj1940487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Palmer J. M. Charge screening by cations affects the conformation of the mitochondrial inner membrane. A study of exogenous MAD(P)H oxidation in plant mitochondria. Biochem J. 1981 Jun 1;195(3):583–588. doi: 10.1042/bj1950583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries S., Grivell L. A. Purification and characterization of a rotenone-insensitive NADH:Q6 oxidoreductase from mitochondria of Saccharomyces cerevisiae. Eur J Biochem. 1988 Sep 15;176(2):377–384. doi: 10.1111/j.1432-1033.1988.tb14292.x. [DOI] [PubMed] [Google Scholar]