Abstract
Liver cancer remains a challenge of global health, being the 4th leading cause of cancer death worldwide. Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, and is usually precipitated by chronic viral infections (hepatitis B and C), non-alcoholic steatohepatitis, heavy alcohol use, and other factors which may lead to chronic inflammation and cirrhosis of the liver. There have been significant advances in the systemic treatment options for HCC over the past decades, with several approvals of both immune checkpoint inhibitors and tyrosine kinase inhibitors in patients with preserved liver function. These advances have led to improvement in survival outcomes, with expected survival of greater than 18 months, in those with sensitive tumors, adequate liver function, and those functionally fit to receive sequential therapies. Several ongoing and promising trials are now evaluating combinational strategies with novel systemic agents and combinations of systemic therapy with locoregional therapy. In view of these trials, further advances in the treatment of HCC are foreseen in the near future.
Keywords: Hepatocellular carcinoma, Liver cancer, Immunotherapy, Tyrosine protein kinase inhibitors
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, encompassing 80–90% of primary liver cancers. Despite significant advances in therapeutics, HCC has high mortality rates in the United States and globally [1,2]. HCC is the 6th most common cancer and 4th leading cause of cancer-related death globally, and thus a significant affliction to public health [3]. Chronic hepatic inflammation and liver cirrhosis from any cause is the strongest risk factor for HCC. Chronic inflammation may arise from heavy alcohol use, nonalcoholic steatohepatitis (NASH), viral infections (hepatitis B [HBV] and hepatitis C [HCV]), chronic toxin exposure (e.g., aflatoxin). Lifestyle factors like chronic alcohol consumption, dietary habits, and sedentary lifestyle, have led to a continued rise in the incidence of HCC despite advances in anti-viral therapies in dampening HBV and HCV related cirrhosis [3,4].
Diagnosis of HCC remains largely based on radiologic findings [5]. This is in the setting of increased need for better molecular characterization of the disease which requires either tissue for analysis or liquid biopsy, or sometimes both. Immune checkpoint inhibitors (ICI) blocking programmed death ligand 1 (PD-L1) or programmed cell death protein 1 (PD-1) have transformed the treatment landscape for HCC and now form the backbone of most systemic therapies in clinical practice and in trials. Several systemic options have been approved for first-line therapies (atezolizumab and bevacizumab [atezo-bev], durvalumab and tremelimumab [durva-treme], sorafenib, lenvatinib). In addition, several second-line agents such as regorafenib, cabozantinib, ramucirumab, nivolumab with ipilimumab, and pembrolizumab are now approved and available. Many ongoing trials are investigating novel strategies involving tyrosine kinase inhibitors in combination with ICI, locoregional treatment in combination with ICI, and ICI combination strategies in the neoadjuvant setting. Herein, we review the current approaches to the management of advanced HCC. We discuss the evolution of systemic therapy for HCC, strategies for treatment selection and sequencing, clinical challenges in the treatment of HCC, and future directions for novel therapeutic strategies for HCC.
APPROACH TO MANAGEMENT
The relatively recent availability of multiple systemic options in the first and subsequent line settings have significantly changed the treatment landscape for HCC. Most patients with HCC have underlying cirrhosis, which significantly impacts their health, performance status, and ability to tolerate surgical, locoregional and systemic treatments. As a result, treatment must be individualized [6]. Currently, there is a widespread global consensus among clinicians to base treatment of HCC on the tumor stage based on the Barcelona Clinic Liver Cancer (BCLC) staging system [7,8]. Patients who have early-stage HCC (BCLC 0 or BCLC A) are candidates for treatment with surgical resection, ablation or liver transplant [9]. Patients with intermediate-stage HCC (BCLC B) are treated with locoregional therapies such as trans-arterial chemoembolization (TACE), trans-arterial radioembolization with yttrium-90 (Y90), and/or systemic therapy [9]. Several randomized studies are ongoing evaluating the combination of systemic treatment with locoregional treatment for intermediate-stage HCC (NCT04246177, NCT03778957, NCT04340193, NCT04268888). Patients with advanced-stage HCC (BCLC C) are treated with systemic therapies upfront. Select patients with BCLC stage B and rarely C can become candidates for liver transplant with adequate downstaging.
Prognosis of patients with HCC correlates well with their BCLC stage. Median survival ranges from greater than 10 years in patients who receive liver transplant for early stage, to more than 6 years for patients who undergo resection or ablation, approximately 26–30 months for patients with intermediate-stage HCC, and approximately 19 months in patients with advanced stage with compensated liver function (Child Pugh A cirrhosis) [3]. These outcomes are significantly improved in the era of immunotherapy as compared to historic data in patients with HCC [10].
SYSTEMIC THERAPIES
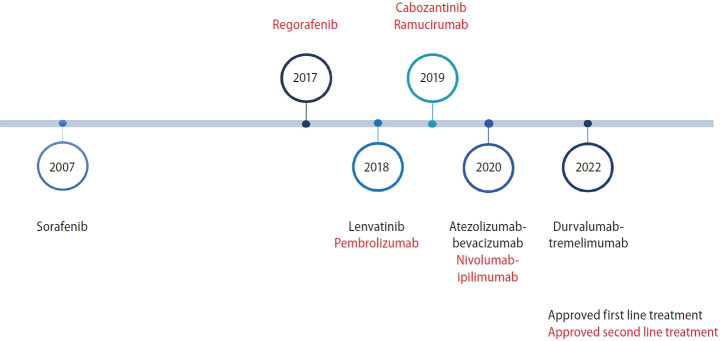
In treatment-naïve patients with unresectable HCC, sorafenib was first shown to prolong survival compared to placebo [11]. Lenvatinib has been shown to be non-inferior to sorafenib [12]. Both atezo-bev and durva-treme improved survival compared to sorafenib [13,14]. Finally, durvalumab monotherapy has been shown to be non-inferior to sorafenib [14]. A summary of the currently approved systemic therapies for the management of advanced HCC is shown in Figure 1.
Figure 1.
Timeline of approved systemic therapies in management of advanced HCC. HCC, hepatocellular carcinoma.
Currently approved tyrosine-kinase inhibitors and anti-angiogenic agents for treatment of HCC
Prior to 2007, there were no standard systemic therapies for HCC. Cytotoxic chemotherapy was used with limited benefit with high rates of toxicity often stemming from underlying pre-existing liver dysfunction in treated patients. The SHARP trial in 2007 was the first clinical trial to show a survival benefit in HCC where sorafenib, a tyrosine kinase inhibitor (TKI), improved median overall survival (OS) from 7.9 months to 10.7 months when compared to placebo (hazard ratio [HR] 0.69, 95% confidence interval [CI] 0.55–0.87; P<0.001) [11].
Lenvatinib, a multi-kinase TKI (targeting VEGF receptor 1–3, FGF receptor 1–4, PDGF receptor alpha, RET, and KIT), was then studied in comparison to sorafenib in the REFLECT trial. Median OS in the lenvatinib group was non-inferior to sorafenib (median OS 13.6 vs. 12.3 months, HR 0.92, 95% CI 0.79–1.06). Lenvatinib had a higher objective response rate (18.8% vs. 6.5%), time-to-progression (7.4 vs. 3.7 months), and median progression-free survival (PFS) (7.3 vs. 3.6 months) [12]. There was a higher incidence of treatment-related adverse events in the lenvatinib arm (43% vs. 30%). Lenvatinib was approved by the US Food and Drug Administration (FDA) in 2018 as the first-line treatment for patients with advanced/unresectable HCC.
In the subsequent-line settings, both cabozantinib and regorafenib are approved. Regorafenib was studied in the RESORCE study which randomized HCC patients who previously progressed on sorafenib with Child Pugh A cirrhosis 2:1 to receive regorafenib or placebo. Regorafenib improved OS (median OS 10.6 vs. 7.8 months, HR 0.63, P<0.0001). The objective response rate for regorafenib was 7% with median duration of response of 3.5 months [15]. Grade 3 or 4 adverse events were reported in 46% of patients. Regorafenib was approved in 2017 for treatment of HCC after progression on sorafenib.
Cabozantinib, a multi-kinase TKI that targets VEGF receptors 1–3, MET and AXL, was studied in the CELESTIAL trial where patients with HCC who had disease progression after 1–2 systemic treatments were randomized 2:1 to receive cabozantinib or placebo [16]. Cabozantinib treated patients showed improved median OS (10.2 vs. 8 months, HR 0.76, 95% CI 0.63–0.92, P=0.005) and median PFS (5.2 vs. 1.9 months, HR 0.44, 95% CI 0.36–0.52, P<0.001). The objective response rate was 4%. Grade 3 or 4 adverse events occurred in 68% of patients in the cabozantinib group. In 2019, cabozantinib was approved for patients with HCC who had previously been treated with sorafenib.
Ramucirumab, a monoclonal antibody which targets VEGF receptor 2, was studied in a placebo-controlled randomized trial in patients with BCLC stage B or C HCC who had shown progression on sorafenib and had alpha-fetoprotein concentrations of 400 ng/mL or greater. This study met its primary endpoint where ramucirumab improved median OS (8.5 vs. 7.3 months, HR 0.71, 95% CI 0.53–0.95, P=0.019) and median PFS (2.8 vs. 1.6 months, HR 0.45, 95% CI 0.34–0.60, P<0.0001) [17]. The objective response rate did not significantly differ between the two groups (5% vs. 1%). The currently approved TKI and anti-angiogenic agents are summarized in Table 1.
Table 1.
Landmark trials evaluating TKIs and anti-angiogenic agents for systemic therapy in HCC
| Trial (reference) | Sample size (n) | Inclusion criteria | Phase and comparator | Primary endpoint(s) | Results |
|---|---|---|---|---|---|
| SHARP [11] | 602 | Patients with advanced HCC without prior systemic therapy, with ECOG PS 0-2 and Child- Pugh liver function class A | 3 | Overall survival | Median OS 10.7 vs. 7.9 months (HR 0.69; P<0.001) |
| Sorafenib 400 mg of twice daily or placebo | Time to symptomatic progression | Median TTP 4.1 vs. 4.9 months (HR 1.08; P=0.77) | |||
| REFLECT [12] | 1,492 | Unresectable HCC with measurable target lesions, BCLC stage B or C, Child-Pugh Class A, and ECOG 0-1 | 3 | Overall survival | Median OS 13.6 vs. 12.3 month (HR 0.92) |
| Lenvatinib 8 mg or 12 mg based on body weight or sorafenib 400 mg twice daily | |||||
| RESORCE [15] | 843 | Patients with BCLC stage B or C HCC not eligible for local treatments with documented radiologic progression during sorafenib treatment, with Child-Pugh class A Liver function. | 3 | Overall survival, analyzedby intention to treat | Median OS 10.6 vs. 7.8 months (HR 0.63; P<0.0001) |
| Regorafenib 160 mg daily or placebo once daily for the first 3 week of each 4-week cycle | |||||
| CELESTIAL [16] | 707 | HCC not amenable to curative treatment with Child-Pugh class A liver function with up to 2 lines of prior treatment for HCC (including sorafenib) with ECOG PS 0-1 | 3 | Overall survival | Median OS 10.2 vs. 8.0 months (HR 0.76; P=0.005) |
| Cabozantinib 60 mg daily or matching placebo | |||||
| REACH-2 [17] | 292 | Patients with BCLC stage B or C HCC treated with prior sorafenib, with AFP ≥400 ng/mL | 3 | Overall survival | Median OS 8.5 vs. 7.3 months (HR 0.71; P=0.0199) |
| Ramucirumab 8 mg/kg or placebo every 14 days |
TKI, tyrosine kinase inhibitor; HCC, hepatocellular carcinoma; ECOG ECOG PS, Eastern Cooperative Oncology Group Performance status; AFP, alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; OS, overall survival; HR, hazard ratio.
Currently approved checkpoint inhibitors in treatment of HCC
The immunotherapy era in the management of HCC began after a pilot study published in 2013 showed the safety and anti-tumor activity of tremelimumab, an inhibitor of cytotoxic T-lymphocyte antigen-4 (CTLA-4), in patients who developed HCC with HCV cirrhosis [18]. In the ensuing decade, the availability of immunotherapy as a treatment option for HCC has had a tremendous impact in the field as demonstrated by its adoption and inclusion of immunotherapy in the majority of treatment algorithms for HCC in clinical practice. Furthermore, most if not all systemic treatments being evaluated in randomized phase III studies in advanced HCC involve a ICI backbone. However, despite major advances and a shift in the treatment paradigm, only a fraction of patients respond to ICI, particularly as monotherapy [19,20], thus highlighting the importance of research in biomarker driven strategies and combination approaches.
Single agents
Nivolumab and pembrolizumab showed activity in phase II trials that evaluated their role as second-line agents when used after progression on sorafenib. These studies showed a response rate of 15–20% (complete response rate of 1–5%) which were durable [19,20]. In the CheckMate 040 trial, the 2 year survival rate among responders to nivolumab was over 80%. Based on these data, both nivolumab and pembrolizumab obtained accelerated approval by regulatory agencies as second-line treatment after progression on or unacceptable toxicity to sorafenib.
CheckMate 459 was a randomized phase III study evaluating nivolumab compared to sorafenib in treatment-naïve patients with advanced HCC and Child Pugh A cirrhosis. At a median follow up of 15.2 months, a trend towards improved OS in the nivolumab arm was reported (median 16.4 vs. 14.7 months, HR 0.85, P=0.075) [21]. Further, patients who received nivolumab had improved durable disease control (median 7.5 vs. 5.7 months) and improved toxicity profile with fewer grade 3 or 4 treatment-related adverse events (22% vs. 49%). However, due to not achieving statistical significance for the primary endpoint of OS, the FDA withdrew its approval of nivolumab for treatment of advanced HCC in 2021.
In a relatively recent randomized phase III placebo-controlled study of patients with advanced HCC previously treated with sorafenib (KEYNOTE-240) pembrolizumab resulted in a median OS of 13.9 months vs. 10.6 months in placebo, objective response rate of 18.3% (vs. 4.4%), and grade 3 or higher treatment-related adverse events rate of 52.7% (vs. 46.3%) [22]. However, statistical significance for improvement in OS was not reached. Pembrolizumab is a category 2B recommendation in the second-line setting for patients with advanced HCC afer progression on TKIs. Another randomized phase III study (KEYNOTE-394) which randomized patients in Asia with advanced HCC with progression on or intolerance to sorafenib to either pembrolizumab or placebo, showed an improvement in OS, PFS, and objective response rate in patients who received pembrolizumab [23]. Overall the results were supportive of the use of pembrolizumab as second-line therapy for advanced HCC.
Combination approaches
The promising activity and favorable safety profile of single-agent ICIs in the management of HCC has spurred the evaluation of various combination strategies, some of which are already being used in clinical practice.
The combination of PD-L1 inhibitor atezolizumab and VEGF inhibitor bevacizumab (atezo-bev) evaluated in the IMbrave150 clinical trial established a new standard of care in 2020 for first-line treatment of patients with unresectable HCC after more than a decade of failing clinical trials. IMbrave150 evaluated sorafenib versus atezo-bev in treatmentnaive patients with unresectable HCC [13]. Compared to sorafenib, Atezo-bev improved both OS and PFS (median OS NE vs. 13.2 months, HR 0.58, P<0.001; median PFS 6.8 vs. 4.5 months, HR 0.59, P<0.001). Atezo-bev also improved objective response rate (27.3% vs. 11.9%, P<0.001) with more durable responses (duration >6 months in 87.6% vs. 59.1% of patients). The most recent analysis shows a median OS of 19.3 months in patients who received atezo-bev and 13 months in patients who received sorafenib (HR 0.66, P<0.001) [24]. Furthermore, health-related quality of life was also significantly improved in the atezo-bev arm where the median time to deterioration in patient-reported quality of life was longer with the combination (11.2 vs. 3.6 months; HR 0.63) [13]. Atezo-bev was approved by the FDA in 2020 for treatment-naïve patients with unresectable or advanced HCC. A global observational study evaluated 433 patients who received atezo-bev in the first line setting for advanced HCC across Europe, Asia, and the United State. At a median follow up of 10 months, the median OS was 15.7 months, median PFS 6.9 months, and overall response rate 30.8% [25]. While this study confirmed reproducible safety and efficacy of atezo-bev in a real world population with results comparable to that of IMBrave150, the median OS was noted to be shorter than that reported in IMbrave150. It is possible that the patient population, with a higher proportion of patients who demonstrated portal vein thrombus and extrahepatic spread in addition to higher albumin-bilirubin grade may have contributed to this finding. The authors reported that within patients with Child Pugh A criteria, the presence of portal vein thrombosis (PVT) and higher albumin-bilirubin grade was associated with poor survival.
The combination of CTLA-4 and PD-1 blockade was studied in the HIMALAYA trial, leading to another approval in the first-line setting in 2022. In this study, patients with unresectable treatment-naïve HCC were randomized to receive tremelimumab 300 mg (one dose) plus durvalumab 1,500 mg every 3 weeks (STRIDE), durvalumab 1,500 mg every 4 weeks, or sorafenib 400 mg BID. Patients who received STRIDE had a higher median OS (16.4 vs. 16.56 vs. 13.77 months; HR 0.78, p=0.0035) [14]. OS with durvalumab alone was noninferior to sorafenib (HR 0.86, non-inferiority margin, 1.08). Grade 3–4 treatment-related adverse events occurred in 50.5% of patients who received STRIDE, 37.1% of patients who received durvalumab and 52.4% of patients who received sorafenib. The combination of durvalumab and tremelimumab is now an approved first-line regimen for patients with advanced HCC. Durvalumab monotherapy can be considered in patients who are not candidates for combination ICI or anti-angiogenic agents.
In CheckMate 040 (phase 1/2 study), patients with advanced HCC were randomized 1:1:1 to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg administered every 3 weeks, followed by nivolumab maintenance (arm A), nivolumab 3 mg/kg plus ipilimumab 1 mg/kg followed by nivolumab maintenance administered every 3 weeks (arm B), or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks (arm C) [26]. Patients in arm A had higher in objective response rate (32% vs. 27% vs. 29% in arms A, B, and C, respectively) and median OS (22.8 vs. 12.5 vs. 12.7 months) [26]; however, this study was not powered to detect differences between treatment arms. Arm A did have higher rate of grade 3 or 4 treatment-related adverse events, and discontinuation of the study drug due to toxic effects. This combination regimen (treatment arm A) was subsequently given accelerated approval by the FDA to treat patients with HCC after progression on sorafenib. This combination is now under investigation as first-line therapy for patients with HCC (NCT04039607). The major trials evaluating ICI alone or as combination therapy in HCC are summarized in Table 2.
Table 2.
Landmark trials evaluating ICI as systemic therapy in the management of HCC
| Trial (reference) | Sample size (n) | Inclusion criteria | Phase and comparator | Primary endpoint(s) | Results |
|---|---|---|---|---|---|
| KEYNOTE-224 [20] | 104 | Patients with BCLC stage B or C HCC who were intolerant to or progressed on sorafenib, that was not amenable to or refractory to curative treatment approach, with ECOG 0–1, and Child-Pugh class A liver function | 2 | ORR per RECIST v1.1 [27] | ORR 18% (95% CI 11–26%) |
| Pembrolizumab 200 mg every 3 weeks for up to 35 cycles | CR 1%, PR 16%, SD 44% | ||||
| CheckMate 040 [19] | 262 | Advanced HCC who progressed on at least one prior line of treatment including sorafenib, with Child-Pugh B7 or A and ECOG 0–1 | I/II | ORR (dose-expansion phase) | ORR 20% (95% CI 15–26) |
| Nivolumab every 2 weeks | CR 1%, PR 18%, SD 45% | ||||
| CheckMate 040 [26] | 148 | HCC not eligible for curative treatment with Child-Pugh class A liver function, ECOG PS 0–1 | 3 | Safety and tolerability, and ORR | ORR 32% (arm A), 27% (arm B), and 29% (arm C) |
| A: Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 doses | |||||
| B: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks | |||||
| C: nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/ kg every 6 weeks | |||||
| CheckMate 459 [21] | 743 | Advanced HCC not eligible for locoregional therapies, Child-Pugh class A liver function, ECOG 0–1, with no prior systemic therapy | 3 | OS | Median OS 16.4 vs. 14.7 months (HR 0.85; P=0.075) |
| Nivolumab 240 mg every 2 weeks or sorafenib 400 mg twice daily | |||||
| CheckMate 240 [22] | 413 | HCC with progression or intolerance to sorafenib treatment, BCLC stage B or C disease, Child-Pugh class A liver disease, ECOG 0–1 | 3 | OS | Median OS 13.9 vs. 10.6 months (HR 0.781; P=0.0238) |
| Pembrolizumab 200 mg every 3 weeks or placebo for up to 35 cycles | PFS | Median PFS 3.0 vs. 4.1 months (HR 0.775; P=0.0186) | |||
| IMBrave150 [13, 24] | 336 | Locally advanced, metastatic, or unresectable HCC with no prior systemic therapy, that was not amenable to curative or locoregional therapies or had progressed thereafter, with Child-Pugh liver function A and ECOG 0–1 | 3 | OS | Median OS 19.2 vs. 13.4 months (HR 0.66; P=0.0009) |
| Atezolizumab 1,200 mg plus bevacizumab 15 mg/kg every 3 weeks or sorafenib 400 mg twice daily | Progression-free survival | Median PFS 6.8 vs. 4.3 months (HR 0.59; P≤0.001) | |||
| HIMALAYA [14] | 1,171 | HCC with no prior systemic therapy that was ineligible for locoregional therapy with ECOG PS 0–1, Child-Pugh class A liver function | 3 | OS | Median OS 16.43 (STRIDE) vs. 13.77 (sorafenib) months (HR 0.78; P=0.0035) |
| Tremelimumab 300 mg for one dose plus durvalumab 1,500 mg every 4 weeks (STRIDE) or tremelimumab 75 mg every 4 weeks for 4 doses plus durvalumab 1500 mg every 4 weeks (T75+D) or durvalumab 1,500 mg every 4 weeks or sorafenib 400 mg twice daily |
ICI, immune checkpoint inhibitors; HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; EECOG PS, Eastern Cooperative Oncology Group Performance status; CR, complete response; PR, partial response; SD, standard deviation; CI, confidence interval; OS, overall survival; HR, hazard ratio.
Several TKI and ICIs have been approved in the first and subsequent line settings for the management of advanced HCC. A general treatment algorithm is shown in Figure 2. In evaluating the optimal first line regimen for each patient, toxicity profiles of the drug in combination with the patient’s medical history and performance status should be taken into consideration, given the lack of randomized data comparing each approved regimen in the treatment-naïve setting. For example, a patient with significant cardiac co-morbidities, bleeding diathesis, and/or history of grade 3 varices with bleeding, may not be a candidate for anti-angiogenic treatment, and thus combination durvalumab and tremelimumab may be considered. Another patient with refractory autoimmune disease would not be a candidate for combination ICI, and thus TKI may be considered. Thus, the treatment of choice is often dependent on clinical factors while weighing the risks and benefits of each regimen for each individual patient.
Figure 2.
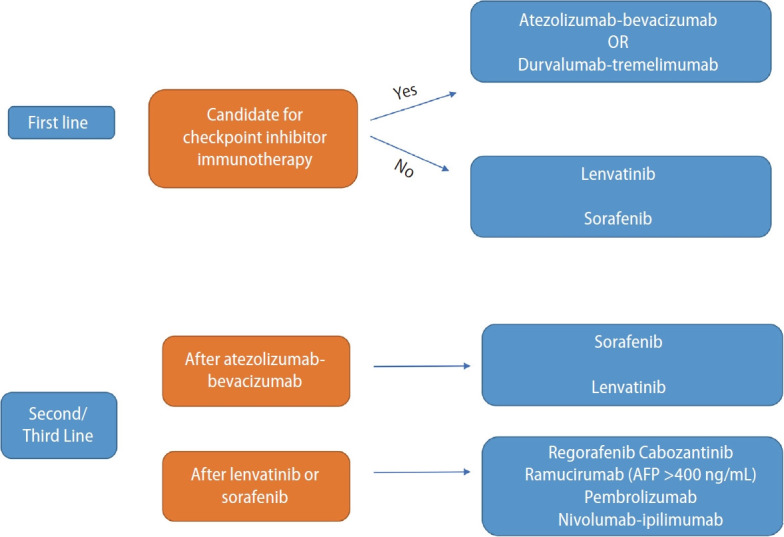
Approach to systemic treatment of advanced HCC. HCC, hepatocellular carcinoma; AFP, alpha fetoprotein.
Ongoing evaluations of combination strategies
ICI plus VEGF blockade
Enhancement of CD8+ T cell function with anti-angiogenic agents has been demonstrated in solid malignancies including HCC and renal cell carcinoma [13,28-30]. VEGF pathway signaling has been implicated to diminish anti-tumoral immunity by several mechanisms, including reducing the cytotoxic activity of peripheral T cells,31 enhancing T regulatory cell activation [32-34], and inducing myeloid derived suppressor cells, which in turn elicit immunosuppressive effects by lymphocyte depletion, generation of oxidative stress, interfering with lymphocyte trafficking and activation of T regulatory cells [35]. VEGF-A also directly induces FASL expression leading to apoptosis of CD8+ T cells [36]. In preclinical models of HCC, anti-PD1 in combination with anti-VEGFR2 antibodies showed enhanced M1 and decreased M2 tumor-associated macrophages, as well as increased level of infiltrating CD8+ T cells [28].
With strong preclinical rationale, ICIs in combination with anti-VEGF TKIs have been evaluated in clinical trials but have shown mixed results. In a randomized double-blind phase III study, lenvatinib plus pembrolizumab was compared to lenvatinib alone in treatment-na ïve HCC, where the primary endpoints of OS and PFS did not meet pre-specified statistical significance (median OS 21.2 vs. 19.0 months, HR 0.84, P=0.02; median PFS 8.2 vs. 8.0 months, HR 0.876, P=0.05) [37]. Similarly, another study evaluated the combination of cabozantinib plus atezolizumab compared to sorafenib in the first-line setting for patients with unresectable or advanced HCC, where the median PFS was improved in the combination arm (6.8 vs. 4.2 months, HR=0.63, P=0.0012), but there was no difference in survival (median OS 15.4 vs. 15.5 months, HR 0.9, P=0.44) [38].
Conversely, the phase III ORIENT-32 trial evaluating PD-1 inhibitor sintilimab with IBI305 (bevacizumab biosimilar) improved OS as compared to sorafenib in patients with untreated hepatitis B virus associated HCC in an exclusively Chinese population (median OS NR vs. 10.4 months, HR 0.57, P<0.0001) [39]. In another phase III trial of PD-1 inhibitor camrelizumab combined with rivoceranib versus sorafenib alone, OS and PFS were superior in the combination arm (median OS 22.1 months vs. 15.2 months, HR 0.62, P<0.001; median PFS 5.6 vs. 3.7 months, HR 0.52, P<0.0001) [40]. This represents the longest OS observed to date in phase III trials involving patients with advanced HCC. Further research is needed to understand the role of ICI with VEGF TKI combination in the treatment of advanced HCC.
ICI plus locoregional treatment
It is hypothesized that combining ICI’s with other treatment modalities (e.g., surgical resection, ablation, transarterial therapies, radiotherapy, etc.) can potentially have an improve overall effect in patients with advanced HCC. Radiotherapy (RT) has been shown to enhance immunotherapeutic effects in various cancers. Radiation can prime the immune system by enhancing antigen presentation, promoting infiltration of cytotoxic T cells, and reprogramming the tumor microenvironment against the immune evasion of cancer [41]. In preclinical models, liver-directed radiotherapy eliminates immunosuppressive hepatic macrophages, increases hepatic T cell survival and reduces hepatic siphoning of T cells [42]. In HCC, preclinical data have shown combination of RT and ICIs to exhibit therapeutic synergism, superior tumor control, and improved OS [43,44]. Despite encouraging preclinical findings, there are a small number of published prospective trials on combination of RT and ICI in HCC. Small series have shown promising clinical activity [45,46]. In a propensity score matching analysis of approximately 64 patients with unresectable or recurrent locally advanced HCC who received Streotactic Body Radiation Therapy in combination with ICI (SBRT-ICI) versus TACE at a single institution, the authors reported a significantly improved objective response rate (87.5% vs. 16.7%), 24-month PFS (77.8% vs. 2.1%) and 24-month OS (80.4% vs. 8.3%) in the SBRT-ICI arm [47]. In a phase I multicenter trial, 14 patients with advanced or unresectable HCC received SBRT (40 Gy in 5 fractions) followed by either nivolumab alone or nivolumab in combination with ipilimumab. Clinical outcomes favored the combination ICI group, with overall response rate of 57% vs. 0%, median PFS of 11.6 months vs. 2.7 months, and median OS of 41.6 months vs. 4.7 months [48]. The study was stopped due to slow accrual. Several ongoing studies are currently evaluating the efficacy of combination of ICI with SBRT in the neoadjuvant setting for early-stage HCC (NCT04857684), and in the first or subsequent line settings for advanced HCC (NCT05488522, NCT04913480).
SBRT has also been studied in combination with sorafenib. In the phase III NRG/RTOG 1112 trial, patients with advanced HCC were treated with sorafenib monotherapy or sorafenib with SBRT. The median OS was 12.3 vs. 15.8 months favoring the combination arm (HR 0.77; P=0.055). The median PFS was 5.5 vs. 9.2 months (HR 0.55; P=0.0001). There was no significant increase of grade 3 or higher adverse events in the combination arm.
Local ablation increases liver immunogenicity and activation of antigen presenting dendritic cells in HCC [49]. In preclinical models, ablation increases T cell infiltration and immune checkpoint expression within and beyond the treatment zone, suggesting that the addition of ICI to ablation may result in synergistic antitumor activity [50-52]. In a study of patients with advanced HCC treated with combination of CTLA-4 inhibitor tremelimumab and tumor ablation (radiofrequency ablation or chemoablation), authors reported a 26.3% tumor response rate and a median time to tumor progression of 7.4 months [53].
Intra-arterial therapies such as TACE and transarterial radioembolization (TARE) with Y90 have been widely adopted over the last two decades and are currently considered fairly standard treatment options in management of intermediate stage HCC in most high volume centers. There is preclinical data to suggest that TACE can improve liver immunogenicity and enhance ICI efficacy. In a cohort of patients with HCC treated with TACE, expression of immune checkpoints PD-1 and PD-L1 on tumor cells increased after treatment with TACE. While PD-L1 has not been shown to be a clinical marker of response to ICI in HCC as in other tumors, the upregulation of immune checkpoints suggests that TACE may induce a immunogenic tumor microenvironment. In a cohort of 34 patients treated with camrelizumab and TACE, the objective response rate was 35.3%, median PFS was 6.1 months and median OS was 13.3 months [54]. Furthermore, in patients with HCC treated with TARE, similar changes were seen within the hepatic tumor microenvironment, where an enhanced number of released tumor antigens leads to local immune activation with infiltration of CD8+ T cells and natural killer cells [55].
Prospective randomized clinical trials are needed to understand the role of combining locoregional treatment with ICI. LEAP-012 is an ongoing randomized phase III trial evaluating combination of lenvatinib and pembrolizumab with TACE versus placebo and TACE in patients with HCC confined to the liver without PVT and otherwise ineligible for other curative treatment options (NCT042461777). Another ongoing study is evaluating Y90 TARE with combination of atezo-bev compared to Y90 TARE alone in patients with unresectable intermediate-stage HCC (NCT04541173 [56]). The results of many such ongoing trials evaluating the combination of ICI with locoregional transarterial therapies may change the treatment algorithm of intermediate-stage HCC in the future.
Combination of TKI and lcoregional treatment have been explored in recent trials with promising results. A recent multicenter, randomized, open-label, parallel group phase III trial investitgated the role of lenvatinib (LEN) combined with TACE (LEN-TACE) as first line treatment for advanced HCC (LAUNCH trial) [57]. A total of 338 patients were randomized to two arms: 170 to LEN-TACE and 168 to LEN. At a prespecified event-driven interim analysis after a median follow-up of 17.0 months, the median OS was significantly longer in the LEN-TACE group (17.8 vs. 11.5 months, HR 0.45, P<0.001). The median PFS was 10.6 months in the LEN-TACE group and 6.4 months in the LEN group (HR 0.43, P<0.001). The grade 3–4 adverse events were more common in the LEN-TACE group and included alanine aminotransferase (ALT) elevation (17.6% vs. 1.2%, P<0.001), aspartate aminotransferase (AST) elevation (22.9% vs. 1.8%, P<0.001), and hyperbilirubinemia (9.4% vs. 3.0%, P=0.014). These data suggest that the combining TACE with lenvatinib may be considered for patients with advanced HCC.
Intra-arterial therapies have also been studied in combination with sorafenib. In the multicenter phase II SORAMIC trial, patients with advanced HCC received sorafenib either alone or in combination with radioembolization. Patients who received combination therapy had higher objective response rate (61.6% vs. 29.8%, P<0.001), complete response rate (13.7% vs. 3.8%, P=0.022), median PFS (8.9 vs. 5.4 months, P=0.022), and hepatic median PFS (9.0 vs. 5.7 months, P=0.014) [58]. However, an improvement in OS was not seen in the combination arm. Similarly, the TACTICS trial evaluated TACE compared to TACE plus sorafenib in patients with unresectable HCC. Here, median PFS was significantly longer in the TACE plus sorafenib arm (25.2 vs. 13.5 months, P=0.006) [59]. One-year OS in the combination group was also prolonged (96.2% vs. 82.7%) [59].
While combining locoregional and systemic therapies has been suggested as a way to enhance efficacy and tumor response rates in treatment of HCC, the ideal strategy has not yet been delineated and remains under investigation.
SYSTEMIC THERAPIES IN NEOADJUVANT AND ADJUVANT SETTINGS
Systemic therapies are now being evaluated in the adjuvant and neoadjuvant settings for early-stage HCC to improve the chance for cure. In a phase IB study, 12 of 15 patients with unresectable HCC who were treated with neoadjuvant cabozantinib and nivolumab underwent successful margin negative resection. Furthermore, 5 of the 12 resected demonstrated major response on final pathologic evaluation [60]. Tumor specimens demonstrated an enrichment in effector T cells and a distinct spatial arrangement of B cells in responders, suggesting the possibility of durable immunologic memory postoperatively conferred by pre-operative immune priming. Several ongoing randomized phase III trials are evaluating whether adjuvant ICI may reduce the risk of recurrence after curative-intent resection (KEYNOTE-937 evaluating pembrolizumab vs. placebo after curative-intent surgical resection or ablation [61], CA209-9DX evaluating nivolumab vs. placebo for tumors at high risk of recurrence after curative-intent surgical resection [62], and IMbrave050 evaluating atezolizumab-bevacizumab vs. active surveillance after resection [63]). At the time of interim analysis with a median follow up of 17.4 months, the primary endpoint (recurrence-free survival [RFS]) was met with HR of 0.72 (P=0.012), making atezo-bev the first adjuvant regimen to demonstrate a statistically significant and clinically meaningful improvement in RFS in patients with high risk of disease recurrence following local curative treatment [64]. Whether this will translate to improving the cure rate and OS in this patient population remains in question. Given the possibility of inducing durable immune responses, ICI will likely begin to play a larger role in the treatment of early-stage HCC. Data from the aforementioned and several other ongoing trials will shed light on the ideal adjuvant and/or neoadjuvant strategies for these patients [65].
Management of HCC in special populations
An area of unmet need in the current understanding of HCC management is the treatment of patients with HCC who have concurrent comorbidities and medical conditions such as advanced cirrhosis (Child Pugh B), history of prior liver transplant, history of immunosuppressive conditions such a HIV infection. While the majority of clinical trials which have led to the approval of systemic agents for HCC have incorporated patients with Child Pugh A cirrhosis only, a significant portion of patients who present with HCC in clinical practice may have advanced cirrhosis. Real-world analyses have sought to answer the question of efficacy of current systemic therapies in patients with advanced cirrhosis. In a retrospective real-world study of 216 patients with HCC who were treated with atezo-bev, 24% were noted to have Child Pugh B cirrhosis. The median OS was significantly longer in the Child Pugh A group (16.8 months) compared to the Child Pugh B group (6.7 months; P=0.0003) [66]. PFS was also longer in the Child Pugh A group (7.6 vs. 3.4 months). However, treatment related adverse events were noted to be similar in both groups [66]. Although more patients with Child Pugh B disease experience grade ≥3 bleeding events and atezolizumab related adverse events compared to the Child Pugh A group (10% vs. 4%; 15% vs. 4%), grade ≥3 atezolizumab-related hepatitis only occurred in patients with Child Pugh A disease (8%). Discontinuation of treatment because of treatment-related adverse events was 11% among all patients, suggesting that atezo-bev may be tolerable for patients with Child Pugh A or B cirrhosis. However, the limited number of patients in this study warrants a larger prospective study to investigate safety of atezo-bev in patients with Child Pugh B disease. Further, nivolumab has been evaluated in a phase I/ II open-label multicenter trial in patients with advanced HCC and Child Pugh B cirrhosis. In 24 sorafenib-treated patients and 25 sorafenib-naïve patients, the objective response rate was 12%. Treatment-related adverse events were reported in 51% of patients, leading to treatment discontinuation in 2% of patients, where safety was comparable to that reported for Child Pugh A patients. Given the limited data to guide in the treatment of patients with advanced HCC and Child Pugh B disease, the recommendation for each individual patient may be variable and dependent on several considerations. For example, those with Child Pugh B7 may be more likely to benefit from treatment than B8 or B9. The etiology of liver dysfunction (i.e., cirrhosis versus tumor burden) may be helpful in understanding whether patients with Child Pugh B8 or B9 may benefit. Furthermore, other markers of liver function including albumin-bilirubin grade and the Model for End-Stage Liver Disease score may be used to stratify patients with cirrhosis.
Up to 10–15% of liver transplant recipients may experience HCC recurrence. TKIs have been evaluated retrospectively in the post-transplant patient population. In a systematic review and meta-analysis of eight studies, median OS of 12 months with acceptable safety profile was reported with sorafenib. In a multi-center retrospective study of 28 post-transplant patients with HCC, regorafenib was evaluated in patients who progressed on sorafenib, with median OS of 12.9 months following treatment initiation [67]. There is a paucity of data examining the efficacy and safety of ICI in the post-transplant setting [68]. In a retrospective pilot evaluation to assess the safety and efficacy of ICI in patients post liver transplant, 7 patients with metastatic cancer with a history of liver transplant were treated with ICI for either HCC or melanoma. 2 of 7 patients developed rejection within a median time of 24 days. 1 patient achieved a complete response, 3 patients had progression of disease, and 3 patients discontinued therapy prior to restaging assessments. Clinical trials are underway evaluating safety and efficacy of ICI in post-transplant HCC, and at this time generally are not given outside of a clinical trial.
CONCLUSIONS
The treatment landscape for advanced HCC has transformed over the last decade. ICIs are now the backbone of most treatment strategies in clinical practice for advanced HCC and continue to be investigated in clinical research in novel combinatorial strategies. Despite these major advances, many challenges still exist in the management of patients with advanced HCC. One such challenge frequently faced in the clinic is the appropriate management of patients with advanced cirrhosis, given that most of the currently approved treatments were studied in patients with and are approved in patients with Child Pugh A cirrhosis. Frequently, patients with HCC tend to be more debilitated from their illness and have more complications from their underlying cirrhosis, than those represented in major clinical trials, and thus a gap still exists in finding the optimal treatment for these patients. Secondly, the optimal sequencing of systemic therapies remains unknown, particularly as it relates to the two ICI-based combination treatments now approved in the first-line setting (atezo-bev and durva-treme). It is also poorly understood whether combination ICI strategies can be effective after progression on PD-1 monotherapy, and whether ICI strategies can improve outcomes when given in the peri-operative setting for patients with early-stage HCC. The data from several ongoing clinical trials will shed light on the optimal combination strategies in these settings. Finally, the identification of biomarkers to assess response and development of resistance to ICIs is crucial and is a significant area of ongoing research. The incorporation of systemic therapy in the management of early-stage and intermediate-stage HCC, and further advances in effective combination strategies for advanced HCC are foreseen in the near future.
Acknowledgments
This work was supported by R01 HL149946, R01 CA273925 grants, Cedars-Sinai Cancer and Cancer Biology Program discovery fund to EKK. EKK also received research funding from Surface Oncology Inc to investigate IL-27 blockade in HCC. JDY’s research is supported by American College of Gastroenterology Junior Faculty Development Award, Department of Defense Peer Reviewed Cancer Research Program Career Development Award (CA191051) and the National Institutes of Health (K08CA259534).
Abbreviations
- HCC
hepatocellular carcinoma
- NASH
nonalcoholic steatohepatitis
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ICI
immune checkpoint inhibitors
- PD-L1
programmed death ligand 1
- PD-1
programmed cell death protein 1
- BCLC
Barcelona Clinic Liver Cancer
- TACE
trans-arterial chemoembolization
- Y90
yttrium-90
- TKI
tyrosine kinase inhibitor
- OS
overall survival
- PFS
progression-free survival
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- RT
radiotherapy
- TARE
transarterial radioembolization
- LEN
lenvatinib
Footnotes
Authors’ contribution
Conception and design: KS, JDY. Administrative support: N/A. Provisions of study materials or patients: N/A. Collection and assembly of data: N/A. Data analysis and interpretation: N/A. Manuscript writing: all authors. Final approval of the manuscript: all authors.
Conflicts of Interest
JG has served as a consultant/advisory role for Amgen, Astellas Pharma, QED Therapeutics, Exelixis, Elsevier, EMD Serono/Merck, Eisai, Pfizer/Myovant, Bayer, Basilea, HalioDx, Natera, Incyte, AVEO, Janssen Biotech, Seagen, MJH Life Sciences. AEH has served as a consultant for Varian, Genentech, Merck, BMS, Abbvie, Valar and Farady. JDY provides a consulting service for AstraZeneca, Eisai, Exact Sciences, Exelixis, Fujifilm Medical Sciences, and Gilead Sciences.
REFERENCES
- 1.Lee YT, Wang JJ, Luu M, Noureddin M, Kosari K, Agopian VG, et al. The mortality and overall survival trends of primary liver cancer in the United States. J Natl Cancer Inst. 2021;113:1531–1541. doi: 10.1093/jnci/djab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee YT, Wang JJ, Luu M, Tseng HR, Rich NE, Lu SC, et al. StateLevel HCC incidence and association with obesity and physical activity in the United States. Hepatology. 2021;74:1384–1394. doi: 10.1002/hep.31811. [DOI] [PubMed] [Google Scholar]
- 5. CT/MRI LI-RADS® v2017 CORE. [Internet]. American College of Radiology [cited 2023 Feb 1]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LIRADS_2017_Core.pdf.
- 6.Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. doi: 10.1136/bmj.m3544. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 18.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 19.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 21.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 23.Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: A randomized, double-blind, phase III trial. J Clin Oncol. 2023;41:1434–1443. doi: 10.1200/JCO.22.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: updated overall survival data from a global, randomized, open-label Phase III study of atezolizumab+ bevacizumab vs sorafenib in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2021;39(3 suppl):267. [Google Scholar]
- 25.Fulgenzi CAM, Cheon J, D’Alessio A, Nishida N, Ang C, Marron TU, et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: Results of the AB-real study. Eur J Cancer. 2022;175:204–213. doi: 10.1016/j.ejca.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with Sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71:1247–1261. doi: 10.1002/hep.30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 31.Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012;130:857–864. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 32.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandalaft LE, Motz GT, Busch J, Coukos G. Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr Top Microbiol Immunol. 2011;344:129–148. doi: 10.1007/82_2010_95. [DOI] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2022;33(Suppl 7):S1401. [Google Scholar]
- 38.Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, openlabel, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 39.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 40.Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35 Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol. 2022;33(Suppl 7):S1401–S1402. [Google Scholar]
- 41.Colton M, Cheadle EJ, Honeychurch J, Illidge TM. Reprogramming the tumour microenvironment by radiotherapy: implications for radiotherapy and immunotherapy combinations. Radiat Oncol. 2020;15:254. doi: 10.1186/s13014-020-01678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophagemediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One. 2016;11:e0157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 radioembolization combined with a PD-1 inhibitor for advanced hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2018;41:1799–1802. doi: 10.1007/s00270-018-1993-1. [DOI] [PubMed] [Google Scholar]
- 46.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: A potential synergistic treatment strategy. Front Oncol. 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang CL, Chiu KW, Lee FA, Kong FS, Chan AC. Combined stereotactic body radiotherapy and immunotherapy versus transarterial chemoembolization in locally advanced hepatocellular carcinoma: A propensity score matching analysis. Front Oncol. 2021;11:798832. doi: 10.3389/fonc.2021.798832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juloori A, Katipally RR, Lemons JM, Singh AK, Iyer R, Robbins JR, et al. Phase 1 randomized trial of stereotactic body radiation therapy followed by nivolumab plus ipilimumab or nivolumab alone in advanced/unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2023;115:202–213. doi: 10.1016/j.ijrobp.2022.09.052. [DOI] [PubMed] [Google Scholar]
- 49.Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, et al. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817–822. doi: 10.1016/j.jhep.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 50.Fei Q, Pan Y, Lin W, Zhou Y, Yu X, Hou Z, et al. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer. Cell Death Dis. 2020;11:589. doi: 10.1038/s41419-020-02787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, Li SS, Zhou M, Jiang AN, He Y, Wang S, et al. Palliative radiofrequency ablation accelerates the residual tumor progression through increasing tumor-infiltrating MDSCs and reducing T-Cell-mediated anti-tumor immune responses in animal model. Front Oncol. 2020;10:1308. doi: 10.3389/fonc.2020.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res. 2016;22:1173–1184. doi: 10.1158/1078-0432.CCR-15-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang JX, Chen P, Liu S, Zu QQ, Shi HB, Zhou CG. Safety and efficacy of transarterial chemoembolization and immune checkpoint inhibition with camrelizumab for treatment of unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:265–272. doi: 10.2147/JHC.S358658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–346. doi: 10.1136/gutjnl-2017-315485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyer RV, Petroziello M, Parikh N, Kim RD, Heumann TR, Brown D, et al. (2023). A phase II study of atezolizumab (ATEZO) and bevacizumab (BEV) in combination with Y90 TARE in patients (pts) with hepatocellular carcinoma (HCC): Y90+/-BEAT. J Clin Oncol. 2023;41(4 Suppl):TPS629. [Google Scholar]
- 57.Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH) J Clin Oncol. 2023;41:117–127. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 58.Öcal O, Schütte K, Zech CJ, Loewe C, van Delden O, Vandecaveye V, et al. Addition of Y-90 radioembolization increases tumor response and local disease control in hepatocellular carcinoma patients receiving sorafenib. Eur J Nucl Med Mol Imaging. 2022;49:4716–4726. doi: 10.1007/s00259-022-05920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat Cancer. 2021;2:891–903. doi: 10.1038/s43018-021-00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu A, Kudo M, Vogel A, Yau T, Zhou J, Kim E, et al. Abstract CT284: Phase 3 KEYNOTE-937: Adjuvant pembrolizumab versus placebo in patients with hepatocellular carcinoma and complete radiologic response after surgical resection or local ablation. Cancer Res. 2020;80(16 Suppl):CT284. [Google Scholar]
- 62.Exposito MJ, Akce M, Alvarez JM, Assenat E, Balart LA, Baron AD, et al. CA209-9DX: phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann Oncol. 2018;29:viii267–viii268. [Google Scholar]
- 63.Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975–989. doi: 10.2217/fon-2020-0162. [DOI] [PubMed] [Google Scholar]
- 64.Chow P, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Abstract CT003: IMbrave050: Phase 3 study of adjuvant atezolizumab+ bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. Cancer Res. 2023;83(8_ Suppl):CT003. [Google Scholar]
- 65.Franses JW, Zhu AX. Neoadjuvant approaches in hepatocellular carcinoma: There’s no time like the present. Clin Cancer Res. 2022;28:2738–2743. doi: 10.1158/1078-0432.CCR-22-0025. [DOI] [PubMed] [Google Scholar]
- 66.D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology. 2022;76:1000–1012. doi: 10.1002/hep.32468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, et al. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019;19:3176–3184. doi: 10.1111/ajt.15551. [DOI] [PubMed] [Google Scholar]
- 68.DeLeon TT, Salomao MA, Aqel BA, Sonbol MB, Yokoda RT, Ali AH, et al. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018;9:1054–1062. doi: 10.21037/jgo.2018.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]