Abstract
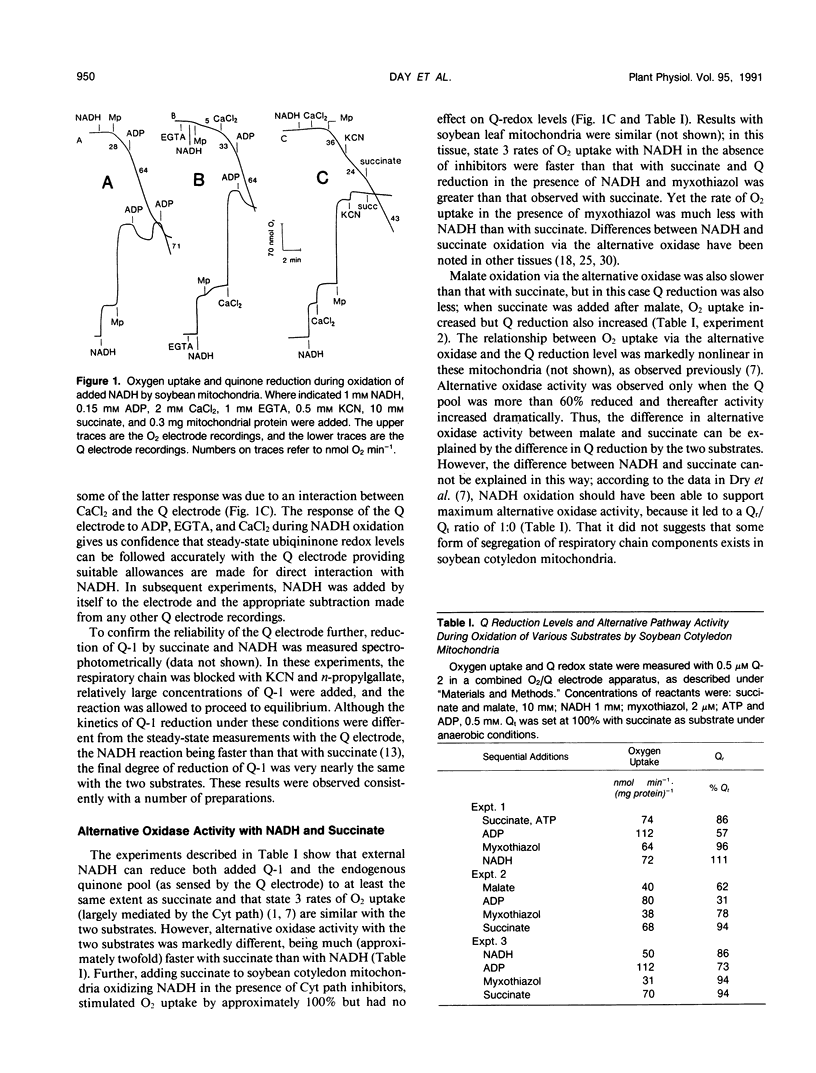
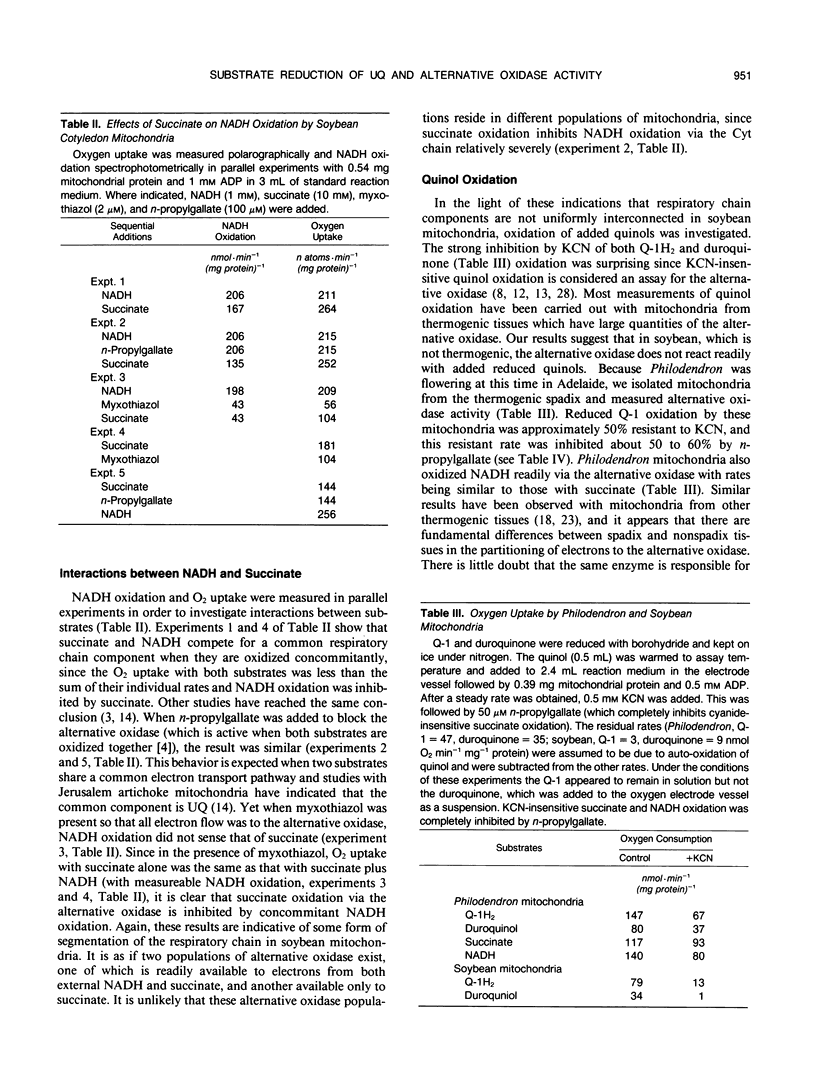
External NADH and succinate were oxidized at similar rates by soybean (Glycine max) cotyledon and leaf mitochondria when the cytochrome chain was operating, but the rate of NADH oxidation via the alternative oxidase was only half that of succinate. However, measurements of the redox poise of the endogenous quinone pool and reduction of added quinones revealed that external NADH reduced them to the same, or greater, extent than did succinate. A kinetic analysis of the relationship between alternative oxidase activity and the redox state of ubiquinone indicated that the degree of ubiquinone reduction during external NADH oxidation was sufficient to fully engage the alternative oxidase. Measurements of NADH oxidation in the presence of succinate showed that the two substrates competed for cytochrome chain activity but not for alternative oxidase activity. Both reduced Q-1 and duroquinone were readily oxidized by the cytochrome oxidase pathway but only slowly by the alternative oxidase pathway in soybean mitochondria. In mitochondria isolated from the thermogenic spadix of Philodendron selloum, on the other hand, quinol oxidation via the alternative oxidase was relatively rapid; in these mitochondria, external NADH was also oxidized readily by the alternative oxidase. Antibodies raised against alternative oxidase proteins from Sauromatum guttatum cross-reacted with proteins of similar molecular size from soybean mitochondria, indicating similarities between the two alternative oxidases. However, it appears that the organization of the respiratory chain in soybean is different, and we suggest that some segregation of electron transport chain components may exist in mitochondria from nonthermogenic plant tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Day D. A., Moore A. L., Dry I. B., Wiskich J. T., Azcon-Bieto J. Regulation of nonphosphorylating electron transport pathways in soybean cotyledon mitochondria and its implications for fat metabolism. Plant Physiol. 1988 Apr;86(4):1199–1204. doi: 10.1104/pp.86.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry I. B., Moore A. L., Day D. A., Wiskich J. T. Regulation of alternative pathway activity in plant mitochondria: nonlinear relationship between electron flux and the redox poise of the quinone pool. Arch Biochem Biophys. 1989 Aug 15;273(1):148–157. doi: 10.1016/0003-9861(89)90173-2. [DOI] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Characterization and Solubilization of the Alternative Oxidase of Sauromatum guttatum Mitochondria. Plant Physiol. 1986 Sep;82(1):1–6. doi: 10.1104/pp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Nickels R. L., McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989 Apr;89(4):1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay C. J., Palmer J. M. Solubilization of the alternative oxidase of cuckoo-pint (Arum maculatum) mitochondria. Stimulation by high concentrations of ions and effects of specific inhibitors. Biochem J. 1985 Jun 1;228(2):309–318. doi: 10.1042/bj2280309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973 Apr;34(2):358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenaz G. Role of mobility of redox components in the inner mitochondrial membrane. J Membr Biol. 1988 Sep;104(3):193–209. doi: 10.1007/BF01872322. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Akerman K. E. Ca2+ stimulation of the external NADH dehydrogenase in Jerusalem artichoke (Helianthus tuberosum) mitochondria. Biochem Biophys Res Commun. 1982 Nov 30;109(2):513–517. doi: 10.1016/0006-291x(82)91751-x. [DOI] [PubMed] [Google Scholar]
- Reed J. S., Ragan C. I. The effect of rate limitation by cytochrome c on the redox state of the ubiquinone pool in reconstituted NADH: cytochrome c reductase. Biochem J. 1987 Nov 1;247(3):657–662. doi: 10.1042/bj2470657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R. Electron and proton transfers through quinones and cytochrome bc complexes. Biochim Biophys Acta. 1984 Apr 9;768(1):53–79. doi: 10.1016/0304-4173(84)90007-7. [DOI] [PubMed] [Google Scholar]


