Abstract
Peripheral nerve injury (PNI) can cause partial or total motor and sensory nerve function, leading to physical disability and nerve pain that severely affects patients’ quality of life. Autologous nerve transplantation is currently the clinically recognized gold standard, but due to its inherent limitations, researchers have been searching for alternative treatments. Nerve guidance conduits (NGCs) have attracted much attention as a favorable alternative to promote the repair and regeneration of damaged peripheral nerves. In this review, we provide an overview of the anatomy of peripheral nerves, peripheral nerve injury and repair, and current treatment methods. Importantly, different design strategies of NGCs used for the treatment of PNI and their applications in PNI repair are highlighted. Finally, an outlook on the future development and challenges of NGCs is presented.
Keywords: Peripheral nerve injury, nerve guidance conduits, nerve regeneration
Introduction
The nervous system is divided into the central nervous system (CNS) and the peripheral nervous system (PNS) [1]. The CNS is the most complex system in the human body and is responsible for integrating and processing the information transmitted by nerves and directing the responses of different parts of the body organs [2]. And the PNS is an extensive network of neurons that connects muscles, glands, and the CNS throughout the body. Its nerves are responsible for conveying the instructions from the CNS [3,4]. The CNS and PNS work together to control sensory input, information integration, and motor output, forming a highly specialized body system that mobilizes all parts of the body to respond to multiple changes in the environment [5].
However, PNI is a major health problem worldwide, with more than 1 million PNI occurring annually worldwide [6]. In the United States alone, half a million surgical procedures are performed each year at a cost of up to $1.5 billion [7], resulting in a significant socio-economic burden. CNS disorders are usually caused by trauma, disease, and surgery, which can lead to motor dysfunction, sensory impairment, and neuropathic pain [8,9]. The CNS injuries do not regenerate spontaneously, and to date, there have been no reports of effective clinical treatment for CNS injury. On the contrary, PNS has the potential to regenerate after injury [10-12]. Autologous transplantation is currently the gold standard for the treatment of PNI [13,14]; however, it has plenty of limitations, such as donor scarcity, donor size mismatch, and immunological problems [15,16]. Therefore, new approaches are urgently needed to restore the structure and function of the injured peripheral nervous system.
With the development of tissue engineering techniques, NGCs have emerged as a prospective method to facilitate nerve repair. NGCs are tubular structures that provide a supportive and appropriate microenvironment for nerve regeneration by bridging the severed ends of nerve injuries. In this review, we review the different designs of NGC for nerve regeneration in recent years. What’s more, the future research directions and development prospects of NGCs are also discussed.
Anatomy and injury of peripheral nerves
Peripheral nerve anatomy
The peripheral nervous system includes a collection of the nerves stemming from the brain and spinal cord, as well as the ganglia associated with them. The nerves that derive from the brain and spinal cord are called cranial and spinal nerves, respectively, and consist of nerve fiber bundles [5]. The nerve fiber bundles consist of multiple longitudinal arrangements of axons and are covered by three layers of connective tissue. These layers play an important role in the movement of nerve fibers in the body. On the one hand, they protect nerve bundles from stretching and compression. On the other hand, it contains blood vessels (neurovessels) that provide nutritional support for nerve fibers [17].
From the inside out, nerve fibers and connective tissue consist of three layers, which are shown in Figure 1. The innermost layer is the endoneurum, including loose collagenous matrix, nerve fibers, Schwann cells (SCs), fibroblasts, endothelial-like cells, mastocytes, and so on, which protect these cells from mechanical forces. The second layer is the perineurium, which contains epithelium-like cells and collagen fibers and will wrap each fascicle individually, providing strong mechanical protection for the nerve bundle. The outermost layer is the epineurium, which contains blood vessels that flush nerves and some fatty tissue. It plays an important role in isolating the external environment and providing mechanical protection [18]. The most basic cells in PNS include endothelial cells, macrophages, mastoid cells, and neurons. In addition, other supportive cells have been found in the PNS, including macrophages, satellite glial cells, and SCs, which are critical to axonal regeneration. Specifically, macrophages have a strong ability to phagocytose foreign bodies, contributing to endocytosis of damaged nerve fibers and myelin fragments [19-21]. Satellite glial cells play an important role in regulating the external microenvironment, thereby promoting axonal regeneration of peripheral nerves [22-24]. SCs produce extracellular matrix (ECM), neurotrophic factors, cell adhesion molecules, and other molecules conducive to nerve regeneration to provide a supportive environment for cells in tissue. SCs are divided into two types: myelinated SCs encircle large diameter axons one by one in the myelin sheath, while unmyelinated SCs surround multiple small diameter axons without producing myelin.
Figure 1.
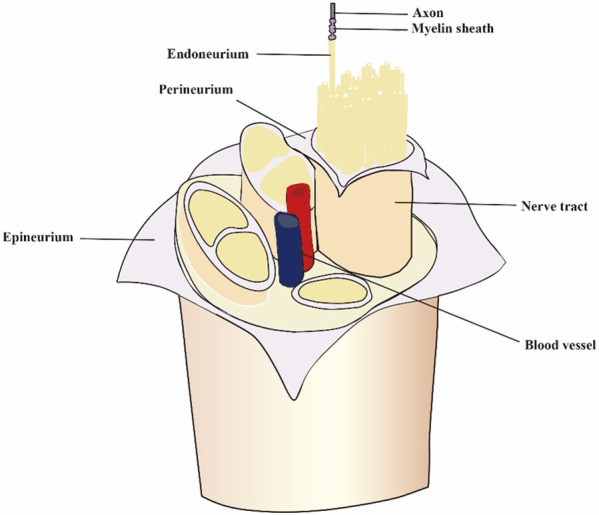
Peripheral nerve anatomy. The three layers of the nerve fiber includes epineurium, perineurium, endoneurium.
Peripheral nerve injuries
PNI can cause damage to the sensory and motor nerves, resulting in partial or complete loss of function and even disability, which seriously affects the quality of life of patients [25-27]. The higher the degree of injury, the more complete the loss of function and the more difficult the repair. According to the severity of the injury, nerve injury is divided into five grades [28]. Grade I PNI is the mildest, caused by ischemia or focal demyelination due to traction or mild compression. For grade II injury, only the axons are damaged with the rest of the structure is intact. For grade III injury, the axons are damaged and the nerve lining is destroyed. For grade IV injury, the epineurium is the only intact structure, and the axons, endoneurum, internal nerves, and nerve bundles are all damaged. Finally, the grade V injury is complete damage to the entire nerve trunk [29]. After axon damage, both myelinated and unmyelinated SCs undergo extensive reprogramming to promote and guide axonal repair. These SCs then secrete chemical molecules including cytokines and neurotrophic factors, to support the survival of injured neurons, promote axon regeneration, and guide the regenerated axons to reconnect with their original targets [30-32].
Current treatment methods
Although the peripheral nervous system has the ability to self-repair and regenerate, it cannot fully recover due to scattered axonal growth, scarring, and neuroma obstruction. Unfortunately, direct suturing is limited by the loss of nerve tissue and the tension on the sutured nerve [33]. Therefore, microsurgery implantation of replacements is required for larger interstitial nerve injuries to achieve nerve regeneration and functional recovery. Current treatments for PNI can be divided into two types: surgical treatments and non-surgical treatments. Electrostimulation [34-39], magnetic stimulation [40-42], laser therapy [43], as well as the introduction of growth factor [44,45] are the main approaches for accelerating nerve repair and regeneration. Benefiting from these non-surgical treatments, short gaps of injured peripheral nerves can regenerate to some extent. However, the disadvantages of these methods should also be considered. For example, the frequency range and duration of electrical stimulation must be carefully selected, as higher frequencies and prolonged stimulation may aggravate nerve injury [5]. Although many studies have reported a variety of applications of magnetic stimulation in nerve regeneration [46-48], which can efficiently facilitate axonal regeneration and functional repair, some studies have questioned the application of variable magnetic fields in nerve repair [49]. Walker et al. used 3 different modes of magnetic stimulation on rats with sciatic nerve injury, and unfortunately, they did not observe any difference in functional recovery [50]. Moreover, the success rate of non-surgical treatment for PNI is unclear [5].
Compared with non-surgical treatment, surgical treatment is more commonly used in the treatment of PNI. Generally, surgical treatments mainly focus on transplantation, including allografts, autografts, and tissue engineering grafts [5,51]. As with other organ transplants, nerve allografts require systemic immunosuppression. However, the widespread use of nerve allografts has been limited by problems associated with immunomodulatory therapy [52]. Despite the problems of immunosuppression, nerve allografts still appeal to scientists. This is due to the fact that the immunosuppression can be prevented by adding host Schwann cells to the nerve allograft [53]. Moreover, Safa et al. reported a meaningful recovery in 82% of patients when the peripheral nerve repair gap was 70 mm, which is comparable to those of autologous nerve transplantation [54]. In addition, the advantages of allografts also include the absence of donor site morbidity and the small wounds caused by surgery, which make it possible to reduce postoperative pain [55].
Autologous nerve transplantation is considered to be the most reliable clinical treatment for PNI [56,57]. One of the advantages of autologous transplantations is their ability to revascularization, which is an essential regeneration process for damaged tissues [58]. Revascularization reduces oxygen deprivation at the damaged site and promotes the delivery of nutrients and cells for nerve regeneration [59]. However, there are several critical and unavoidable drawbacks in the application of autologous nerve transplantation, including the damage of donor site, insufficient supply of donor nerves, and mismatch between donor and recipient nerves [60,61]. Therefore, researchers have been exploring better methods to treat PNI that overcome the limitations of autologous transplantation. Tissue-engineered grafts have been a hot research topic for the treatment of PNI in recent years. NGCs synthesizing various cues have even received more and more researchers’ attention and extensive studies (Figure 2).
Figure 2.
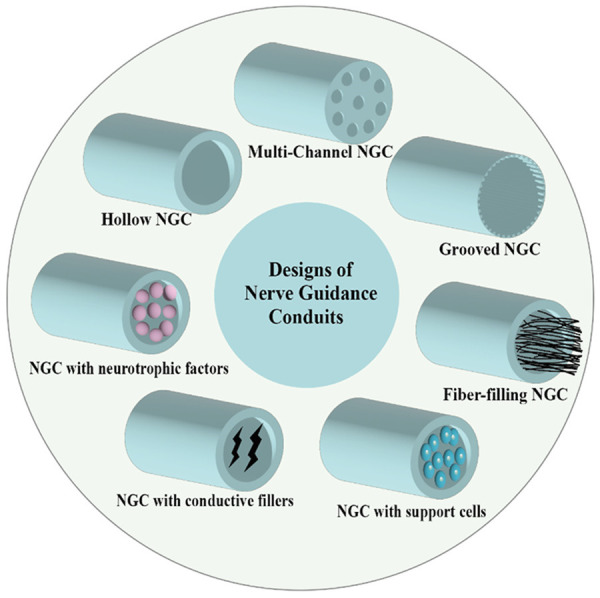
Different designs of NGC include hollow NGC, multi-channel NGC, grooved NGC, fiber-filling NGC, and NGC loaded with neurotrophic factor, conductive fillers and support cells.
NGCs with mechanical cues for PNI
The most typical tissue engineering strategy is to construct NGCs by natural, synthetic, or semi-synthetic biopolymers to repair nerve injury, which may contain biochemical cues to promote nerve regeneration and repair [7,62]. Importantly, NGCs can address the problems of donor site disease, limited supply, and the secondary incision of autografts [5]. The US Food and Drug Administration (FDA) has approved the construction of NGCs based on collagen, polyglycolic acid, and polylactide-ε-caprolacton [33], which have shown good performance in PNI repair, suggesting that artificial NGCs may be a promising solution for PNI regeneration. NGCs with different morphological and mechanical cues have attracted much attention. The main designs of NGCs include hollow design, multi-channel design, grooved design, and NGCs with fillers [63].
Hollow NGCs
Due to the approval of the FDA, hollow NGCs are more clinically acceptable compared to other types of NGCs [64]. Studies have shown that the hollow NGCs can prevent the infiltration of fibroblasts and allow the accumulation of neurotrophic factors (NTFs), thus providing clues for nerve regeneration. It can also prevent the formation of neuromas and scarring at the wound site, promote axial sprouting, and prevent the invasion of fibroblasts [65]. The most commonly used techniques for preparing hollow NGCs include electrospinning, solvent casting, cross-linking, physical film winding, melt extrusion, and weaving [66]. NGCs can also be prepared by selecting a certain method based on the desired properties [67]. However, hollow NGCs still have limitations in that they can lead to incomplete nerve regeneration due to the axonal dispersion and innervation of the regenerated nerves [68].
Multi-channel NGCs
Multi-channel conduits achieve neural orientation by restricting the space for axonal regeneration by sub-channels, preventing the dispersion of biochemical signals and providing topographical guidance [69,70]. Therefore, many researchers have studied the application of multi-channel NGCs in nerve repair. Lee et al. designed a multi-channel NGC based on the functional Arg-Gly-Asp. They performed a 10 mm sciatic nerve transection in a rat model and evaluated the effectiveness of the NGCs after 8 weeks. The results showed a significant improvement in nerve electrophysiological activity of NGC-guided regenerated nerve compared to autologous nerve grafts. Furthermore, NGCs were comparable to autologous nerve grafts in terms of functional recovery and target muscle [71]. In another study, Frost et al. prepared electrospun multi-channel NGCs using polycaprolactone (PCL) or poly-L-lactic acid (PLLA) and used them to bridge a 10 mm defect in the rat sciatic nerve to study their repair effects. It was demonstrated that these multi-channel NGCs could promote axonal regeneration in vivo [72]. Zhang L et al. developed an extrusion-stretching method to prepare multi-channel NGCs without the use of solvents and electric fields, which avoids the potential effects of traditional preparation methods on cells or tissues. The prepared multi-channel NGCs loaded with multi-walled carbon nanotubes (MWCNTs) have improved flexibility and multifunctionality, which can enhance the axonal growth and promote the formation of anisotropic tissue [73].
Grooved NGCs
Grooved NGCs have anisotropic groove structures on their inner walls which can provide topographic cues for cell migration and neurite extension [74,75]. The material topology plays a significant role in nerve regeneration, and the simulation of this structure will facilitate the directional growth of nerve axons [76,77]. Wang Z et al. prepared polyacrylonitrile NGCs by the dry-spraying and wet-spinning method and incorporated microgroove structures on their inner surface to repair PNI in Sprague Dawley (SD) rats. It was found that the repair ability of the prepared NGCs was similar to that of autologous transplantation. In another study, researchers designed a composite NGC by filling decellularized rat nerves or kidneys into poly(lactic-hydroxyglycolic acid) (PLGA) grooved conduit and introducing biochemical signals. The obtained NGCs were then transplanted to repair sciatic nerve injury in rats for 16 weeks, the results showed that decellularized nerves promoted nerve regeneration more effectively than decellularized kidneys. Moreover, compared with the simple decellularized tissue, the decellularized tissue combined with grooved PLGA conduit was significantly more effective in repairing PNI [78]. These findings provide support for the design of NGCs and provide guidance for improving neural tissue engineering strategies.
Fiber-filling NGCs
Unfilled NGCs have enough space to allow free nerve growth and to reconnect with their proper targets. In contrast, NGCs with fillings provide physical and biological support to direct cell growth and extension [65]. Therefore, in order to achieve long-gap PNI repair, the internal filling of NGCs may be a decisive factor. Many types of nanofibers have attracted special attention due to their properties similar to natural ECM and have been used to fill the NGCs [79].
Jeon J et al. prepared porous patterned NGC filled with aligned fibers (PA-NGC) with microgrooves on the inner surface of the lumen. The prepared NGCs were then implanted into a 10 mm deficient rat sciatic nerve model to investigate their ability to support nerve regeneration. The findings indicated that the growth rate of neurofilament in the PA-NGC group was significantly better than that the group of NGCs filled with random fibers. It also displayed the best performance on electrophysiology, muscle wet weight, and muscle fiber diameter [80]. In another study, the researchers designed an original spiral-shaped NGC with an array of nanofibers and wrapped with outer nanofiber tubes. In vivo application in a 10 mm model of sciatic nerve defect in SD rats showed that the novel spiral NGC promoted nerve regeneration. The results of gait analysis, electrophysiological examination, histological evaluation, and gastrocnemius measurements indicated that the constructed NGCs offered a more ideal microenvironment for peripheral nerve regeneration than conventional NGC [81]. The results of this study have important implications for improving tissue engineering strategies for PNI treatment.
NGCs with biochemical cues for PNI
In addition to physical cues, it is also important to create a microenvironment with high biological attractiveness that is suitable for nerve growth through biochemical cues. The electrical conductivity and neurotrophic capacity of NGCs are also crucial for the repair of PNI.
NGC with conductive fillers
Except for some fundamental properties of NGC, such as biocompatibility and biodegradability, electrical conductivity is one of the crucial properties that promote nerve regeneration [82-84]. The biological processes of wound healing, muscle contraction, and nerve signaling in the human body are greatly impacted by the presence of bioelectricity. Therefore, the introduction of conductive materials to NGCs helps to facilitate tissue regeneration by establishing connections for the natural flow of bioelectricity within the body [85,86].
In one study, researchers fabricated electrically conductive NGCs using a mixture of PCL and polypyrrole - polycaprolactone conductive block copolymer (PPy-b-PCL) by a novel 3D printing technique of electrohydrodynamic jetting. The technique overcomes the difficulties of controlling pore size, porosity, fiber size, and orientation during electrospinning. The authors investigated the effects of the prepared NGCs on the growth and differentiation of human embryonic stem cell-derived neural crest stem cells (hESC-NCSCs). The results showed that hESC-NCSCs could attach to and differentiate into peripheral neurons on conduits containing PCL and PCL/PPy. Furthermore, NGCs containing higher PCL/PPy content were more effective in promoting the growth and maturation of nerve cells and [87]. Hu et al. prepared conductive topological scaffolds by modifying Morpho butterfly wings with reduced graphene oxide nanosheets and brain-derived neurotrophic factor and manually curling into NGCs for PNI repair. Their results suggested that the modified wings could facilitate the growth and orientation of nerve cells. Additionally, the obtained conductive NGCs showed a great performance in repairing rat sciatic nerve 10 mm defects [88]. In conclusion, these findings show that the conductive NGCs have potential clinical application value for PNI repair and regeneration.
NGCs with neurotrophic factors
NTFs can bind to cell receptors to regulate and direct cellular activities. Meanwhile, NTFs can promote neuronal survival, axonal regeneration, and SC migration [89-91]. Therefore, the introduction of NTFs in NGCs can further promote the regeneration and repair of injured nerves. By combing various therapies for promoting peripheral nerve regeneration, Chang et al. designed a naturally degradable multi-channel scaffold with oriented electrospun nanofibers and a neurotrophic gradient (MC/AN/NG). In vitro results suggested that the neural stem cell differentiated cells extended their neurites along the aligned nanofibers. What’s more, the cell density was higher in areas with higher nerve growth factor concentrations, and the neurotrophic factors significantly improved myelination. When transplanted into rabbit sciatic nerve injuries for repair, the results showed that their fabricated MC/AN/NG NGCs exhibited better performance in nerve and muscle functional recovery than autologous nerve grafts [92]. In a study by Carvalho et al., they prepared fibroin protein-based NGCs capable of controlled release of growth factors for PNI repairing. They implanted fibroin NGCs loaded with neurotrophic factor derived from glial cell lines at 10 mm of sciatic nerve defect in rats. The results showed better performance in nerve repair compared with autografts and free fibroin conduits [93]. These studies demonstrated that NGCs loaded with neurotrophic factors is one of the most promising and prominent possibilities for promoting nerve regeneration.
NGCs with support cells for PNI
Cell-based therapy has become a potential method to promote the repair of nerve injury [94]. In the field of tissue engineering, a variety of cells have been used for PNI repair, mainly including SCs and stem cells.
Schwann cells
SCs are known as natural seed cells because they are the main glial cells of the peripheral nervous system and are key cells for peripheral nerve regeneration [95]. Therefore, SC-based NGCs are ideal scaffolds for PNI repair. The role of SCs in nerve regeneration is undisputed, so how to better transplant autologous or allogeneic SCs into injury sites has become the focus of many researchers. For example, Salehi’s team [96] constructed biodegradable and conductive NGCs by combining polylactic acid, MWCNTs, and gelatin nanofibers. In addition, they used chitosan nanoparticles coated with recombinant human erythropoietin for transplantation of SCs. The results showed that the NGC successfully delivered SCs to the site of the sciatic nerve defect in rats and demonstrated a regenerative capacity comparable to that of autologous nerve transplantation. In another study, Jahromi et al. prepared NGCs using PLLA and surface-modified MWCNT as carriers, filled with SCs and nanocurcumin, which reduced apoptosis of SCs. It was found that the number of nerve fibers at the injured site of the rat sciatic nerve increased significantly, suggesting that this NGC is an appropriate method to promote nerve regeneration after PNI [97]. Although SCs are known as the gold standard for neural bridging [96], their disadvantages such as limited supply, long culture cycles, and immune rejection problems have limited their wide application.
Stem cells
Researchers have turned their attention to undifferentiated stem cells because of their potential to self-renew and differentiate. There have been various types of stem cells used for nerve regeneration, such as bone marrow stromal cells (BMSCs) [98], neural stem cells [99,100], adipose-derived stem cells [101], mesenchymal stem cells [8,102], and embryonic stem cells [103]. Studies have shown that the combination of stem cells and NGCs has a remarkable therapeutic effect on PNI repair. It has been reported that rat BMSCs were used as supporting cells to fill silk fibroin-based NGCs, which were then implanted into the rat sciatic nerve defects to bridge the 10 mm-long space. The finding of research demonstrated that the scaffold up-regulated the expression of multiple growth factor genes and significantly improved the outcome of nerve regeneration and functional recovery [95]. In summary, stem cells have significant potential for clinical application as seed cells to construct cell-based NGCs.
Conclusion and perspective
In recent years, due to the outstanding performance of NGCs in PNI repair, it has been increasingly recognized as the next gold standard that can replace autologous nerve grafts. In this review, the repair effects of NGCs designed with different strategies on PNI were reviewed from the perspectives of mechanical cues, biochemical cues, and supporting cells. The NGCs with different mechanical cues including hollow NGCs, multi-channel NGCs, grooved NGCs, and fiber-filling NGCs. They all have certain limitations. Regenerated nerve axons are dispersed in hollow NGCs, making them less likely to connect with the target. The production processes of multi-channel NGC and grooved NGC are complicated. Fiber-filling NGCs require the development of more easily degradable fibers to avoid secondary surgery removal of the conduit. Therefore, more materials or fillers that are more suitable for guiding nerve growth need to be designed in the future. Furthermore, the application of 3-dimensional or 4-dimensional printing techniques for the preparation of NGCs allows for customized nerve repair approaches for each PNI patient. Moreover, NGCs combined with multiple physical and biochemical cues may surpass the efficacy of autologous nerve transplantation and has the potential to be developed into clinical applications in the future. Nerve regeneration is an extremely complicated process in which axons can grow in response to topological, electrical, or biochemical cues and supporting cells that guide their directed growth. Therefore, future design strategies for NGCs should focus on composite scaffolds that synthesize various cues. In conclusion, although new technologies are emerging in the field and other alternative treatments for PNI are being investigated, further research and more attention are needed to overcome the barriers to translating these technologies into the clinic.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (82371157, 82371164, 82201292), the General Program of Jiangsu Commission of Health (M2021026), the Natural Science Foundation of Jiangsu Province (BK20190121), the Natural Science Foundation of Anhui Province (2208085MH231), the China Postdoctoral Science Foundation (2020M681555), and the Distinguished Young Scholar supported by Medical Science and technology development Foundation, Nanjing Department of Health (JQX20003).
Disclosure of conflict of interest
None.
References
- 1.Cho Y, Park S, Lee J, Yu KJ. Emerging materials and technologies with applications in flexible neural implants: a comprehensive review of current issues with neural devices. Adv Mater. 2021;33:e2005786. doi: 10.1002/adma.202005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domínguez A, Ãlvarez A, Hilario E, Suarez-Merino B, Goñi-de-Cerio F. Central nervous system diseases and the role of the blood-brain barrier in their treatment. Neurosci Discov. 2013;1:3. [Google Scholar]
- 3.Jakob MO, Kofoed-Branzk M, Deshpande D, Murugan S, Klose CSN. An integrated view on neuronal subsets in the peripheral nervous system and their role in immunoregulation. Front Immunol. 2021;12:679055. doi: 10.3389/fimmu.2021.679055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang PL, Yim AKY, Kim KW, Avey D, Czepielewski RS, Colonna M, Milbrandt J, Randolph GJ. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat Commun. 2020;11:2552. doi: 10.1038/s41467-020-16355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomater. 2020;106:54–69. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Yang W, Xie H, Wang J, Zhang L, Wang Z, Wang L. CNT/Sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model. ACS Appl Mater Interfaces. 2020;12:36860–36872. doi: 10.1021/acsami.0c08457. [DOI] [PubMed] [Google Scholar]
- 7.Lackington WA, Ryan AJ, O’Brien FJ. Advances in nerve guidance conduit-based therapeutics for peripheral nerve repair. ACS Biomater Sci Eng. 2017;3:1221–1235. doi: 10.1021/acsbiomaterials.6b00500. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Nguyen PD, Shi S, Burrell JC, Cullen DK, Le AD. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci Rep. 2018;8:6634. doi: 10.1038/s41598-018-24888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagher Z, Ehterami A, Nasrolahi M, Azimi M, Salehi M. Hesperidin promotes peripheral nerve regeneration based on tissue engineering strategy using alginate/chitosan hydrogel: in vitro and in vivo study. International Journal of Polymeric Materials and Polymeric Biomaterials. 2021;70:299–308. [Google Scholar]
- 10.Hu Y, Zhang H, Wei H, Cheng H, Cai J, Chen X, Xia L, Wang H, Chai R. Scaffolds with anisotropic structure for neural tissue engineering. Engineered Regeneration. 2022;3:154–162. [Google Scholar]
- 11.Pisciotta A, Bertoni L, Vallarola A, Bertani G, Mecugni D, Carnevale G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen Res. 2020;15:373–381. doi: 10.4103/1673-5374.266043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelbasset WK, Jasim SA, Sharma SK, Margiana R, Bokov DO, Obaid MA, Hussein BA, Lafta HA, Jasim SF, Mustafa YF. Alginate-based hydrogels and tubes, as biological macromolecule-based platforms for peripheral nerve tissue engineering: a review. Ann Biomed Eng. 2022;50:628–653. doi: 10.1007/s10439-022-02955-8. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang L, Zhang F, Liu J, Cui S, Chen K, Wang J, Zhou L. Application of oblique nerve coaptation in autologous nerve transplantation. Ann Transl Med. 2020;8:1300. doi: 10.21037/atm-20-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Lu CF, Liu ZY, Han S, Wei P, Zhang DY, Kou YH, Jiang BG. Chitin scaffold combined with autologous small nerve repairs sciatic nerve defects. Neural Regen Res. 2022;17:1106–1114. doi: 10.4103/1673-5374.324859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore AM, Ray WZ, Chenard KE, Tung T, Mackinnon SE. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand (N Y) 2009;4:239–244. doi: 10.1007/s11552-009-9183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Guo J, Wang Y, Shang L, Chai R, Zhao Y. Natural polymer-derived bioscaffolds for peripheral nerve regeneration. Advanced Functional Materials. 2022;32:2203829.1–2203829.21. [Google Scholar]
- 17.Wang ML, Rivlin M, Graham JG, Beredjiklian PK. Peripheral nerve injury, scarring, and recovery. Connect Tissue Res. 2019;60:3–9. doi: 10.1080/03008207.2018.1489381. [DOI] [PubMed] [Google Scholar]
- 18.Alvites R, Rita Caseiro A, Santos Pedrosa S, Vieira Branquinho M, Ronchi G, Geuna S, Varejão ASP, Colette Maurício A. Peripheral nerve injury and axonotmesis: state of the art and recent advances. Cogent Medicine. 2018;5:1466404. [Google Scholar]
- 19.Liu P, Peng J, Han GH, Ding X, Wei S, Gao G, Huang K, Chang F, Wang Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen Res. 2019;14:1335–1342. doi: 10.4103/1673-5374.253510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An N, Yang J, Wang H, Sun S, Wu H, Li L, Li M. Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci. 2021;11:41. doi: 10.1186/s13578-021-00554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SM, Chung G, Kim YH, Park CK. The role of maresins in inflammatory pain: function of macrophages in wound regeneration. Int J Mol Sci. 2019;20:5849. doi: 10.3390/ijms20235849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avraham O, Deng PY, Jones S, Kuruvilla R, Semenkovich CF, Klyachko VA, Cavalli V. Satellite glial cells promote regenerative growth in sensory neurons. Nat Commun. 2020;11:4891. doi: 10.1038/s41467-020-18642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izmiryan A, Li Z, Nothias F, Eyer J, Paulin D, Soares S, Xue Z. Inactivation of vimentin in satellite glial cells affects dorsal root ganglion intermediate filament expression and neuronal axon growth in vitro. Mol Cell Neurosci. 2021;115:103659. doi: 10.1016/j.mcn.2021.103659. [DOI] [PubMed] [Google Scholar]
- 24.Hanani M, Spray DC. Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci. 2020;21:485–498. doi: 10.1038/s41583-020-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics. 2017;40:e141–e156. doi: 10.3928/01477447-20161019-01. [DOI] [PubMed] [Google Scholar]
- 26.Dong R, Liu Y, Yang Y, Wang H, Xu Y, Zhang Z. MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. Biomed Res Int. 2019;2019:6458237. doi: 10.1155/2019/6458237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC. Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res. 2020;98:780–795. doi: 10.1002/jnr.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Liu SY, Pi W, Zhang PX. Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen Res. 2021;16:1518–1523. doi: 10.4103/1673-5374.303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes B, Sousa P, Alvites R, Branquinho M, Sousa AC, Mendonça C, Atayde LM, Luís AL, Varejão ASP, Maurício AC. Peripheral nerve injury treatments and advances: one health perspective. Int J Mol Sci. 2022;23:918. doi: 10.3390/ijms23020918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nocera G, Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020;77:3977–3989. doi: 10.1007/s00018-020-03516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R, Li D, Wu C, Ye L, Wu Y, Yuan Y, Yang S, Xie L, Mao Y, Jiang T, Li Y, Wang J, Zhang H, Li X, Xiao J. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. 2020;10:1649–1677. doi: 10.7150/thno.40919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Li Q, Li L, Chen S, Zhao Y, Hu Y, Wang L, Lan X, Zhong L, Lu D. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells function. Bioact Mater. 2021;8:355–367. doi: 10.1016/j.bioactmat.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou Y, Wang X, Zhang Z, Luo J, Cai Z, Wang Y, Li Y. Repairing transected peripheral nerve using a biomimetic nerve guidance conduit containing intraluminal sponge fillers. Adv Healthc Mater. 2019;8:e1900913. doi: 10.1002/adhm.201900913. [DOI] [PubMed] [Google Scholar]
- 34.Zuo KJ, Gordon T, Chan KM, Borschel GH. Electrical stimulation to enhance peripheral nerve regeneration: update in molecular investigations and clinical translation. Exp Neurol. 2020;332:113397. doi: 10.1016/j.expneurol.2020.113397. [DOI] [PubMed] [Google Scholar]
- 35.Guo R, Liao M, Ma X, Hu Y, Qian X, Xiao M, Gao X, Chai R, Tang M. Cochlear implant-based electric-acoustic stimulation modulates neural stem cell-derived neural regeneration. J Mater Chem B. 2021;9:7793–7804. doi: 10.1039/d1tb01029h. [DOI] [PubMed] [Google Scholar]
- 36.Guo R, Xiao M, Zhao W, Zhou S, Hu Y, Liao M, Wang S, Yang X, Chai R, Tang M. 2D Ti3C2TxMXene couples electrical stimulation to promote proliferation and neural differentiation of neural stem cells. Acta Biomater. 2022;139:105–117. doi: 10.1016/j.actbio.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 37.Liao M, Cui Q, Hu Y, Xing J, Wu D, Zheng S, Zhao Y, Yu Y, Sun J, Chai R. Recent advances in the application of MXenes for neural tissue engineering and regeneration. Neural Regen Res. 2024;19:258–263. doi: 10.4103/1673-5374.379037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao M, Hu Y, Zhang Y, Wang K, Fang Q, Qi Y, Shen Y, Cheng H, Fu X, Tang M, Sun S, Gao X, Chai R. 3D Ti3C2Tx MXene-matrigel with electroacoustic stimulation to promote the growth of spiral ganglion neurons. ACS Nano. 2022;16:16744–16756. doi: 10.1021/acsnano.2c06306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Hu W, Tang L, Wang Y. Fundamental neurocircuit of anti-inflammatory effect by electroacupuncture stimulation identified. Neurosci Bull. 2022;38:837–839. doi: 10.1007/s12264-022-00849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian Y, Cheng Y, Cai J, Zhao X, Ouyang Y, Yuan WE, Fan C. Advances in electrical and magnetic stimulation on nerve regeneration. Regen Med. 2019;14:969–979. doi: 10.2217/rme-2018-0079. [DOI] [PubMed] [Google Scholar]
- 41.Xia L, Zhao X, Ma X, Hu Y, Zhang Y, Li S, Wang J, Zhao Y, Chai R. Controllable growth of spiral ganglion neurons by magnetic colloidal nanochains. Nano Today. 2022;44:101507. [Google Scholar]
- 42.Hu Y, Li D, Wei H, Zhou S, Chen W, Yan X, Cai J, Chen X, Chen B, Liao M, Chai R, Tang M. Neurite extension and orientation of spiral ganglion neurons can be directed by superparamagnetic iron oxide nanoparticles in a magnetic field. Int J Nanomedicine. 2021;16:4515–4526. doi: 10.2147/IJN.S313673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreo L, Ribeiro BG, Alves AN, Martinelli ASA, Soldera CB, Horliana ACRT, Bussadori SK, Fernandes KPS, Mesquita-Ferrari RA. Effects of photobiomodulation with low-level laser therapy on muscle repair following a peripheral nerve injury in wistar rats. Photochem Photobiol. 2020;96:1124–1132. doi: 10.1111/php.13255. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Li DH, Zhang HY, Wang J, Li XK, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41:1289–1300. doi: 10.1038/s41401-019-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Ren L, Zhang H, Hu Y, Liao M, Shen Y, Wang K, Cai J, Cheng H, Guo J, Qi Y, Wei H, Li X, Shang L, Xiao J, Sun J, Chai R. Basic fibroblast growth factor-loaded methacrylate gelatin hydrogel microspheres for spinal nerve regeneration. Smart Medicine. 2023;2:e20220038. doi: 10.1002/SMMD.20220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riggio C, Calatayud MP, Giannaccini M, Sanz B, Torres TE, Fernández-Pacheco R, Ripoli A, Ibarra MR, Dente L, Cuschieri A, Goya GF, Raffa V. The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomedicine. 2014;10:1549–1558. doi: 10.1016/j.nano.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Dong T, Li X, Zhao H, Yang L, Xu R, Fu Y, Li L, Gai X, Qin D. Research progress on the application of transcranial magnetic stimulation in spinal cord injury rehabilitation: a narrative review. Front Neurol. 2023;14:1219590. doi: 10.3389/fneur.2023.1219590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Zhou XJ, Sun RB. Effect of the combination of high-frequency repetitive magnetic stimulation and neurotropin on injured sciatic nerve regeneration in rats. Neural Regen Res. 2020;15:145–151. doi: 10.4103/1673-5374.264461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bannaga A, Guo T, Ouyang X, Hu D, Lin C, Cao F, Deng Y, Guo Z, Luo Y. Magnetic stimulation accelerating rehabilitation of peripheral nerve injury. J Huazhong Univ Sci Technol (Med Sci) 2002;22:135–139. doi: 10.1007/BF02857676. [DOI] [PubMed] [Google Scholar]
- 50.Walker JL, Kryscio R, Smith J, Pilla A, Sisken BF. Electromagnetic field treatment of nerve crush injury in a rat model: effect of signal configuration on functional recovery. Bioelectromagnetics. 2007;28:256–263. doi: 10.1002/bem.20302. [DOI] [PubMed] [Google Scholar]
- 51.Han GH, Peng J, Liu P, Ding X, Wei S, Lu S, Wang Y. Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation. Neural Regen Res. 2019;14:1343–1351. doi: 10.4103/1673-5374.253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roballo KCS, Bushman J. Evaluation of the host immune response and functional recovery in peripheral nerve autografts and allografts. Transpl Immunol. 2019;53:61–71. doi: 10.1016/j.trim.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:7785. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Safa B, Jain S, Desai MJ, Greenberg JA, Niacaris TR, Nydick JA, Leversedge FJ, Megee DM, Zoldos J, Rinker BD, McKee DM, MacKay BJ, Ingari JV, Nesti LJ, Cho M, Valerio IL, Kao DS, El-Sheikh Y, Weber RV, Shores JT, Styron JF, Thayer WP, Przylecki WH, Hoyen HA, Buncke GM. Peripheral nerve repair throughout the body with processed nerve allografts: results from a large multicenter study. Microsurgery. 2020;40:527–537. doi: 10.1002/micr.30574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown MJ, Carter T. ACL allograft: advantages and when to use. Sports Med Arthrosc Rev. 2018;26:75–78. doi: 10.1097/JSA.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 56.Kornfeld T, Borger A, Radtke C. Reconstruction of critical nerve defects using allogenic nerve tissue: a review of current approaches. Int J Mol Sci. 2021;22:3515. doi: 10.3390/ijms22073515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitsuzawa S, Ikeguchi R, Aoyama T, Takeuchi H, Yurie H, Oda H, Ohta S, Ushimaru M, Ito T, Tanaka M, Kunitomi Y, Tsuji M, Akieda S, Nakayama K, Matsuda S. The efficacy of a scaffold-free Bio 3D conduit developed from autologous dermal fibroblasts on peripheral nerve regeneration in a canine ulnar nerve injury model: a preclinical proof-of-concept study. Cell Transplant. 2019;28:1231–1241. doi: 10.1177/0963689719855346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saffari TM, Mathot F, Friedrich PF, Bishop AT, Shin AY. Revascularization patterns of nerve allografts in a rat sciatic nerve defect model. J Plast Reconstr Aesthet Surg. 2020;73:460–468. doi: 10.1016/j.bjps.2019.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathot F, Rbia N, Bishop AT, Hovius SER, Shin AY. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery. 2020;40:585–592. doi: 10.1002/micr.30579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Zhang H, Wang H, Zhao Y, Chai R. Natural proteins-derived asymmetric porous conduit for peripheral nerve regeneration. Applied Materials Today. 2022;27:101431. [Google Scholar]
- 61.Wang ZZ, Sakiyama-Elbert SE. Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp Neurol. 2019;319:112837. doi: 10.1016/j.expneurol.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang NU, Lee SJ, Gwak SJ. Fabrication techniques of nerve guidance conduits for nerve regeneration. Yonsei Med J. 2022;63:114–123. doi: 10.3349/ymj.2022.63.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Houshyar S, Bhattacharyya A, Shanks R. Peripheral nerve conduit: materials and structures. ACS Chem Neurosci. 2019;10:3349–3365. doi: 10.1021/acschemneuro.9b00203. [DOI] [PubMed] [Google Scholar]
- 64.Liu K, Yan L, Li R, Song Z, Ding J, Liu B, Chen X. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv Sci (Weinh) 2022;9:e2103875. doi: 10.1002/advs.202103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carvalho CR, Oliveira JM, Reis RL. Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Front Bioeng Biotechnol. 2019;7:337. doi: 10.3389/fbioe.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarker M, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol J. 2018;13:e1700635. doi: 10.1002/biot.201700635. [DOI] [PubMed] [Google Scholar]
- 67.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng Part A. 2009;15:3605–3619. doi: 10.1089/ten.TEA.2008.0689. [DOI] [PubMed] [Google Scholar]
- 68.Sun B, Zhou Z, Wu T, Chen W, Li D, Zheng H, El-Hamshary H, Al-Deyab SS, Mo X, Yu Y. Development of nanofiber sponges-containing nerve guidance conduit for peripheral nerve regeneration in vivo. ACS Appl Mater Interfaces. 2017;9:26684–26696. doi: 10.1021/acsami.7b06707. [DOI] [PubMed] [Google Scholar]
- 69.Yao L, de Ruiter GC, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ, Pandit A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials. 2010;31:5789–5797. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Xiong H, Zhu T, Liu Y, Pan H, Fan C, Zhao X, Lu WW. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano. 2020;14:12579–12595. doi: 10.1021/acsnano.0c03570. [DOI] [PubMed] [Google Scholar]
- 71.Lee DJ, Fontaine A, Meng X, Park D. Biomimetic nerve guidance conduit containing intraluminal microchannels with aligned nanofibers markedly facilitates in nerve regeneration. ACS Biomater Sci Eng. 2016;2:1403–1410. doi: 10.1021/acsbiomaterials.6b00344. [DOI] [PubMed] [Google Scholar]
- 72.Frost HK, Andersson T, Johansson S, Englund-Johansson U, Ekström P, Dahlin LB, Johansson F. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci Rep. 2018;8:16716. doi: 10.1038/s41598-018-34699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L, Sun R, Wang B, Lang Y, Chang MW. Polycaprolactone/multi-walled carbon nanotube nerve guidance conduits with tunable channels fabricated via novel extrusion-stretching method for peripheral nerve repair. International Journal of Polymeric Materials and Polymeric Biomaterials. 2023:1–9. [Google Scholar]
- 74.Lee MS, Lee DH, Jeon J, Oh SH, Yang HS. Topographically defined, biodegradable nanopatterned patches to regulate cell fate and acceleration of bone regeneration. ACS Appl Mater Interfaces. 2018;10:38780–38790. doi: 10.1021/acsami.8b14745. [DOI] [PubMed] [Google Scholar]
- 75.Yang X, Liu X, Xu F, Ji S, Sun Y, Song Z, Song J, Wu Y, Yin J. Fabrication of microgroove poly(lactic-co-glycolic acid) nerve guide conduit using dry-jet wet spinning for rat laryngeal recurrent nerve regeneration. Materials & Design. 2022;223:111151. [Google Scholar]
- 76.Liu F, Xu J, Wu L, Zheng T, Han Q, Liang Y, Zhang L, Li G, Yang Y. The influence of the surface topographical cues of biomaterials on nerve cells in peripheral nerve regeneration: a review. Stem Cells Int. 2021;2021:8124444. doi: 10.1155/2021/8124444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei H, Chen Z, Hu Y, Cao W, Ma X, Zhang C, Gao X, Qian X, Zhao Y, Chai R. Topographically conductive butterfly wing substrates for directed spiral ganglion neuron growth. Small. 2021;17:e2102062. doi: 10.1002/smll.202102062. [DOI] [PubMed] [Google Scholar]
- 78.Yu E, Chen Z, Huang Y, Wu Y, Wang Z, Wang F, Wu M, Xu K, Peng W. A grooved conduit combined with decellularized tissues for peripheral nerve regeneration. J Mater Sci Mater Med. 2023;34:35. doi: 10.1007/s10856-023-06737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keshvardoostchokami M, Majidi SS, Huo P, Ramachandran R, Chen M, Liu B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials (Basel) 2020;11:21. doi: 10.3390/nano11010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeon J, Lee MS, Lim J, Park S, Kim SM, Kim D, Tae G, Yang HS. Micro-grooved nerve guidance conduits combined with microfiber for rat sciatic nerve regeneration. J Ind Eng Chem. 2020;90:214–223. [Google Scholar]
- 81.Chang W, Shah MB, Lee P, Yu X. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. 2018;73:302–311. doi: 10.1016/j.actbio.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 82.Hu Y, Chen W, Yin H, Chen X, Cai J, Guo J, Zhou S, Chai R, Tang M. Super-aligned carbon nanotubes and GelMA hydrogel composite scaffolds promote spiral ganglion neuron growth and orientation. Materials Today Nano. 2022;18:100181. [Google Scholar]
- 83.Li Y, Hu Y, Wei H, Cao W, Qi Y, Zhou S, Zhang P, Li H, Li GL, Chai R. Two-dimensional Ti3C2Tx MXene promotes electrophysiological maturation of neural circuits. J Nanobiotechnology. 2022;20:398. doi: 10.1186/s12951-022-01590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo R, Ma X, Liao M, Liu Y, Hu Y, Qian X, Tang Q, Guo X, Chai R, Gao X, Tang M. Development and application of cochlear implant-based electric-acoustic stimulation of spiral ganglion neurons. ACS Biomater Sci Eng. 2019;5:6735–6741. doi: 10.1021/acsbiomaterials.9b01265. [DOI] [PubMed] [Google Scholar]
- 85.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J Tissue Eng Regen Med. 2011;5:e17–35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 86.Cai J, Zhang H, Hu Y, Huang Z, Wang Y, Xia Y, Chen X, Guo J, Cheng H, Xia L, Lu W, Zhang C, Xie J, Wang H, Chai R. GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair. J Nanobiotechnology. 2022;20:460. doi: 10.1186/s12951-022-01669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vijayavenkataraman S, Kannan S, Cao T, Fuh JYH, Sriram G, Lu WF. 3D-printed PCL/PPy conductive scaffolds as three-dimensional porous nerve guide conduits (NGCs) for peripheral nerve injury repair. Front Bioeng Biotechnol. 2019;7:266. doi: 10.3389/fbioe.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu Y, Chen Z, Wang H, Guo J, Cai J, Chen X, Wei H, Qi J, Wang Q, Liu H, Zhao Y, Chai R. Conductive nerve guidance conduits based on morpho butterfly wings for peripheral nerve repair. ACS Nano. 2022;16:1868–1879. doi: 10.1021/acsnano.1c11627. [DOI] [PubMed] [Google Scholar]
- 89.Tajdaran K, Chan K, Gordon T, Borschel GH. Matrices, scaffolds, and carriers for protein and molecule delivery in peripheral nerve regeneration. Exp Neurol. 2019;319:112817. doi: 10.1016/j.expneurol.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Pi W, Zhang Y, Li L, Li C, Zhang M, Zhang W, Cai Q, Zhang P. Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration. Biofabrication. 2022;14 doi: 10.1088/1758-5090/ac57a6. [DOI] [PubMed] [Google Scholar]
- 91.Xu YL, Zhu L, Chen ZJ, Deng XF, Liu PD, Li S, Lin BC, Yang CZ, Xu W, Zhou KK, Zhu YJ. Release of endogenous brain-derived neurotrophic factor into the lateral entorhinal cortex from the paraventricular thalamus ameliorates social memory deficits in a mouse model of Alzheimer’s disease. Neurosci Bull. 2022;38:1425–1430. doi: 10.1007/s12264-022-00900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang YC, Chen MH, Liao SY, Wu HC, Kuan CH, Sun JS, Wang TW. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl Mater Interfaces. 2017;9:37623–37636. doi: 10.1021/acsami.7b12567. [DOI] [PubMed] [Google Scholar]
- 93.Carvalho CR, Chang W, Silva-Correia J, Reis RL, Oliveira JM, Kohn J. Engineering silk fibroin-based nerve conduit with neurotrophic factors for proximal protection after peripheral nerve injury. Adv Healthc Mater. 2021;10:e2000753. doi: 10.1002/adhm.202000753. [DOI] [PubMed] [Google Scholar]
- 94.Yousefi F, Lavi Arab F, Nikkhah K, Amiri H, Mahmoudi M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019;221:99–108. doi: 10.1016/j.lfs.2019.01.052. [DOI] [PubMed] [Google Scholar]
- 95.Yi S, Zhang Y, Gu X, Huang L, Zhang K, Qian T, Gu X. Application of stem cells in peripheral nerve regeneration. Burns Trauma. 2020;8:tkaa002. doi: 10.1093/burnst/tkaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salehi M, Naseri-Nosar M, Ebrahimi-Barough S, Nourani M, Khojasteh A, Hamidieh AA, Amani A, Farzamfar S, Ai J. Sciatic nerve regeneration by transplantation of Schwann cells via erythropoietin controlled-releasing polylactic acid/multiwalled carbon nanotubes/gelatin nanofibrils neural guidance conduit. J Biomed Mater Res B Appl Biomater. 2018;106:1463–1476. doi: 10.1002/jbm.b.33952. [DOI] [PubMed] [Google Scholar]
- 97.Jahromi HK, Farzin A, Hasanzadeh E, Barough SE, Mahmoodi N, Najafabadi MRH, Farahani MS, Mansoori K, Shirian S, Ai J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater Sci Eng C Mater Biol Appl. 2020;109:110564. doi: 10.1016/j.msec.2019.110564. [DOI] [PubMed] [Google Scholar]
- 98.Hou B, Ye Z, Ji W, Cai M, Ling C, Chen C, Guo Y. Comparison of the effects of BMSC-derived Schwann cells and autologous Schwann cells on remyelination using a rat sciatic nerve defect model. Int J Biol Sci. 2018;14:1910–1922. doi: 10.7150/ijbs.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ye W, Li H, Yu K, Xie C, Wang P, Zheng Y, Zhang P, Xiu J, Yang Y, Zhang F, He Y, Gao Q. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Materials & Design. 2020;192:108757. [Google Scholar]
- 100.Han S, Sun J, He S, Tang M, Chai R. The application of graphene-based biomaterials in biomedicine. Am J Transl Res. 2019;11:3246–3260. [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang R, Rosen JM. The role of undifferentiated adipose-derived stem cells in peripheral nerve repair. Neural Regen Res. 2018;13:757–763. doi: 10.4103/1673-5374.232457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang RC, Du WQ, Zhang JY, Yu SX, Lu FZ, Ding HM, Cheng YB, Ren C, Geng DQ. Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review. Neural Regen Res. 2021;16:2170–2176. doi: 10.4103/1673-5374.310941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kubiak CA, Grochmal J, Kung TA, Cederna PS, Midha R, Kemp SWP. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve. 2020;61:449–459. doi: 10.1002/mus.26760. [DOI] [PubMed] [Google Scholar]


