Abstract
Background: Keloids and hypertrophic scars are some of the most common skin conditions globally, associated with poor treatment response and high recurrence rates. Autologous adipose-derived stromal vascular fraction (SVF) is increasingly recognized as an emerging therapy albeit limited literature on its outcome in scar treatment. This review aimed to describe the current practices and outcomes of adipose-derived stromal Vascular Fraction in scar treatment. Methods: This systematic review assessed articles describing the use of SVF in scar treatment published between 2000 and 2023. Article searches of Medline/PubMed, Cochrane Library, and Embase databases using Mesh terms and the Boolean operators (“AND”, “OR”) by two independent researchers were done whilst following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Clinical studies assessing SVF in scar treatment with a primary outcome measure being an improvement in scar characteristics including the thickness, scar assessment scores were included. Results: Among the 1425 studies identified in the search, 20 studies met the inclusion criteria with a total of 493 patients included. Eight of these were clinical trials with the rest being observational studies. Follow-up ranged from 3 months to 24 months. In all studies, there was an improvement in scar characteristics following single-dose treatment with SVF or its equivalent. All studies reported SVF to be safe. Conclusion: The review found that autologous adipose-derived SVF is a clinically effective therapy for keloids and scar treatment.
Keywords: Stromal vascular fraction, keloids, scars, adipose stem cells, systematic review
Introduction
Keloids and hypertrophic scars are among the most prevalent disfiguring skin conditions, typically affecting individuals with pigmented skin, particularly those of African descent. In the United States, these conditions are estimated to occur at a frequency ranging from 4.5% to 16% [1].
While hypertrophic scars are more common, keloids are a severe version often characterized by invasive properties such as growing into the surrounding tissues [2] and behaving similarly to malignant tumors. On the other hand, hypertrophic scars have a milder course of growth often restricted to the previous wound site [3].
Despite ongoing research efforts, a definitive cure for keloids has yet to be identified, and there is no single, consistently effective therapy available [4]. Instead, a combination of treatments is typically required to achieve satisfactory results. However, recurrence of keloids following treatment termination is a common occurrence even in the most effective therapies [5,6].
Commonly utilized therapies for keloids and hypertrophic scars include silicone pressure compression, topical or injected corticosteroids (such as Triamcinolone), anticancer agents (such as 5-Fluorouracil), cryotherapy, laser therapy (e.g., pulsed dye laser, CO2 laser), interferon therapy, radiation therapy, imiquimod cream, and bleomycin injection [6-10]. However, as no single therapy has consistently shown to be effective, a combination of therapies is often used. Surgery may be performed following adjuvant treatment, but it is not recommended as a single therapy [11].
Stromal Vascular Fraction (SVF) derived from adipose tissue is an emerging therapy for scar treatment that shows promise in the regression and flattening of hypertrophic scars. This innovative approach has the potential to improve the appearance of scars and is being increasingly recognized as a valuable treatment option [12,13].
The SVF is a heterogeneous mixture of adipose-derived stem cells, endothelial cells, immune cells, and other cell types obtained from the processed lipoaspirate following liposuction. These cells can potentially promote tissue repair and regeneration through various mechanisms, including the secretion of growth factors and cytokines and the induction of angiogenesis and immune modulation [14]. Due to its regenerative properties [15], SVF has been explored as a potential therapy for various medical conditions, including wound healing, osteoarthritis, and autoimmune diseases [16-18].
In the context of keloid and scar treatment, SVF has shown promising results in preclinical and clinical studies. In a study by Li et al [19], SVF was shown to suppress fibrosis in scars via the p38/MAPK signaling pathway. Similar clinical studies report improvement in scarring following SVF injections [20].
The use of SVF in keloid and scar treatment is still in its infancy, and several gaps and limitations exist. Such gaps include the lack of standardized protocols for the isolation and preparation of SVF. The composition and potency of SVF can vary depending on the donor, the method of isolation, and the processing techniques used. Similarly, the reporting of outcomes is heterogeneous. Therefore, more rigorous and standardized protocols are needed to ensure reproducibility and efficacy.
Despite this growing recognition, the role of adipose-derived stromal vascular fraction or related products is yet to be fully understood.
We therefore set out with the primary objective of reviewing existing studies to establish the efficacy of adipose-derived stromal vascular fraction in comparison to other therapies.
We also described the methods used to isolate and process the lipoaspirate to obtain the Stromal Vascular Fraction and reported any adverse events.
Methods/Design
Study design, protocol, and registration
We designed this systematic review to evaluate the existing clinical research involving the use of autologous adipose-derived stromal vascular fraction in the treatment of scars.
The systematic review protocol was published by the American Journal of Stem Cells [21]. The systematic review was also submitted for registration under the PROSPERO International Register of Systematic Reviews. The study was conducted following the principles of Preferred Report Items of Systematic Reviews and meta-analysis protocols (PRISMA-P) [22].
In as much as we intended to conduct a meta-analysis comparing different therapies, the study outcomes were heterogeneous among the clinical trials. Instead, we conducted a meta-analysis of proportions for the observational studies.
Eligibility criteria
Inclusion and exclusion criteria
Using the PICOS (Participants, Interventions, Comparisons, outcomes, and study design) framework we identified relevant articles that were included in the review.
For articles to be included in the review, they had to meet the requirements as described below: 1. The studies had to be clinical studies including randomized controlled clinical trials, cohorts, and case controls evaluating (I) Adipose-derived stromal vascular fraction independently or in comparison to an established (C) treatment modality including intralesional corticosteroids, cryotherapy, anti-cancer, cutaneous radiotherapy, laser therapy among others. 2. The studies had to have been conducted among participants of all ages from any part of the world and published in peer-reviewed journals between 2000 to 2023. 3. The outcome measure of the study had to be a Scar Assessment Score or the scar volume changes between baseline and end of follow-up.
Exclusion criteria
Studies involving the use of stromal vascular fraction in non-healed wounds or for lipo-filling of defects were excluded.
Language
There was no restriction on language applied to the review.
Information sources
We searched the following databases: Medline/PubMed, SCOPUS, and EMBASE.
We also searched for grey literature using Google Scholar.
Search strategy
We initially searched for studies published from January 2000 to December 2020 as there was no consensus on the definition of Adipose-derived stem cells before the year 2000. Subsequently, we repeated the search to involve studies conducted between 2020 to 2023. Hence the search involved any studies from 2000 to 2023.
Key search terms
We used keywords including Medical Subject Heading (MeSH) terms related to Adipose-Derived Stromal Vascular Fraction (SVF) and keloids to identify the articles in the electronic databases. Boolean operators “AND” and “OR” were used to combine the search terms.
The keywords and a preliminary MEDLINE search string have been included (Supplementary File 1).
Study records
Data management, selection, and collection processes
The results of the literature search were exported to Rayyan software. Two reviewers (AK and SR) searched the extract for relevant search titles and abstracts and selected those deemed relevant. Duplicated studies were merged when found. The reviewers went ahead and screened all articles based on the pre-set eligibility criteria. The PRISMA Flow guide elaborated on the selection process of the articles (See Figure 1). Excluded studies included preclinical studies, animal studies, and reviews. All disagreements between the reviewers were solved by consensus between the reviewers.
Figure 1.
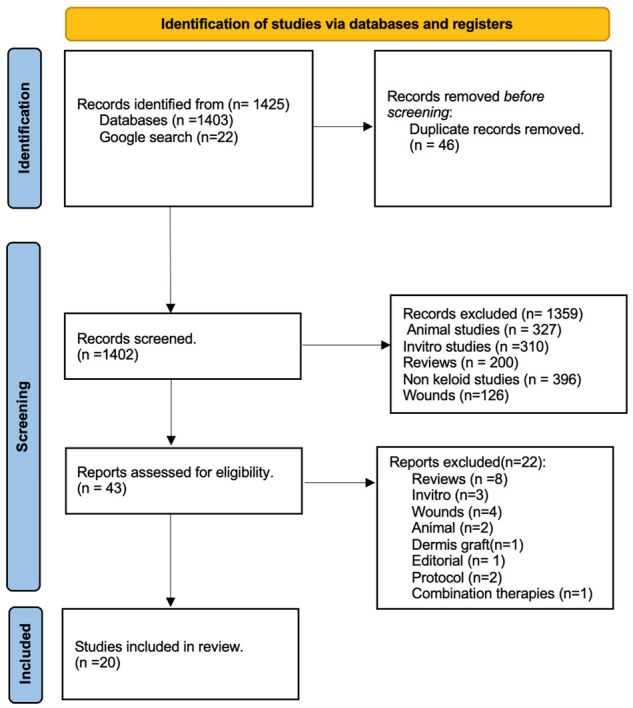
PRISMA flow chart of studies screened and included.
Data collection process
Data extraction
For studies that met the inclusion criteria, data extraction was performed. The data items were collected as described below:
Study characteristics: a. Study design, participant characteristics: Study design, sample size, length of follow-up, and participant demographic characteristics including the mean age were obtained. b. Baseline keloid and scar characteristics: The scar volume, height, surface area, and Scar assessment scores. c. The SVF intervention methodologies: Description of the intervention methods including harvesting, process, and infiltration techniques. The comparison/control arm methods. Processing technique for obtaining SVF or ADSCs. d. End of follow-up keloid and scar characteristics: The scar volume (mm3), height (mm), and the Scar assessment scores at the end of the study follow-up. e. End of Follow-up adverse effects: Any recorded adverse effects and their categorization.
Outcomes
The treatment outcome following scar treatment with SVF described as a mean change in scar assessment score or volume was the primary outcome of interest.
The development of adverse events as reported in each study constituted the secondary outcome of interest.
Evaluation of risk of bias
Risk of bias in individual studies/Internal validity
The two researchers participated in the evaluation of the risk of bias for each of the selected studies. The risk of bias assessment was based on the Cochrane Collaboration tools for assessing for risk of Bias.
For Randomized control trials, we used the “Revised Cochrane risk-of-bias tool for randomized trials (RoB2)” [23] while for non-randomized studies, the “Risk of Bias in Non-Randomized studies of Intervention (ROBINS-I) tool” [24] was used. Observational studies were classified as having a high risk of bias.
The risk of bias in each study was reported as ‘Low’ risk or ‘High’ risk or ‘Some concern’.
Individually for each randomized controlled trial, Selection bias, performance bias, detection bias, attrition bias, reporting bias, and any other source of bias were reviewed.
Assessment of external validity
To assess how generalizable, the findings in the different studies were, we evaluated how the study populations were selected including the sampling methods and sample characteristics.
Summary measures
Differences of means were used as the summary of measures for the Scar assessment scores from baseline and at the end of the follow-up of the study.
Data synthesis and statistical analysis
We summarized the characteristics of the included studies based on the PICOS elements with the summary presented in the “characteristics of included studies” table.
The primary and secondary outcome measures: The scar assessment scores as well as the scar volume were analyzed using descriptive statistics of means and proportions. Analysis of the effect measures was conducted to obtain mean differences and the standardized mean differences with a confidence interval of 95% for all the continuous variables. Due to the heterogeneity of the administered clinical therapies and methodological design as well as the outcome measures, it was not possible to conduct a meta-analysis of the clinical trials. As are result, we performed a qualitative synthesis to describe a narrative of the studies regarding the outcomes of interest. However quantitative subgroup analysis for studies that used the POSAS score as the outcome measure of assessment was performed. The involved studies in the quantitative analysis were observational and once summarized, a meta-analysis for prevalence (one proportion) was conducted which allows for meta-analysis of studies that don’t have are control arm but only the outcome arm. In this case, STATA software version 18 was used to conduct the meta-analysis using the “metan” command. Specifically, to generate the Forrest plots of the six included observational studies, the mean differences of the POSAS scores at three months and baseline were obtained. The standard error of the mean (SEM) of these mean differences was obtained from the standard deviation (SD), and sample size using the formula SEM = SD/√n.
Where SEM = Standard error of the Mean, SD = Standard Deviation of the mean, and n = sample size.
The Forrest plot was therefore generated using the command below: Metan mean difference standard error of the mean, random.
We used the Random effects Model. The I2 statistic was reported for the degree of variability.
Results
Study selection process
The initial search of articles from 2000 to 2020 resulted in a total of 978 articles from PubMed, Embase, and Medline search while an extra 20 other articles were obtained from a Google search. Of these, 20 papers were included for full-text review of which 19 papers were included in the main study. See the flow chart in Figure 1.
The same search was repeated to include studies published from 2020 to 2023. In total 453 articles were obtained of which 5 were included in the full text review and finally one study was included in the final review hence having 20 articles included in this systematic review.
Baseline description of the included studies
Twenty studies were included in the review. These included 3 randomized controlled clinical trials, and 5 non-randomized clinical trials while the rest of the 12 included studies were observational. Among the observational studies were 3 case reports/case series, 2 retrospective studies, and 6 prospective observational studies.
The study sample sizes ranged between 3 and 50 while the study follow-up time ranged from 3 to 24 months with are total of 493 patients included in this review. Detailed characteristics of the included studies are presented in Table 1.
Table 1.
Characteristics of included studies
| Author | Sample size | Age (years) | Gender (M:F) | Design | Scar type | Intervention Arm (n) | Control Arm (n) | Dose frequency | Outcome type | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Zahorec et al (2020) Slovakia | 8 | 9 to 42 | n/a | NCT | Post burn scars | ADSCs (8) | None | Varied | VSS | 6 |
| Eitta et al (2019) Egypt | 10 | 33.20 (±6.51) | 1:9 | NCT | Post acne scars | SVF (5) | FxCR (5) | Single | Scar area percentage, Patient self-assessment and satisfaction | 3 |
| L’Orphelin et al (2020) France | 43 | n/a | n/a | Retrospective study | Acquired or congenital scars | Fat graft (55) | n/a | Single | Patient and surgeon satisfaction | 6 |
| Elkahky et al (2016) Egypt | 20 | 20-45 | 7:13 | RCT | Post acne scars | SVF (10) | PRP (10) | Single | Surface area of scars | 3 |
| Lee et al (2018) Korea | 17 | 37 (14-74) | 12:5 | Retrospective Study-study 1 | Diverse distribution | SVF (17) | n/a | Single | OSAS, VSS, VAS | 6 |
| 15 | 34 (14-65) | 12:3 | Retrospective Cohort Study 2 | Face | SVF (7) | Control (8) | OSAS, VSS, VAS | |||
| Bhoosan et al (2019) India | 34 | 32.2 (±12) | 22:12 | Prospective study | Post traumatic scars | Nano-fat graft (34) | None | Single | POSAS | 3 |
| Rao et al (2020) India | 60 | 30.8 (±9.8) | 4:32 | Prospective study | Facial scars | Nano-fat graft (30) | Non nano-fat (unclear) (30) | Single dose in 83.3% | VAS | 12 |
| Svolacchia et al (2015) Italy | 3 | 28-36 | 1:02 | Case series | Hypertrophic scar or keloids | Nano fat graft (micro fat graft) (3) | None | Single | VSS | 24 |
| Pallua et al (2014) Germany | 26 | 22-64 | 10:16 | Prospective study | Facial scars | Fat graft (26) | None | Single | POSAS | 1 |
| Klinger et al (2013) Italy | 20 | 38.3 (±12.4) | Not clear | Prospective study | Post traumatic scars | Fat graft (20) | Placebo (Normal saline) (20) | Single | POSAS | 3 |
| Kim et al (2011) Korea | 31 | 39.5 | 14:17 | NCT | Depressed scars | ADSCs (31) | None | Single | Scar volume | 3 |
| Zhou et al (2016) China | 22 | 36.4 | 10:12 | NCT | Post acne | ADSC + FxCR (22) | FxCR (22) | Single | Subjective satisfaction Scale | 3 |
| Gentile et al (2014) Italy | 20 | 21-69 | na | NCT | Post burn and post traumatic | SVF enriched fat graft (10) | Fat graft with PRP (10) | Single | Process description | 12 |
| Jaspers et al (2015) Netherlands | 40 | 45 (±15.3) | 9:31 | Prospective study | Post surgical and post skin graft | Fat graft (40) | None | Single | POSAS | 3 |
| Tenna et al (2017) Italy | 30 | 18-52 | n/a | RCT | Acne scars | Nanofat + PRP + FxCR (15) | Nanofat + PRP (15) | Single | Ultrasound scar thickness, satisfaction | 6 |
| Jan et al (2018) Pakistan | 48 | 22.25 (±5.79) | 20:28 | Prospective study | Post burn facial scars | Nanofat graft (48) | None | Single | POSAS | 6 |
| Kim et al (2019) Korea | 1 | 21 | Male | Case report | Hypertrophic scars | SVF + FxCR (1) | na | Single SVF and FxCR | Photography | 12 |
| Carstens et al (2017) Nicaragua | 5 | 18-37 | 2:3 | Case series | Post burn fibrosis | SVF (5) | None | Single | VSS | 6 |
| Amir Bajouri et al (2018) Iran | 20 | na | na | RCT | Post burn scars | SVF (20) | Normal saline (20) | Single | VSS | 4 |
| Kwon et al (2023) | 20 | 19-65 | Revision scars | SVF | Normal saline | POSAS | 6 |
Abbreviations: NCT = non-randomized clinical trial, RCT = Randomized clinical trial, SVF = Stromal vascular fraction, FxCR = Fractionated cryotherapy, VSS = Vancouver scar scale, POSAS = Patients and Observers Scar Assessment Score, ADSC = Adipose derived stem cells, PRP = Platelet rich plasma, HTS = Hypertrophic scar, Msc = Mesenchymal stem cells.
Quality assessment
Risk of Bias assessment was conducted for the 7 clinical trials using the Revman tool that relies on the ROBINS-I risk assessment tool. Since the studies by Zahorec [25], Eitta [26], Kim [27], and Gentile [28] were not randomized studies, they were judged as high risk of bias for random sequence generation. Overall, all studies had some concerns in at least one of the five domains assessed while the study by Bajouri [29] had a high overall risk of bias. All observational studies were categorized as having a high risk of bias and hence not included in the risk of bias assessment. A summary of the clinical trials assessed for risk of bias is presented in Figure 2.
Figure 2.
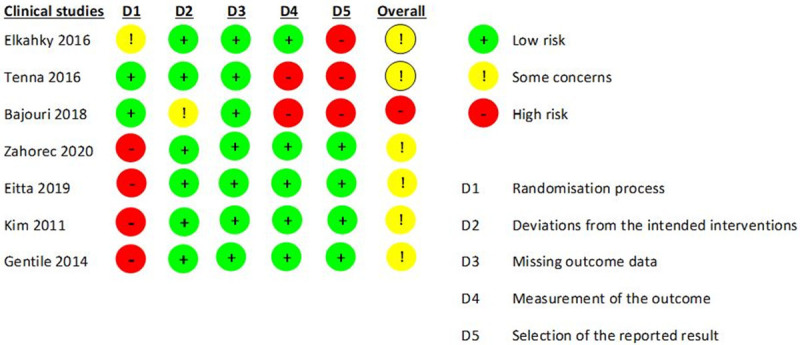
Risk of bias assessment of included clinical trials.
Form of stromal vascular fraction used
Of the 20 studies, 8 studies used point of care stromal vascular fraction (SVF), 3 studies used culture-expanded autologous adipose-derived stem cells (ADSC) while 9 studies used nano-graft.
Stromal vascular fraction harvesting and processing techniques
Fat harvesting
All studies described the method of collection of fat as liposuction with all but one study reporting tumescence liposuction as the technique of choice. Carstens reported dry liposuction as the method of fat collection [30].
The liposuction site was reported as abdominal in 13 of the included studies while other sites included trochanteric, outer thigh, gluteal and underarm. Only four studies described the volume of tumescent solution infiltrated ranging between 20 ml to 1013.5 ml while only six studies reported the mean lipoaspirate volume collected ranging between 50 to 350 ml.
Lipoaspirate processing to obtain the SVF was only performed in 11 studies while the rest of the studies used nano-fat graft which didn’t require this step.
SVF and ADSC lipoaspirate processing
Among the 10 studies that processed lipo-aspirate to obtain the SVF, 8 described the lipo-aspirate techniques to obtain the SVF while 2 didn’t have a description. Of the 8 studies that described the process, 7 reported enzymatic digestion with collagenase as the method for fat break down while one study by Gentile [28] described using the Celution system for mechanical breakdown of the fat. Five of the 10 SVF studies described a mean cell concentration harvested from the lipoaspirate. Four of the five studies reported mean cell concentrations ranging between 1-6×106 cells/ml and 1×108 cells/ml of SVF suspension.
All studies that used SVF reported an infiltration volume of less than 5 ml however there is no clear description of the factors that determined the volume infiltrated.
Single-dose infiltration was performed in all studies except one by Zahorec [25] in which 3 patients in one arm got a single dose while the 2 patients in the other arm received multiple doses. Three studies described the use of SVF in combination with another therapy. Zhou described the use of ADSCs in combination with Fractionated cryotherapy [31]. Similarly, Kim described SVF use in combination with fractionated cryotherapy [32] while Gentile described SVF-enriched fat graft [28].
Nano-fat processing
Among the nine studies that used nano-fat, the lipoaspirate following centrifugation was immediately infiltrated the scars without any further processing.
Treatment outcomes
Scar assessment scores
All the studies reviewed described at least one form of scar assessment to determine the improvement in scar characteristics following the treatment intervention. The most used scar assessment score tools were the Patient and Observer Scar Assessment Score (POSAS) and the Vancouver Scar Assessment Score (VSS) used in 7 and 5 of the included studies respectively (see Table 1). Other scar assessment scores used included the visual analog scale and patient satisfaction scales.
Five studies did not employ a scar assessment score and instead used scar volume, area, or thickness [26,27,32-34]. The surface area was determined using photography in the study by Kim [27] while height, and ultrasound were used in 4 of the studies.
POSAS score
Six studies used the POSAS score as the outcome assessment measure [35-40]. The follow-up time in all studies excluding Jan’s [39] and Lee [40] was 3 months. All six studies that used the POSAS score had an observational study design without having conducted a clinical trial. Therefore, these studies except that conducted by Lee didn’t have a comparison (control) arm.
All studies that used 3 months of follow-up demonstrated improvement in the POSAS scores with a baseline mean total patient and total observer POSAS scores of 15.8 (±13) and 12.9 (±11.3) and 3 months scores of 9.4 (±7.8) and 7.9 (±6.8) respectively (See Figures 3 and 4). The mean difference was 6.4 (±5.6) and 5 (±4.7) among total patient and observer POSAS scores respectively (Figures 3 and 4).
Figure 3.
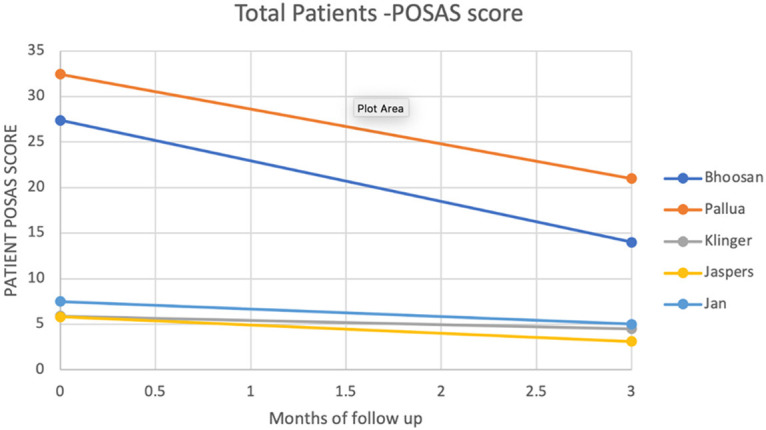
Total patient POSAS score.
Figure 4.
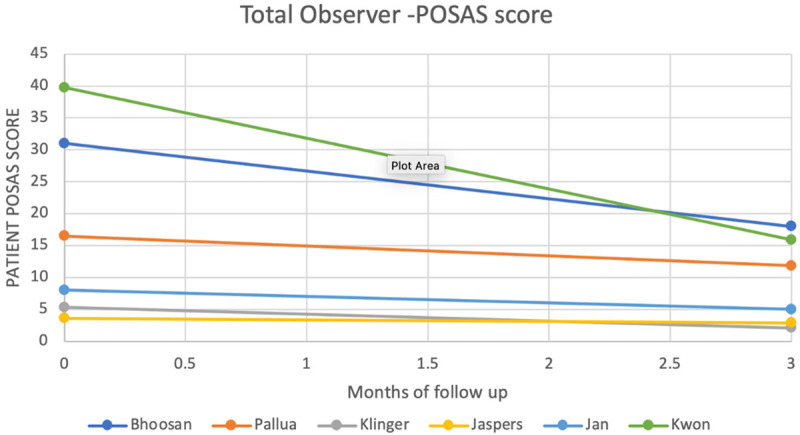
Total observer POSAS score.
In Lee’s study [40], where he compared SVF use following revision surgery to revision surgery without SVF, patients were followed up for 6 months. He reported a mean drop in the Observer POSAS score of 3 in his first sub-study and 2 in the second sub-study. In Kwon’s study, he noted improvements in the Observer Scar Assessment scores (OSAS) from 39.75±7.06 to 15.88±2.58 with are mean difference of 23.87.
When a meta-analysis of proportions was done, there was a statistically significant improvement in the POSAS score with an overall mean difference between baseline and 3 months of MD=8.05 (95% CI, 1.68-14.42, P=0.000, I2=98.4%) (See Figure 5). This indicates that in all studies, there was a significant improvement in the POSAS scores following the treatment with SVF. To assess the risk of bias in the publication for the meta-analysis, a funnel plot was obtained. This was found to be symmetrical indicating no significant publication bias (See Figure 6). However, for meta-analysis of proportions, funnel plots are not as accurate in describing publication bias [41,42].
Figure 5.
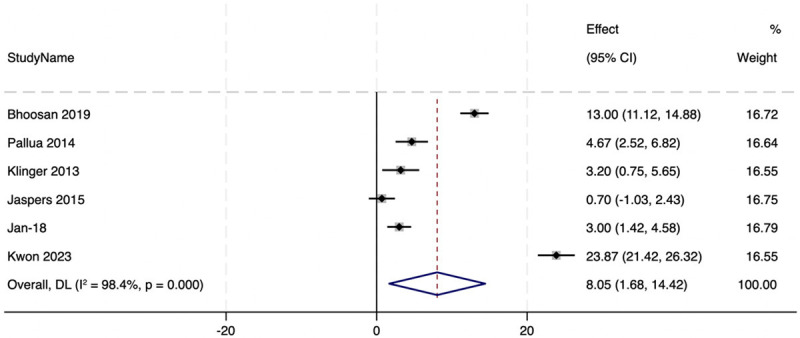
Meta-analysis of the observational studies that reported POSAS scores.
Figure 6.
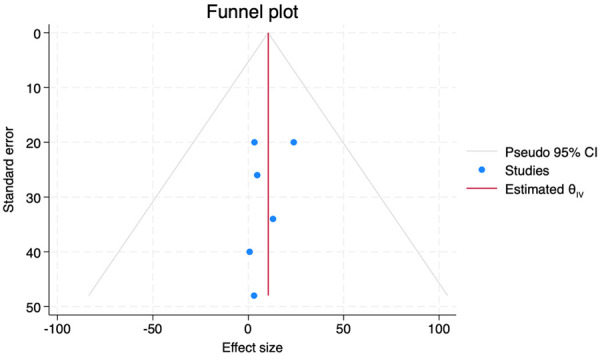
Funnel plot for publication bias.
Vancouver scar score (VSS)
In total, five studies used the Vancouver scar score (VSS) [25,29,30,40,43] with all except Bajouri’s [29] being observational studies.
All these studies followed patients for at least 6 months, and this was the follow-up time to compare outcomes.
All studies reported improvement in the VSS scores with three studies [25,29,30] reporting both baseline and 6-month VSS scores. The other two studies merely reported the mean difference between the baseline and 6-month scores. The mean VSS scores were 8.02 (±0.16) and 3.85 (±2.04) at baseline and six months among the three studies.
The mean difference between the baseline and 6 months follow-up of all studies was 6.65 (±2.04) with all studies reporting positive improvements in the VSS scar scores.
In the second sub-study conducted by Lee [40] in which comparison between the exposed arm (SVF) and the control (Non-SVF), there was a comparable improvement in the VSS scores in the SVF arm. In his study, the SVF group had a 2-point improvement in the VSS score while the control (non-SVF) group did not have any improvement in the VSS score during the 6-month follow-up time.
Other outcome assessments
Scar surface area
Three studies [26,27,34] used either scar surface area, volume, or thickness. Eitta used the NIH Image J to measure the scar surface area while Elkany and Kim used a USB digital microscope and 3D scanning system (Opto-TOP-HE Scanner, Breukmann, Germany) respectively.
In the study by Eitta, he reported significant improvements in the scar characteristics at 3 months with improvement from a mean scar area percentage of 2.24±1.33 at baseline to 1.24±1.15 at three months. Notably, he reported no significant scar area difference at one month following the injection. Similarly, Eitta noted scar surface area reduction most marked at 3 months following the administration of the adipose-derived stem cells. Eitta reported statistically significant mean changes from baseline 1403855±752277 µm2 to 3 months 489739±317481 µm2.
On the other hand, Elkany reported significant mean area percentage scar reduction at 2 to 3 months following the infiltration of adipose stem cells [26].
Included clinical trials comparison to other therapies
In Elkahky’s study, he compared Autologous adipose stem cells to Platelet-rich plasma. Elkany reported a reduction in the percentage surface area in both treatment arms with the PRP arm performing better than the SVF arm. He noted a statistically significant superiority in the efficacy of PRP with an 80.2% percentage change compared to a 66.45% change in the SVF arm at 3 months. Elkahky found both therapies effective with the PRP being superior to the SVF [34]. On the other hand, Eitta in a split face study compared a single injection of the autologous adipose-derived stromal vascular fraction to a three-session fractional carbon-dioxide laser therapy and reported comparable outcomes in the total surface area, trans-epidermal water loss, and skin hydration [26] concluding that single-dose therapy is as effective as three sessions of fractionated carbon-dioxide laser therapy.
Bajouri compared Autologous adipose-derived stromal vascular fraction to Normal saline (Placebo). In her findings the VSS scores improved from the baseline scores of 8.0±1.2 and 7.3±1.6 in the SVF and Control arm respectively to 6.4±1.4 and 6.7±1.7 respectively.
In Tenna’s study, a comparison on the extra benefit of using fractional CO2 laser resurfacing among patients who had received nano-fat enriched PRP and those who hadn’t, revealed equally beneficial outcomes in both arms describing that fractional laser did not add any extra benefit in reducing scar thickness as compared to nano-fat enriched Platelet Rich Plasma (PRP). In both arms, the scar thickness was reduced by 0.668 cm and 0.63 cm in nano-fat with PRP alone and with fractional CO2 laser with the difference found to not be statistically significant (P=0.7289) whilst all patients reported good treatment benefits. In all these clinical studies there was evidence of the therapeutic benefits of the SVF/Fat graft.
The study outcomes for the clinical trials were however found to be so heterogeneous that it was not possible to compare their measurements and hence not possible to conduct a meta-analysis for the clinical trial studies.
Adverse events
All studies reported that the use of autologous adipose-derived stromal vascular fraction was safe with no study reporting any adverse events associated with the treatments.
Discussion
The potential therapeutic role of autologous adipose-derived stromal vascular fraction (SVF) in keloid and scar treatment has garnered significant interest over the last couple of years with studies reporting promising outcomes. We conducted a systematic review to evaluate the efficacy of the SVF in treating keloids and scars by assessing all clinical studies conducted from 2000 to 2023. Initially, 973 studies were obtained, and 475 studies were selected. Of the 475 studies, only 20 studies met our inclusion criteria. We excluded in-vitro, animal, and non-scar/keloid studies while studies such as one by Nango’le that evaluated for recurrence of the keloids following excision [44] instead of regression of the keloid were also excluded. Similarly, a study by Suroweicka was excluded as it described three cases where each received either Platelet-rich plasma, lipofilling, or stromal vascular fraction in combination with radiofrequency CO2 laser therapy [45]. Clinical trial protocols such as one by Vriend [46] were also excluded.
The review aimed to evaluate the efficacy of SVF, adipose-derived stem cells, and nano fat in keloid and scar treatment, by causing an improvement in the Patient and Observer Scar Assessment Score (POSAS) and other measures of assessing keloid/scar resolution as reported by the different authors.
Study design and risk of bias
Among the 20 included studies, only three were randomized controlled studies while five were non-randomized clinical trials and the rest were observational studies. All studies were found to have concerns about the risk of biased assessment. The fact that only three studies randomized participants poses are risk of selection bias in most studies. All observational studies were considered to have been at high risk for selection bias. Overall, all studies were found to have a high risk of bias.
Efficacy
The review found that autologous adipose-derived SVF was effective in causing keloid and scar regression, with statistically significant improvement in POSAS scores and other parameters in all included studies. Notably, the therapy was administered as a single dose, with maximal improvement in scores observed at three months. Where POSAS scores were used as are measure of scar improvement, it’s of interest to note that studies involving participants with facial scars seemed to have better improvement in the POSAS scores [35,36]. Other systematic reviews also describe the similar efficacy of SVF in scar treatment [47-49].
It was found that no study comprehensively compared the efficacy of SVF or nanofat to established therapies, making it difficult to know how well SVF would compare to conventional treatments. In one study, platelet-rich plasma (PRP) performed better than SVF [34] in improving scar outcomes however the reason for this is unclear. It’s important to note that all studies describe SVF to be a safe therapy with no report of adverse events. However, due to the study designs, reporting bias cannot be ruled out.
On conducting the meta-analysis, it is evident that SVF had statistically significant improvements in the POSAS scores which underscores its therapeutic potential. The kind of meta-analysis performed for studies that don’t have comparator arms [50,51] called meta-analysis for proportions was performed. Despite a symmetrical funnel plot indicating a low risk of publication bias, funnel plots are generally not considered to be accurate in the meta-analysis for proportions methods [41,42].
The lack of well-designed clinical trials that could comparatively assess the efficacy of SVF in comparison to established therapies, such as Triamcinolone Acetanoide, was identified as a major limitation in this review. Other reviews agree with the limitation of well-designed clinical trials [48]. There was also no clear standardization on the dosing constitution, and each researcher provided a dosing constitution based on independent protocols [52]. This variability created difficulties in understanding how dosing would be calculated. Additionally, the lack of clear target endpoints that are consistent made it difficult to fully assess the maximal time of efficacy of the therapy. Despite most studies reporting 3 months as a key study endpoint, it’s not clear how this was derived.
To address these limitations, there is a need to develop well-organized efficacy clinical trials that can compare SVF to established standard therapies such as Triamcinolone Acetonide. Similarly, dosing studies need to be conducted to obtain an optimal efficacious dose. The studies included in the review reported a single dosage and follow-up of up to three months and six months in others, but there is a need to explore the therapeutic potential of multiple dosing strategies.
Conclusion
In conclusion, the review found that autologous adipose-derived SVF is a clinically effective therapy for keloids and scar treatment. However, limited information on its effectiveness in comparison to existing standard therapies such as Triamcinolone Acetonide and limited standard methods of dose constitution and administration were identified as limitations. Therefore, well-structured clinical trials are needed to compare the efficacy of SVF to existing conventional therapies.
Acknowledgements
The Government of Uganda through the Makerere Research Innovation Fund (MakRIF)-PhD support grants provided support for conducting this systematic review.
Disclosure of conflict of interest
None.
Abbreviations
- ADSC
Adipose-Derived Stem Cells
- PRISMA-P
Preferred Report Items of Systematic Reviews and Meta-Analyses Protocols
- SVF
Adipose-derived stromal Vascular Fraction
- TIDIeR
Template for Intervention Description and Replication
Supporting Information
References
- 1.Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84:827–37. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Tan S, Khumalo N, Bayat A. Understanding keloid pathobiology from a quasi-neoplastic perspective: less of a scar and more of a chronic inflammatory disease with cancer-like tendencies. Front Immunol. 2019;10:1810. doi: 10.3389/fimmu.2019.01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmieder SJ, Ferrer-Bruker SJ. Hypertrophic Scarring. 2023 Sep 4. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. Jan, [PubMed] [Google Scholar]
- 4.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–25. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betarbet U, Blalock TW. Keloids: a review of etiology, prevention, and treatment. J Clin Aesthet Dermatol. 2020;13:33–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa R, Mitsuhashi K, Hyakusoku H, Miyashita T. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–553. doi: 10.1097/01.PRS.0000040466.55214.35. discussion 554-555. [DOI] [PubMed] [Google Scholar]
- 8.Klotz T, Munn Z, Aromataris EC, Greenwood JE. Imiquimod to prevent keloid recurrence postexcision: a systematic review and meta-analysis. Wound Repair Regen. 2020;28:145–56. doi: 10.1111/wrr.12766. [DOI] [PubMed] [Google Scholar]
- 9.Huu ND, Huu SN, Thi XL, Van TN, Minh PPT, Minh TT, Van TH, Cam VT, Huyen ML, Hau KT, Gandolfi M, Satolli F, Feliciani C, Tirant M, Vojvodic A, Lotti T. Successful treatment of intralesional bleomycin in keloids of vietnamese population. Open Access Maced J Med Sci. 2019;7:298–9. doi: 10.3889/oamjms.2019.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4:235–43. doi: 10.2165/00128071-200304040-00003. [DOI] [PubMed] [Google Scholar]
- 11.Siotos C, Uzosike AC, Hong H, Seal SM, Rosson GD, Cooney CM, Cooney DS. Keloid excision and adjuvant treatments: a network meta-analysis. Ann Plast Surg. 2019;83:154–62. doi: 10.1097/SAP.0000000000001951. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Park SH, Lee SJ, Kim SH, Suh IS, Jeong HS. Clinical impact of highly condensed stromal vascular fraction injection in surgical management of depressed and contracted scars. Aesthetic Plast Surg. 2018;42:1689–98. doi: 10.1007/s00266-018-1216-9. [DOI] [PubMed] [Google Scholar]
- 13.Domergue S, Bony C, Maumus M, Toupet K, Frouin E, Rigau V, Vozenin MC, Magalon G, Jorgensen C, Noël D. Comparison between stromal vascular fraction and adipose mesenchymal stem cells in remodeling hypertrophic scars. PLoS One. 2016;11:e0156161. doi: 10.1371/journal.pone.0156161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Febbo I, Bunnell BA. Immunomodulatory effects of adipose stromal vascular fraction cells promote alternative activation macrophages to repair tissue damage. Stem Cells. 2017;35:2198–207. doi: 10.1002/stem.2689. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo P, Lombardi F, Siragusa G, Cifone MG, Cinque B, Giuliani M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ASCs): an overview. Int J Mol Sci. 2018;19:1897. doi: 10.3390/ijms19071897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi H, Li H, Zhang C, Mao Y, Nie F, Xing Y, Sha W, Wang X, Irwin DM, Tan H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res Ther. 2019;10:302. doi: 10.1186/s13287-019-1415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boada-Pladellorens A, Avellanet M, Pages-Bolibar E, Veiga A. Stromal vascular fraction therapy for knee osteoarthritis: a systematic review. Ther Adv Musculoskelet Dis. 2022;14:1759720X221117879. doi: 10.1177/1759720X221117879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Manayan RC, Bunnell BA. Adipose stromal vascular fraction attenuates T(H)1 cell-mediated pathology in a model of multiple sclerosis. J Neuroinflammation. 2018;15:77. doi: 10.1186/s12974-018-1099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, Yang X, He T, Guan H, Zheng Z, Han S, Dong M, Han J, Shi J, Hu D. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther. 2016;7:102. doi: 10.1186/s13287-016-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattei A, Magalon J, Bertrand B, Grimaud F, Revis J, Velier M, Veran J, Dessi P, Sabatier F, Giovanni A. Autologous adipose-derived stromal vascular fraction and scarred vocal folds: first clinical case report. Stem Cell Res Ther. 2018;9:202. doi: 10.1186/s13287-018-0842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mbiine R, Wayengera M, Ocan M, Kiwanuka N, Munabi I, Muwonge H, Lekuya HM, Kawooya I, Nakanwagi C, Kinengyere AA, Joloba M, Galukande M. Adipose-derived stromal vascular fraction (SVF) in scar treatment: a systematic review protocol. Am J Stem Cells. 2022;11:56–63. [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahorec P, Sarkozyova N, Ferancikova N, Bukovcan P, Danisovic L, Bohac M, Tomas M, Koller J. Autologous mesenchymal stem cells application in post-burn scars treatment: a preliminary study. Cell Tissue Bank. 2021;22:39–46. doi: 10.1007/s10561-020-09862-z. [DOI] [PubMed] [Google Scholar]
- 26.Abou Eitta RS, Ismail AA, Abdelmaksoud RA, Ghezlan NA, Mehanna RA. Evaluation of autologous adipose-derived stem cells vs. fractional carbon dioxide laser in the treatment of post acne scars: a split-face study. Int J Dermatol. 2019;58:1212–22. doi: 10.1111/ijd.14567. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Kim I, Lee SK, Bang SI, Lim SY. Clinical trial of autologous differentiated adipocytes from stem cells derived from human adipose tissue. Dermatol Surg. 2011;37:750–9. doi: 10.1111/j.1524-4725.2011.01765.x. [DOI] [PubMed] [Google Scholar]
- 28.Gentile P, De Angelis B, Pasin M, Cervelli G, Curcio CB, Floris M, Di Pasquali C, Bocchini I, Balzani A, Nicoli F, Insalaco C, Tati E, Lucarini L, Palla L, Pascali M, De Logu P, Di Segni C, Bottini DJ, Cervelli V. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg. 2014;25:267–72. doi: 10.1097/01.scs.0000436746.21031.ba. [DOI] [PubMed] [Google Scholar]
- 29.Bajouri A, Salahi Kajoor A, Fallah N, Latifi NA. 1397 Autologous human stromal vascular fraction injection in post- burn hypertrophic scar: a double-blinded placebo-controlled ClinicalTrial, Third National Festival and International Congress of Rehabilitation Stem Cells and Medical Stems and Technologies, Tehran. https://civilica.com/doc/818904.
- 30.Carstens MH, Pérez M, Briceño H, Valladares S, Correa D. Treatment of late sequelae of burn scar fibrosis with adipose-derived stromal vascular fraction (SVF) cells: a case series. CellR4. 2017;5:e2404. [Google Scholar]
- 31.Zhou BR, Zhang T, Bin Jameel AA, Xu Y, Xu Y, Guo SL, Wang Y, Permatasari F, Luo D. The efficacy of conditioned media of adipose-derived stem cells combined with ablative carbon dioxide fractional resurfacing for atrophic acne scars and skin rejuvenation. J Cosmet Laser Ther. 2016;18:138–48. doi: 10.3109/14764172.2015.1114638. [DOI] [PubMed] [Google Scholar]
- 32.Kim DG, Park ES, Kim SH. Combined treatment of stromal vascular fraction and ablative fractional CO2 laser for hypertrophic foot scar. Med Lasers. 2019;8:90–93. [Google Scholar]
- 33.Tenna S, Cogliandro A, Barone M, Panasiti V, Tirindelli M, Nobile C, Persichetti P. Comparative study using autologous fat grafts plus platelet-rich plasma with or without fractional CO(2) laser resurfacing in treatment of acne scars: analysis of outcomes and satisfaction with FACE-Q. Aesthetic Plast Surg. 2017;41:661–6. doi: 10.1007/s00266-017-0777-3. [DOI] [PubMed] [Google Scholar]
- 34.Elkahky HO, Fathy G, Abuzahra F, Afify A. Autologous adipose-derived adult stem cells injection versus platelet-rich plasma injection in the treatment of rolling postacne scars. Journal of the Egyptian Women’s Dermatologic Society. 2016;13:165–72. [Google Scholar]
- 35.Bhooshan LS, Devi MG, Aniraj R, Binod P, Lekshmi M. Autologous emulsified fat injection for rejuvenation of scars: a prospective observational study. Indian J Plast Surg. 2018;51:77–83. doi: 10.4103/ijps.IJPS_86_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallua N, Baroncini A, Alharbi Z, Stromps JP. Improvement of facial scar appearance and microcirculation by autologous lipofilling. J Plast Reconstr Aesthet Surg. 2014;67:1033–7. doi: 10.1016/j.bjps.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 37.Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci V. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–5. doi: 10.1097/SCS.0b013e3182a24548. [DOI] [PubMed] [Google Scholar]
- 38.Jaspers MEH, Brouwer KM, van Trier AJM, Groot ML, Middelkoop E, van Zuijlen PPM. Effectiveness of autologous fat grafting in adherent scars: results obtained by a comprehensive scar evaluation protocol. Plast Reconstr Surg. 2017;139:212–9. doi: 10.1097/PRS.0000000000002891. [DOI] [PubMed] [Google Scholar]
- 39.Jan SN, Bashir MM, Khan FA, Hidayat Z, Ansari HH, Sohail M, Bajwa AB, Shami HB, Hanif A, Aziz F, Choudhery MS. Unfiltered nanofat injections rejuvenate postburn scars of face. Ann Plast Surg. 2019;82:28–33. doi: 10.1097/SAP.0000000000001631. [DOI] [PubMed] [Google Scholar]
- 40.Lee JW, Park SH, Lee SJ, Kim SH, Suh IS, Jeong HS. Clinical impact of highly condensed stromal vascular fraction injection in surgical management of depressed and contracted scars. Aesthetic Plast Surg. 2018;42:1689–98. doi: 10.1007/s00266-018-1216-9. [DOI] [PubMed] [Google Scholar]
- 41.Cheema HA, Shahid A, Ehsan M, Ayyan M. The misuse of funnel plots in meta-analyses of proportions: are they really useful? Clin Kidney J. 2022;15:1209–10. doi: 10.1093/ckj/sfac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Svolacchia F, De Francesco F, Trovato L, Graziano A, Ferraro GA. An innovative regenerative treatment of scars with dermal micrografts. J Cosmet Dermatol. 2016;15:245–53. doi: 10.1111/jocd.12212. [DOI] [PubMed] [Google Scholar]
- 44.Nang’ole WF, Omu A, Ogeng’o JA, Agak GW. Do mesenchymal stem cells influence keloid recurrence? Stem Cells Cloning. 2022;15:77–84. doi: 10.2147/SCCAA.S373551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surowiecka A. Combined therapies in scar treatment-The role of autologous derived agents in scar remodeling: a series of cases. Dermatol Ther. 2022;35:e15877. doi: 10.1111/dth.15877. [DOI] [PubMed] [Google Scholar]
- 46.Vriend L, van Dongen JA, Pijpe A, Nieuwenhuis MK, Jongen SJM, Harmsen MC, van Zuijlen PPM, van der Lei B. Stromal vascular fraction-enriched fat grafting as treatment of adherent scars: study design of a non-randomized early phase trial. Trials. 2022;23:575. doi: 10.1186/s13063-022-06514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stachura A, Paskal W, Pawlik W, Mazurek MJ, Jaworowski J. The use of adipose-derived stem cells (ADSCs) and stromal vascular fraction (SVF) in skin scar treatment-a systematic review of clinical studies. J Clin Med. 2021;10:3637. doi: 10.3390/jcm10163637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walocko FM, Eber AE, Kirsner RS, Badiavas E, Nouri K. Systematic review of the therapeutic roles of adipose tissue in dermatology. J Am Acad Dermatol. 2018;79:935–44. doi: 10.1016/j.jaad.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Gentile P, Sterodimas A, Calabrese C, Garcovich S. Systematic review: advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment. Stem Cell Res Ther. 2021;12:318. doi: 10.1186/s13287-021-02397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, Munn Z. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21:189. doi: 10.1186/s12874-021-01381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karina K, Rosliana I, Rosadi I, Schwartz R, Sobariah S, Afini I, Widyastuti T, Remelia M, Wahyuningsih KA, Pawitan JA. Safety of technique and procedure of stromal vascular fraction therapy: from liposuction to cell administration. Scientifica (Cairo) 2020;2020:2863624. doi: 10.1155/2020/2863624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


