ABSTRACT
Clean air actions (CAAs) in China have been linked to considerable benefits in public health. However, whether the beneficial effects of CAAs are equally distributed geographically is unknown. Using high-resolution maps of the distributions of major air pollutants (fine particulate matter [PM2.5] and ozone [O3]) and population, we aimed to track spatiotemporal changes in health impacts from, and geographic inequality embedded in, the reduced exposures to PM2.5 and O3 from 2013 to 2020. We used a method established by the Global Burden of Diseases Study. By analyzing the changes in loss of life expectancy (LLE) attributable to PM2.5 and O3, we calculated the gain of life expectancy (GLE) to quantify the health benefits of the air-quality improvement. Finally, we assessed the geographic inequality embedded in the GLE using the Gini index (GI). Based on risk assessments of PM2.5 and O3, during the first stage of CAAs (2013 to 2017), the mean GLE was 1.87 months. Half of the sum of the GLE was disproportionally distributed in about one quarter of the population exposed (GI 0.44). During the second stage of CAAs (2017 to 2020), the mean GLE increased to 3.94 months and geographic inequality decreased (GI 0.18). According to our assessments, CAAs were enhanced, from the first to second stages, in terms of not only preventing premature mortality but also ameliorating health inequalities. The enhancements were related to increased sensitivity to the health effects of air pollution and synergic control of PM2.5 and O3 levels. Our findings will contribute to optimizing future CAAs.
Keywords: clean air action, public health, inequality, fine particulate matter, ozone
This study finds that during the first stage of clean air actions in China (2013 to 2017), the mean gain of life expectancy (GLE) was 1.87 months. Half of the sum of the GLE was disproportionally distributed in about one quarter of the population exposed (Gini Index: 0.44). During the second stage (2017 to 2020), the mean GLE increased to 3.94 months and geographic inequality decreased (Gini Index: 0.18). Clean air actions were enhanced, from the first to second stages, in terms of not only preventing premature mortality but also ameliorating health inequalities in China.
INTRODUCTION
Clean air actions (CAAs) in China from 2013 to 2020 improved air quality [1–3], and may have prevented premature mortality caused by air pollution exposure. In the first stage of CAA implementation (2013 to 2017), the focus was on reducing primary emissions of fine particulate matter (PM2.5) in the Beijing-Tianjin-Hebei region (BTH), the Yangtze River Delta (YRD), the Pearl River Delta (PRD) and other regions. The key measures during this stage included controlling industrial emissions, promoting the use of clean energy sources, improving vehicle emissions standards, and optimizing air-quality monitoring systems [4]. During this stage, a nationwide monitoring network has also been established, which provided key inputs for accurate assessments on exposure to air pollutants and their health impacts. The second stage (2018 to 2020) aimed to further improve air quality nationwide. Tailored measures (e.g. reducing emissions of volatile organic compounds [VOCs]) targeting multiple air pollutants, including PM2.5 and O3, were implemented [5]. Overall, CAAs have markedly reduced air pollution levels in China, thereby significantly enhancing public health. From 2013 to 2020, a 48% decrease in PM2.5 concentration was associated with a 21% reduction in attributable deaths (1.75 [95% confidence interval 1.64–1.85] million in 2013 to 1.39 [95% confidence interval 1.27–1.51] million in 2020) [3]. CAAs were associated with improvements in multiple health indicators, including lung function [6], lung-cancer incidence [7] and survival rate [8], kidney function [9], blood lipids [10], physical functions [11], household medical expenditure [12], and mental health [13–15]. However, increases in O3 concentrations, particularly during the first stage, increased mortality in populous eastern China [16].
The health benefits of air pollution control should be maximized, and the environmental inequalities minimized as well. Although interpretation on environmental inequality can be complex (for more details, please see the discussion section), in this study, we utilized the terminology to describe the phenomenon that a large fraction of exposed population, disease burden, or another additive measure of environmental impact is disproportionally attributable to a small identifiable subgroup. In high-income countries, actions targeting toward environmental equality, such as the Justice40 Initiative in the United States (US), have been taken to identify disadvantaged communities, and to prioritize them for public policies on air pollution control and climate mitigation [17]. There is considerable inequality embedded in exposure to, and the health effects of, ambient air pollution, particularly in low- and middle-income countries (LMICs) [18]. For instance, in China, exposure to nitrogen dioxide (NO2) and PM2.5 tends to scale with increasing socioeconomic status [19]. However, few studies have evaluated this trend. In our previous study, we assessed attributable deaths linked to long-term exposure to NO2, and the geographic inequality therein. The distribution of NO2-related deaths was disproportional, with the top 20% high-risk individuals contributing 85.7% of attributable deaths [20]. In addition to the attributable burden of air pollution exposure, policymakers and the public are also interested in the relevant inequality. For instance, PM2.5 exposure in the US exhibits inequalities among races and income levels. In addition, from 2000 to 2016, the fraction of the population exposed to PM2.5 >8 μg/m3 decreased from 89% to 41% as the degree of inequality between racial groups gradually increased. Therefore, the health benefit of a reduction in air pollution may not be distributed equally [21], and warrants a study to quantitively examine whether a strategy of environmental management increases or decreases such inequalities, particularly among the LMICs, such as China.
Environmental inequality can have several causes, and intervening in some of those could be prohibitively costly. For instance, individuals living close to desert areas are more frequently affected by exposure to dust particles; migration is the ultimate solution to this but may be unaffordable. Therefore, investigations of the distributions of the absolute health effects of air pollution exposure [20] may overestimate environmental health inequality. It would be useful to assess how temporal changes in air pollution-related disease burden (also known as relative disease burden) are differentially geographically distributed. Additionally, to establish air pollution-control targets at the population level and to optimize their health benefits [22], stakeholders are interested in the geographic inequality (also known as spatial inequality or spatial disparity) caused by regional differences in, for instance, population characteristics. However, no study has assessed the geographic inequalities of the health benefits caused by CAAs.
In this study, we developed a health metric (gain of life expectancy (GLE)) to monitor the health benefits associated with CAAs based on classical risk assessments, and evaluated the geographic inequality embedded in the metric using Lorenz curves and the Gini index (GI). The Lorenz curve visualizes cumulative distribution of the health benefits against the corresponding population distribution, and GI is a summary statistic of the curve. The risk assessments focused on the long-term exposures to PM2.5 and O3, which had been used as additive risk factors in previous studies [23–25]. We utilized the metric of attributable deaths or years of life lost (YLL) to measure the sum of health impact from air pollution exposure among different subgroups, distinguished by sex, age, residence, and spatiotemporal coordinate. YLL was further transformed into loss of life expectancy (LLE) to be indicative for the health impact per capita. We investigated the magnitude and inequalities in health benefits during the two stages of the CAAs, from 2013 to 2020.
RESULTS
PM2.5 and O3 exposure
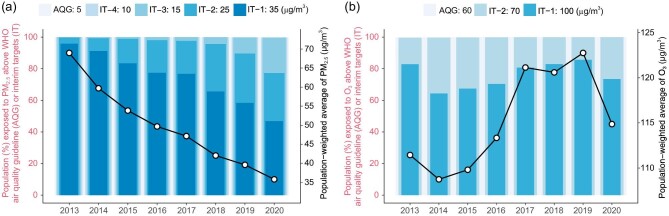
The population-weighted concentration of PM2.5 decreased from 68.98 μg/m3 to 47.13 μg/m3 in the first stage of the CAAs and to 35.77 μg/m3 after the second stage (Fig. 1). The proportion of adults exposed to a polluted level of PM2.5 (greater than the national ambient air quality standard, i.e. 35 μg/m3, equal to the first WHO interim target) decreased from 95.86% to 76.77% and 46.83% after the first and second stages, respectively. The gridded map of trends from 2013 to 2020 indicated geographic heterogeneity in the effect of CAAs on PM2.5 exposure (Supplementary Fig. S1).
Figure 1.
PM2.5 (a) and O3 (b) exposures in China from 2013 to 2020. The black lines and circles (referring to right y-axis) show nationwide population-weighted average exposure, and the colored bars (referring to left y-axis) show percentages of population exposed to pollution above levels recommended by the World Health Organization (WHO). We also present the least-square trends in exposure by grid in Supplementary Fig. S1.
The values for O3 increased from 111.4 μg/m3 to 121.1 μg/m3 in the first stage and decreased slightly to 114.9 μg/m3 after the second stage. The proportion of adults exposed to a polluted level of O3 showed a similar temporal trend. A gridded map of trends showed that hotspots of O3 growth spanned north and northwest China (Supplementary Fig. S1), suggesting considerable geographic inequality in the effect of CAAs on O3 exposure.
Attributable burden of mortality
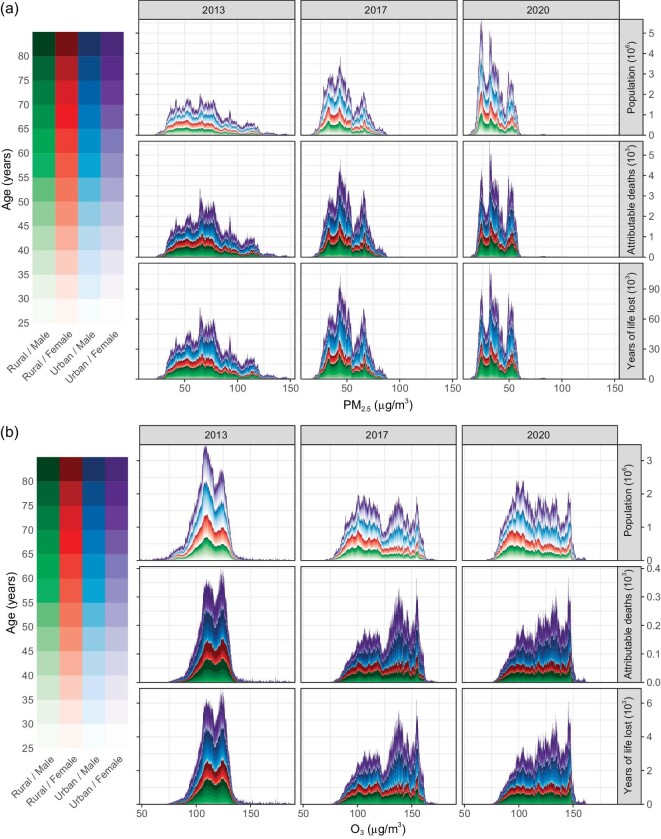
Figure 2 shows the population distribution by exposure level or health impact of air pollutants in 2013, 2017, and 2020. In 2013, 1.31 (95% confidence interval [CI]: 1.26–1.39) million attributable deaths and 28.87 (95% CI: 27.88–30.71) million YLLs were associated to PM2.5 exposure. These values decreased to 1.23 (95% CI: 1.14–1.26) and 26.97 (95% CI: 24.90–27.60) in 2017 and to 1.06 (95% CI: 1.00–1.12) and 22.62 (95% CI: 21.41–23.92) in 2020, respectively. Compared to PM2.5, O3 exposure made a smaller contribution to the burden of mortality. In 2013, 0.102 (95% CI: 0.100–0.103) million attributable deaths and 1.76 (95% CI: 1.72–1.77) million YLLs were associated to O3 exposure. These values increased to 0.124 (95% CI: 0.121–0.125) and 2.10 (95% CI: 2.05–2.17) in 2017 and decreased to 0.116 (95% CI: 0.113–0.116) and 1.93 (95% CI: 1.88–1.94) in 2020, respectively. For PM2.5 and O3, the burden on males was heavier than on females, that on the elderly was heavier than on the young, and that on urban residents was heavier than on rural residents.
Figure 2.
Distributions of exposure to and the health effects of PM2.5 (a) and O3 (b) by sex, age, and area of residence among Chinese adults in 2013, 2017, and 2020. For more distributions standardized by sex, age, and residence, please see Supplementary Fig. S2.
Health benefits
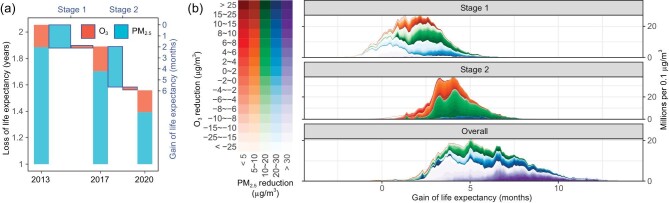
The burden of mortality was dominated by PM2.5 exposure, which had LLEs of 1.86 (95% CI: 1.80–1.98), 1.69 (95% CI: 1.56–1.72), and 1.38 (95% CI: 1.30–1.46) in 2013, 2017, and 2020, respectively. Based on only the PM2.5-associated disease burden, the first and second stages of CAAs contributed to GLEs of 2.11 and 3.68 months, respectively. Combining the health effects of PM2.5 and O3 exposure, the GLE was estimated to be 1.87 months for the first stage and 3.94 months for the second stage of CAAs.
Figures 3 and S3 show the distributions of GLE associated with CAAs. During the first stage, GLE was distributed unevenly, being low in the north and high in the south. Although the PM2.5 concentrations in the North China Plain and Fenwei Plain (NCP-FP) decreased markedly during the first stage, the GLE of joint exposure to PM2.5 and O3 was low for two reasons. First, the increment in O3 exposure offset the health benefits caused by PM2.5 reduction. Second, due to the sublinear curvature of the relationship between PM2.5 and mortality, the marginal effect on health of the same reduction from baseline of high-concentration exposure was smaller than that for low-concentration exposure. By contrast, during the second stage, GLE was evenly distributed geographically. Throughout CAAs, GLE was high in the YRD and Sichuan Basin. Similarly, because of the synergic control of PM2.5 and O3 and the improved baseline air quality, the GLE was higher in the second than the first stage, although the reduction in PM2.5 concentration in the second stage (11.36 μg/m3) was smaller than in the first stage (21.85 μg/m3).
Figure 3.
Gain of life expectancy (GLE) caused by CAAs, 2013 to 2020. (a) Temporal trend in GLE, determined by changes in loss of life expectancy attributable to PM2.5 and O3 exposure. (b) Distributions of GLE by level of air-quality improvement, codetermined by PM2.5 and O3 reductions. The detailed geographic distributions of GLE are documented in Supplementary Fig. S3.
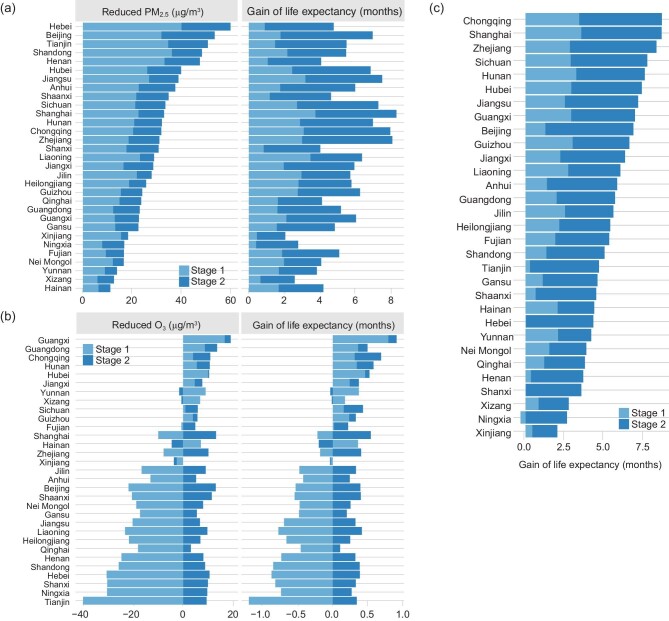
Analyses by province yielded similar results (Fig. 4). The provincial average GLEs did not reflect changes in exposure to PM2.5. For O3, which was linked to mortality by a uniform function for adults of all ages, the provincial average GLEs were proportional to the corresponding changes in exposure. The GLE during CAAs was codetermined by changes in air pollutants and demographic characteristics, as well as their nonlinear interactions. The average baseline mortality rate, standardized by the 2013 demographic structure, was reduced from 7.6 per 1000 adults in 2013 to 6.9 in 2020, meaning that the population became less vulnerable due to improvements in many aspects such as healthcare and medical accessibility. The decreased vulnerability would shrink the health benefits of CAAs. In contrast, without standardization by demographic structure, the raw baseline mortality rate was increased to 7.8 per 1000 adults in 2020. The different trend in mortality rate during 2013–2020 after standardization showed the changes in demographic structure, particularly population aging (Supplementary Fig. S2). Briefly speaking, the rapidly aging population in China increased the sensitivity to air pollution, and thus would amplify the health benefits of CAAs.
Figure 4.
Gain of life expectancy during CAAs by province, 2013 to 2020. (a) and (b) Improvements in air quality and health for PM2.5 and O3, respectively. (c) Total gain of life expectancy attributable to PM2.5 and O3 reductions in combination.
Geographic inequality embedded in health benefits
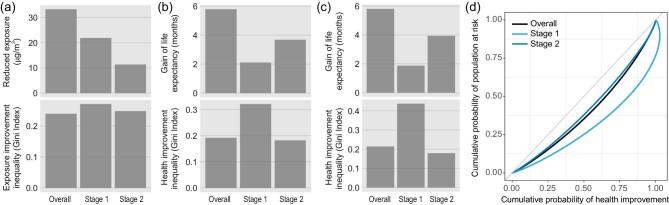
Use of different air pollution-control targets and their interactions with spatially varying demographic characteristics resulted in a complex geographic pattern in CAA-mediated health benefits (Fig. 2). During the first stage, half of the health benefits was evident in 25% of the population (GI 0.44). This increased to 37.5% during the second stage (GI 0.18). The degree of inequality embedded in the reduced PM2.5 concentration was stable (Fig. 5). In other words, the decreased inequality in health benefits could be partially attributable to control of O3 pollution. Furthermore, as shown in Fig. 2, the burden of diseases was disproportionally distributed between different age groups. As expected, a large fraction of attributable deaths was highly clustered among the elderly, who are recognized as being susceptible to cardiorespiratory diseases. Given the trend of population aging in China (Supplementary Fig. S2), the increased sensitivity to the beneficial effects of air pollution reduction can also partially explain the decreased geographic inequality.
Figure 5.
Magnitudes of geographic inequality embedded in the health benefits at the two stages of CAAs. (a) Improvement in PM2.5 exposure (top panel) and Gini indexes for their geographic inequalities (bottom panel). (b) PM2.5-attributable health benefits (top) and its inequality (bottom). (c) Health benefits (top) and its inequality (bottom) attributable to PM2.5 and O3 in combination. (d) Lorenz curves underlying the Gini indexes in (c).
DISCUSSION
To the best of our knowledge, this is the first assessment of the effect of CAAs on health benefits and health equality. Compared to the first stage of the CAAs, the second stage had a smaller reduction in PM2.5 exposure but a greater benefit in health, which was distributed more equally. Therefore, CAAs not only reduced attributable deaths but also improved relevant health equality. Our findings will facilitate the optimization of air pollution-control policies to better protect public health.
Sensitivity to the health effects of air pollution
The health benefits of CAAs were codetermined by air pollution control and human sensitivity. In this study, human sensitivity was captured by baseline mortality, age structure, and baseline exposure level. Subgroups with higher baseline mortality, advanced age, and lower PM2.5 exposure were more sensitive to air quality changes. High sensitivity could enhance the health benefits caused by air pollution reduction, explaining the greater health benefits in the second stage of the CAAs. Therefore, Chinese adults are increasingly sensitive to air pollution exposure.
Other factors contribute to sensitivity to the health effects of air pollution. First, chemical profiles modify the toxicity of PM2.5, thereby modulating the health effects. For instance, desert dust, biofuel burning, and open fires were the three largest contributors to PM2.5-related deaths in children under 5 years of age [26]. Similar results have been reported for birthweight reductions attributable to PM2.5 [27]. Second, sociodemographic factors can affect susceptibility or vulnerability to the adverse effects of PM2.5 and O3. Bell et al. systematically reviewed epidemiological evidence on susceptibility to morbidity or mortality related to short-term O3 exposure. Elderly individuals were significantly more susceptible to O3 exposure (excess risk 1.27%, 95% CI: 0.76–1.78) than young people (0.60%, 95% CI: 0.40–0.80). Individuals of low socioeconomic status (such as unemployed/low occupational status, ethnic/racial minorities, low educational level, and poverty) are potentially susceptible to O3 exposure [28]. Findings on susceptibility to the short-term health effects of PM2.5 are similar [29]. Third, behavioral factors can also modify the association between ambient exposure and a health outcome by affecting personal exposure patterns such as respiration rate. For instance, active commuting enhances, and physical activity attenuates, the association between PM2.5 exposure and cerebrovascular disease. By ignoring such factors, we might have underestimated the magnitude of geographic inequality.
Additionally, we might have underestimated geographic inequality by ignoring adverse effects on newborns and children, who are sensitive to air pollution. Gestational PM2.5 or O3 exposure increases the risk for pregnancy loss [30–32], but there is little evidence on this risk in China. According to a previous study, each 10 μg/m3 increment of PM2.5 exposure during pregnancy increases the risk for stillbirth by 10% (95% CI: 7–13) [31]. In China, PM2.5 and O3 adversely affect birth outcomes, leading to preterm birth and low birthweight outcomes [33–36]. For example, in a study, each 10 μg/m3 increment of gestational PM2.5 exposure was associated with a 26.2% (95% CI: 8.7–46.5) increased risk for preterm birth [33]. In another study, each 10 μg/m3 increment of gestational O3 exposure increased the risk for term low birthweight by 3% (95% CI: 0.1–5.2) [36]. Moreover, acute exposure to PM2.5 could increase the risk of mortality in children under 5 years of age in China, with a greater effect in neonates [37]. However, the lack of gridded data on the baseline prevalence of stillbirth, low-birthweight livebirth, or preterm birth enabled us to estimate the disease burden attributable to PM2.5 and O3 among adults only.
Policy implications
The high degree of inequality in the first stage of the CAAs may be explained by the slight improvement in health across some regions, such as NCP-FP. In the second stage, one focus was controlling O3 pollution by synergically reducing emissions of VOCs and nitrogen oxides (NOx), which enhanced the health benefits. In contrast, the lack of VOC control, particularly in urban areas, the reduction in NOx concentration [38] contributed to an increased O3 exposure during the first stage, due to VOC-limited regimes [39]. It is worth mentioning that some studies have proven a positive interaction between the health effect of PM2.5 and that of O3 [40]. The PM2.5-O3 interaction is also known as the synergic health effect. Given that, the burdens of diseases separately estimated for PM2.5 and O3 are not additive, and the current method might even underestimate the health benefits brought by the synergic emission control for PM2.5 and O3. Furthermore, it is worth mentioning that the synergic strategies could change source sectors and chemical compositions of the PM2.5 mixture. For instance, the fraction of VOC in PM2.5 mixture and VOC-related sources (e.g. diesel vehicular exhaust) will be reduced, in order to synergically control for O3. However, the current risk assessment approach ignored inequal toxicities between different PM2.5 components, and thus couldn’t evaluate the health impacts from the synergic emission controls, accurately.
Additionally, with the in-depth reduction in PM2.5 pollution, residents in heavily polluted areas, such as NCP-FP, became sensitive to the resulting beneficial effects. Furthermore, the second stage focused on three key regions likely contributing to the reduced geographic inequality (NCP, FP, and YRD). In terms of maximizing the health benefits or minimizing the inequality therein, the second stage of CAAs outperformed the first stage. Therefore, optimized strategies including the synergic control of PM2.5 and O3, and in-depth reduction in air pollution, should be considered for future air quality management.
Although the CAAs in China can promote equality, warranted are further additional investigations and policies specifically targeted to minimizing environmental injustice. In high-income countries, such strategies have been put into action. For instance, the recent Integrated Science Assessment for particulate matter and its supplement, the scientific reports to support the proposal to strengthen the annual PM2.5 standard in the US from 12 μg/m3 to 9–10 μg/m3, had identified disadvantaged subpopulations by socioeconomic status and race (https://www.federalregister.gov/d/2020-01223 and https://www.federalregister.gov/d/2022-07938). The US Environmental Protection Agency also proposed to modify the PM2.5 monitoring network design criteria to include an environmental justice factor. The Justice40 Initiative developed the Climate and Economic Justice Screening Tool (CEJST) to identify disadvantaged communities and to allocate future resources for environmental improvement. Although studies have found CEJST could not effectively reduce inequalities embedded in air pollution exposure [17], studies of quantitative inequality assessment, like ours, will contribute to develop an optimized tool, and to design justice policies.
Complexities underlying environmental inequality
Improving environmental equality is an aim of environmental governance. There is growing interest in the distribution of the effects of environmental policies [41,42]. However, the concepts of equality are often interpreted and operationalized differently. As a result, multiple dimensions or indicators of inequality are needed to evaluate air pollution-control policies [43].
To achieve equal distribution of clean air, it has been assumed that all individuals should be exposed to an equal level of air pollution. Therefore, exposure levels by subpopulations, such as multiple ethnic groups, need to be examined. For instance, a previous study combined US demographic and PM2.5 data from 2004 to 2016, and examined whether minority (Black, Asian, and Hispanic or Latino) areas had higher levels of PM2.5 [21]. Second, to achieve equality in terms of individual health, it has been assumed that the disease burden attributable to air pollution should be equally borne by all individuals. Therefore, health inequalities, such as the effect of air pollution on mortality and hospitalization, need to be examined. For example, Fann et al. evaluated the effects of air pollution-control strategies on the inequalities of mortality and the risk for hospitalization due to asthma [44]. Xue et al. studied the inequalities in the distributions of attributable deaths and YLLs caused by NO2 exposure in China from 2013 to 2020 [20]. Third, equality can also be interpreted to mean that the exposure level or health effects of air pollution should be proportional to socioeconomic status. This is based on the criterion of equality in promoting the welfare of socially disadvantaged or vulnerable groups. For example, Tomar et al. examined whether lower socioeconomic status groups experience more premature deaths due to PM2.5 exposure in India [45]. Finally, inequality has also been utilized to describe that different source sectors disproportionally contributed to the burden of air pollution, due to the unequal toxicities for different chemical components [46]. However, to reveal such inequality, source- or component-specific air pollution concentrations and customized joint-exposure-response functions [47] are warranted, which is beyond the capability of commonly-utilized risk assessment approaches.
Policies with a focus on the abovementioned inequalities are critical for promoting environmental equality and justice. Although the CAAs improved air quality and alleviated the associated health burdens, the distribution of health benefits had not been examined, before this study. We evaluated the geographic inequality of the CAAs, which assumed that the health benefits of an air pollution-control policy are equally shared by all individuals. Our findings provide insights into the equitable geographic distribution of clean air and health benefits. However, we did not consider detailed demographic and sociological characteristics or assess health benefits in vulnerable groups. Therefore, further investigation is needed.
CONCLUSION
The health effects of the second stage of CAAs were greater than the first stage, in terms of both increased efficacy and decreased inequality. Our findings suggest that continuously improving air quality throughout China will benefit public health.
METHODS
Environmental and population data
PM2.5 and O3 concentration (1 × 1 km resolution) data were taken from previous studies [48,49], which combined multiple datasets using machine-learning algorithms (random forest, extremely randomized trees, and extreme gradient boosting). The inputs were satellite atmospheric measurements, meteorological fields, chemical-transport model simulations, and other geographical variables. The models for predicting daily levels of PM2.5 and O3 in China were evaluated by cross-validation based on monitoring data, and their overall performances were good (R2 0.92 and 0.89 for long-term concentrations of PM2.5 and O3, respectively) [48,49].
We analyzed the effects of PM2.5 and O3 on health, considering factors such as sex, age, and place of residence. Therefore, population maps based on sex and age were combined with urbanization maps. We used WorldPop (https://www.worldpop.org/) data to obtain information on sex and age, which were combined and aggregated into a regular 1 × 1 km grid over China. Population data were estimated by combining spatial population datasets with national age and sex data, as described previously [50,51]. An urbanization map of China was obtained from satellite images of impervious surfaces using a product developed by Gong et al. [52]. We combined those maps to develop a series of gridded maps for each subpopulation based on sex, age, and urban/rural residence. For details of data processing, see a previous study [20].
Assessment of mortality burden
We applied the method developed by the 2019 GBD Study to assess the mortality burden attributable to long-term ambient air pollution exposure among all Chinese adults (>25 years old) from 2013 to 2020. The risk assessments for long-term PM2.5 and O3 exposures were performed within a 1 × 1 km grid by subpopulations of sex, age, and residence (urban or rural), and were parametrized using the following equations:
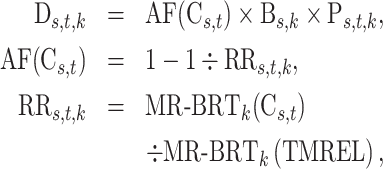 |
(1) |
where subscripts s, t, and k denote the indexes for spatial grid, calendar year, and sex-age-residence subpopulations, respectively; Ds,t,k represents the attributable deaths among the kth subpopulation in the sth grid cell during the tth year; C, B, and P are the three inputs, i.e. annual concentration of PM2.5 or O3, baseline mortality rate, and population size, respectively; AF is the attributable fraction; RR is the relative risk; MR-BRT is the nonlinear exposure-response function; and TMREL is the theorical minimum risk exposure level. The Meta-regression–Bayesian, Regularized Trimmed (MR-BRT) tool estimates the function that links a risk factor to mortality risk. The MR-BRT and TMREL parameters were obtained from the 2019 GBD Study [23]. The MR-BRT considers six cause-specific deaths attributable to PM2.5 exposure—ischemic heart disease, stroke, lung cancer, chronic obstructive pulmonary disease (COPD), lower-respiratory-tract infection, and type-2 diabetes for age-specific adults—and considers COPD-caused deaths for long-term O3 exposure. The long-term concentration (Cs,t) was set as the 12-month average PM2.5, or the peak-season value of O3. We referred to the recent air quality guidelines of the World Health Organization to evaluate long-term O3 exposure [53]. We obtained the daily 8 h average from the gridded estimates and calculated the monthly averages. Then the peak-season value was calculated as the maximum 6-month moving average, based on the monthly average concentrations. The premature deaths associated with peak-season O3 concentrations have been reported [54]. We also obtained gridded estimates of baseline total mortality rates by combining the data in the annual reports of the National Disease Surveillance Program and the gridded maps for sex-age-residence subpopulations. The gridded mortality estimates were validated against the county-level values in the census data. We used the mortality causes profile from the GBD Study [23] to obtain disease-specific baseline estimates.
Assessment of health benefits by gain of life expectancy
We transformed the number of attributable mortalities to loss of life expectancy (LLE), a disease burden metric that is scalable and computationally additive. LLE can be estimated using the following equation:
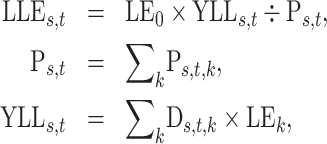 |
(2) |
where LEk denotes the theorical life expectancy at a given age for the kth subpopulation and YLLs,t represents years of life lost. The LLE at birth can be viewed as the re-scaled average of the YLL given a specific age-structure and has been used in previous works [20,55]. To quantify health benefits, we derived an indicator, gain of life expectancy (GLE):
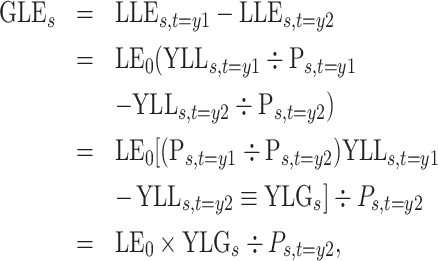 |
(3) |
where y1 and y2 are the temporal indexes for the beginning and ending years of air pollution control, respectively (e.g. for the first stage of CAAs, y1 = 2013 and y2 = 2017), and YLG denotes years of life gain, which measures the reduction in YLL with adjustment for population size.
Assessment of inequality
We viewed the CAA-associated GLE as a type of health income, and used the classical econometric method to evaluate the relevant inequality. We created a Lorenz curve to assess the geographic inequality embedded in the health benefits (GLEs) caused by a reduction in PM2.5 exposure, O3 exposure, or their combination, during a given stage of air pollution control. The Lorenz curve, a graphical representation of income distribution that plots the cumulative share of total income held by a given percentage of the population, is expected to be a diagonal line, indicating absolute equality. Correspondingly, to generate health Lorenz curves, income was replaced by the health benefits metric (i.e. GLE). The coordinates (xi, yi) of points on health Lorenz curves can be expressed as:
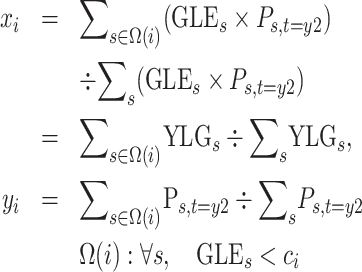 |
(4) |
where Ω(i) denotes the subset of the population with a GLE below a given constant level (ci). We calculated the GI to measure the deviation from the diagonal line, where a greater value indicates a more disproportionate distribution of health benefits.
Analyses were performed in R (version 4.2.2). We used the Monte Carlo approach to evaluate the uncertainties embedded in the mortality-burden metrics, and calculated the empirical CIs. Due to computational complexity, we were unable to calculate uncertainties for the Lorenz curves and GIs.
Supplementary Material
Contributor Information
Tao Xue, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China; Advanced Institute of Information Technology, Peking University, Hangzhou, 311215, China.
Ruohan Wang, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China.
Meng Wang, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, Buffalo, NY, 14214, USA.
Yanying Wang, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China.
Dan Tong, Department of Earth System Science, Tsinghua University, Beijing, 100084, China.
Xia Meng, School of Public Health, Key Laboratory of Public Health Safety of the Ministry of Education, and Key Laboratory of Health Technology Assessment of the Ministry of Health, Fudan University, Shanghai, 200433, China.
Conghong Huang, College of Land Management, Nanjing Agricultural University, Nanjing 210095, China; National & Local Joint Engineering, Research Center for Rural Land Resources Use and Consolidation, Nanjing 210095, China.
Siqi Ai, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China.
Fangzhou Li, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China.
Jingyuan Cao, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China.
Mingkun Tong, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China.
Xueqiu Ni, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China.
Hengyi Liu, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China.
Jianyu Deng, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China.
Hong Lu, Institute of Reproductive and Child Health, National Health Commission Key Laboratory of Reproductive Health/Department of Epidemiology and Biostatistics, Ministry of Education Key Laboratory of Epidemiology of Major Diseases (PKU), School of Public Health, Peking University Health Science Centre, Beijing, 100191, China.
Wei Wan, Clean Air Asia, Beijing, 100600, China.
Jicheng Gong, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China.
Shiqiu Zhang, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China.
Tong Zhu, State Key Joint Laboratory of Environment Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University, Beijing, 100871, China; State Environmental Protection Key Laboratory of Atmospheric Exposure, and Health Risk Management and Center for Environment and Health, Peking University, Beijing, 100871, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (42175182, 42375179 and 42293324), the Ministry of Science and Technology of China (2022YFC3703000), the National Institute of Environmental Health Sciences of the United States (ES031986), and Clean Air Asia.
AUTHOR CONTRIBUTIONS
T.X. and T.Z. designed the study. T.X., Y.W., D.T., X.M., W.W., J.G., S.Z., and T.Z. contributed to the methodology. R.W., Y.W., S.A., F.L., J.C., M.T., X.N., H.L., J.D., and H.L. performed the analysis. R.W., M.W., and C.H. contributed to the data curation. T.X., R.W., and M.W. wrote the first draft of the manuscript, with revisions from all authors.
Conflict of interest statement. None declared.
REFERENCES
- 1. Xue T, Liu J, Zhang Qet al. Rapid improvement of PM2.5 pollution and associated health benefits in China during 2013–2017. Sci China Earth Sci 2019; 62: 1847–56. 10.1007/s11430-018-9348-2 [DOI] [Google Scholar]
- 2. Zheng Y, Xue T, Zhang Qet al. Air quality improvements and health benefits from China's clean air action since 2013. Environ Res Lett 2017; 12: 114020. 10.1088/1748-9326/aa8a32 [DOI] [Google Scholar]
- 3. Xiao Q, Geng G, Xue Tet al. Tracking PM2.5 and O3 pollution and the related health burden in China 2013–2020. Environ Sci Technol 2022; 56: 6922–32. 10.1021/acs.est.1c04548 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Q, Zheng Y, Tong Det al. Drivers of improved PM2.5 air quality in China from 2013 to 2017. Proc Natl Acad Sci USA 2019; 116: 24463–9. 10.1073/pnas.1907956116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo B, Wu H, Pei Let al. Study on the spatiotemporal dynamic of ground-level ozone concentrations on multiple scales across China during the blue sky protection campaign. Environ Int 2022; 170: 107606. 10.1016/j.envint.2022.107606 [DOI] [PubMed] [Google Scholar]
- 6. Xue T, Han Y, Fan Yet al. Association between a rapid reduction in air particle pollution and improved lung function in adults. Ann Am Thorac Soc 2021; 18: 247–56. 10.1513/AnnalsATS.202003-246OC [DOI] [PubMed] [Google Scholar]
- 7. Yang L, Xue T, Wang Net al. Burden of lung cancer attributable to ambient fine particles and potential benefits from air quality improvements in Beijing, China: a population-based study. Sci Total Environ 2020; 738: 140313. 10.1016/j.scitotenv.2020.140313 [DOI] [PubMed] [Google Scholar]
- 8. Yang L, Wang N, Liu Set al. The PM2.5 concentration reduction improves survival rate of lung cancer in Beijing. Sci Total Environ 2023; 858: 159857. 10.1016/j.scitotenv.2022.159857 [DOI] [PubMed] [Google Scholar]
- 9. Han Y, Xue T, Kelly FJet al. Association of PM2.5 reduction with improved kidney function: a nationwide quasiexperiment among Chinese adults. Health Data Sci 2022; 2022: 9846805. 10.34133/2022/9846805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Yao Y, Xie Wet al. Association of long-term exposure to PM2.5 with blood lipids in the Chinese population: findings from a longitudinal quasi-experiment. Environ Int 2021; 151: 106454. 10.1016/j.envint.2021.106454 [DOI] [PubMed] [Google Scholar]
- 11. Wang H, Liu H, Guo Fet al. Association between ambient fine particulate matter and physical functioning in middle-aged and older Chinese adults: a nationwide longitudinal study. J Gerontol A Biol Sci Med Sci 2022; 77: 986–93. 10.1093/gerona/glab370 [DOI] [PubMed] [Google Scholar]
- 12. Xue T, Zhu T, Peng Wet al. Clean air actions in China, PM2.5 exposure, and household medical expenditures: a quasi-experimental study. PLoS Med 2021; 18: e1003480. 10.1371/journal.pmed.1003480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xue T, Guan T, Zheng Yet al. Long-term PM2.5 exposure and depressive symptoms in China: a quasi-experimental study. Lancet Reg Health West Pac 2021; 6: 100079. 10.1016/j.lanwpc.2020.100079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xue T, Zhu T, Zheng Yet al. Declines in mental health associated with air pollution and temperature variability in China. Nat Commun 2019; 10: 2165. 10.1038/s41467-019-10196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao Y, Lv X, Qiu Cet al. The effect of China's Clean Air Act on cognitive function in older adults: a population-based, quasi-experimental study. Lancet Health Longev 2022; 3:e98–e108. 10.1016/S2666-7568(22)00004-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue T, Zheng YX, Geng GNet al. Estimating spatiotemporal variation in ambient ozone exposure during 2013–2017 using a data-fusion model. Environ Sci Technol 2020; 54:14877–88. 10.1021/acs.est.0c03098 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Apte JS, Hill JDet al. Air quality policy should quantify effects on disparities. Science 2023; 381: 272–4. 10.1126/science.adg9931 [DOI] [PubMed] [Google Scholar]
- 18. Xue T, Li J, Tong Met al. Stillbirths attributable to open fires and their geographic disparities in non-Western countries. Environ Pollut 2023; 334: 122170. 10.1016/j.envpol.2023.122170 [DOI] [PubMed] [Google Scholar]
- 19. Wang YZ, Wang YF, Xu Het al. Ambient air pollution and socioeconomic status in China. Environ Health Perspect 2022; 130: 067001. 10.1289/EHP9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue T, Tong MK, Wang Met al. Health impacts of long-term NO2 exposure and inequalities among the Chinese population from 2013 to 2020. Environ Sci Technol 2023; 57: 5349–57. 10.1021/acs.est.2c08022 [DOI] [PubMed] [Google Scholar]
- 21. Jbaily A, Zhou XD, Liu Jet al. Air pollution exposure disparities across US population and income groups. Nature 2022; 601: 228–33. 10.1038/s41586-021-04190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng Y, Xue T, Zhao Het al. Increasing life expectancy in China by achieving its 2025 air quality target. Environ Sci Ecotechnol 2022; 12: 100203. 10.1016/j.ese.2022.100203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray CJL, Aravkin AY, Zheng Pet al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet North Am Ed 2020; 396:1223–49. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, West JJ, Mathur Ret al. Long-term trends in the ambient PM2.5- and O3-related mortality burdens in the United States under emission reductions from 1990 to 2010. Atmos Chem Phys 2018; 18: 15003–16. 10.5194/acp-18-15003-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anenberg SC, Horowitz LW, Tong DQet al. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature Human mortality using atmospheric modeling. Environ Health Perspect 2010; 118: 1189–95. 10.1289/ehp.0901220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li P, Wu J, Wang Ret al. Source sectors underlying PM2.5-related deaths among children under 5 years of age in 17 low-and middle-income countries. Environ Int 2023; 172: 107756. 10.1016/j.envint.2023.107756 [DOI] [PubMed] [Google Scholar]
- 27. Li P, Wu J, Tong Met al. The association of birthweight with fine particle exposure is modifiable by source sector: findings from a cross-sectional study of 17 low-and middle-income countries. Ecotoxicol Environ Saf 2023; 253:114696. 10.1016/j.ecoenv.2023.114696 [DOI] [PubMed] [Google Scholar]
- 28. Bell ML, Zanobetti A, Dominici F. Who is more affected by ozone pollution? A systematic review and meta-analysis. Am J Epidemiol 2014; 180:15–28. 10.1093/aje/kwu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bell ML, Zanobetti A, Dominici F. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol 2013; 178:865–76. 10.1093/aje/kwt090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tong M, Li P, Wang Met al. Time-varying association between fetal death and gestational exposure to ambient fine particles: a nationwide epidemiological study of 49 million fetuses in the contiguous US from 1989 to 2004. Int J Epidemiol 2022; 51: 1984–99. 10.1093/ije/dyac103 [DOI] [PubMed] [Google Scholar]
- 31. Zhang H, Zhang X, Wang Qet al. Ambient air pollution and stillbirth: an updated systematic review and meta-analysis of epidemiological studies. Environ Pollut 2021; 278: 116752. 10.1016/j.envpol.2021.116752 [DOI] [PubMed] [Google Scholar]
- 32. Xue T, Tong M, Li Jet al. Estimation of stillbirths attributable to ambient fine particles in 137 countries. Nat Commun 2022; 13: 6950. 10.1038/s41467-022-34250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chu C, Zhu Y, Liu Cet al. Ambient fine particulate matter air pollution and the risk of preterm birth: a multicenter birth cohort study in China. Environ Pollut 2021; 287: 117629. 10.1016/j.envpol.2021.117629 [DOI] [PubMed] [Google Scholar]
- 34. Yuan L, Zhang Y, Wang Wet al. Critical windows for maternal fine particulate matter exposure and adverse birth outcomes: the Shanghai birth cohort study. Chemosphere 2020; 240: 124904. 10.1016/j.chemosphere.2019.124904 [DOI] [PubMed] [Google Scholar]
- 35. Chen J, Guo L, Liu Het al. Modification effects of ambient temperature on associations of ambient ozone exposure before and during pregnancy with adverse birth outcomes: a multicity study in China. Environ Int 2023; 172:107791. 10.1016/j.envint.2023.107791 [DOI] [PubMed] [Google Scholar]
- 36. Wang Q, Miao H, Warren JLet al. Association of maternal ozone exposure with term low birth weight and susceptible window identification. Environ Int 2021; 146: 106208. 10.1016/j.envint.2020.106208 [DOI] [PubMed] [Google Scholar]
- 37. He C, Liu C, Chen Ret al. Fine particulate matter air pollution and under-5 children mortality in China: a national time-stratified case-crossover study. Environ Int 2022; 159: 107022. 10.1016/j.envint.2021.107022 [DOI] [PubMed] [Google Scholar]
- 38. Xue T, Tong M, Wang Met al. Health impacts of long-term NO2 exposure and inequalities among the Chinese population from 2013 to 2020. Environ Sci Technol 2023; 57: 5349–57. 10.1021/acs.est.2c08022 [DOI] [PubMed] [Google Scholar]
- 39. Liu Y, Geng G, Cheng Jet al. Drivers of increasing ozone during the two phases of clean air actions in China 2013–2020. Environ Sci Technol 2023; 57: 8954–64. 10.1021/acs.est.3c00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin H, Guo Y, Ruan Zet al. Ambient PM2.5 and O3 and their combined effects on prevalence of presbyopia among the elderly: a cross-sectional study in six low- and middle-income countries. Sci Total Environ 2019; 655:168–73. 10.1016/j.scitotenv.2018.11.239 [DOI] [PubMed] [Google Scholar]
- 41. Fischer C, Pizer WA. Horizontal equity effects in energy regulation. J Assoc Environ Resour Econ 2019; 6: S209–37. 10.1086/701192 [DOI] [Google Scholar]
- 42. Levinson A. Energy efficiency standards are more regressive than Energy taxes: theory and evidence. J Assoc Environ Resour Econ 2019; 6: S7–36. 10.1086/701186 [DOI] [Google Scholar]
- 43. Deryugina T, Fullerton D, Pizer WA. An introduction to energy policy trade-offs between economic efficiency and distributional equity. J Assoc Environ Resour Econ 2019; 6: S1–6. 10.1086/701515 [DOI] [Google Scholar]
- 44. Fann N, Roman HA, Fulcher CMet al. Maximizing health benefits and minimizing inequality: incorporating local-scale data in the design and evaluation of air quality policies. Risk Anal 2011; 31: 908–22. 10.1111/j.1539-6924.2011.01629.x [DOI] [PubMed] [Google Scholar]
- 45. Tomar G, Nagpure AS, Jain Yet al. High-resolution PM2.5 emissions and associated health impact inequalities in an Indian district. Environ Sci Technol 2023; 57: 2310–21. 10.1021/acs.est.2c05636 [DOI] [PubMed] [Google Scholar]
- 46. Wu D, Zheng H, Li Qet al. Toxic potency-adjusted control of air pollution for solid fuel combustion. Nat Energy 2022; 7: 194–202. 10.1038/s41560-021-00951-1 [DOI] [Google Scholar]
- 47. Li P, Wu J, Ni Xet al. Associations between hemoglobin levels and source-specific exposure to ambient fine particles among children aged < 5 years in low-and middle-income countries. J Hazard Mater 2023; 459:132061. 10.1016/j.jhazmat.2023.132061 [DOI] [PubMed] [Google Scholar]
- 48. Huang CH, Hu JL, Xue Tet al. High-resolution spatiotemporal modeling for ambient PM2.5 exposure assessment in China from 2013 to 2019. Environ Sci Technol 2021; 55: 2152–62. 10.1021/acs.est.0c05815 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Huang C, Hu Jet al. Development of high-resolution spatio-temporal models for ambient air pollution in a metropolitan area of China from 2013 to 2019. Chemosphere 2022; 291: 132918. 10.1016/j.chemosphere.2021.132918 [DOI] [PubMed] [Google Scholar]
- 50. Tatem AJ, Gaughan AE, Stevens FRet al. Quantifying the effects of using detailed spatial demographic data on health metrics: a systematic analysis for the AfriPop, AsiaPop, and AmeriPop projects. Lancet North Am Ed 2013; 381: 142. 10.1016/S0140-6736(13)61396-3 [DOI] [Google Scholar]
- 51. Pezzulo C, Hornby GM, Sorichetta Aet al. Sub-national mapping of population pyramids and dependency ratios in Africa and Asia. Sci Data 2017; 4: 15. 10.1038/sdata.2017.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gong P, Li XC, Zhang W. 40-Year (1978–2017) human settlement changes in China reflected by impervious surfaces from satellite remote sensing. Sci Bull 2019; 64: 756–63. 10.1016/j.scib.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 53. World Health Organization . WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Geneva: World Health Organization, 2021. [PubMed] [Google Scholar]
- 54. Xue T, Geng G, Meng Xet al. New WHO global air quality guidelines help prevent premature deaths in China. Natl Sci Rev 2022; 9: nwac055. 10.1093/nsr/nwac055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lelieveld J, Pozzer A, Pöschl Uet al. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res 2020; 116: 1910–7. 10.1093/cvr/cvaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.