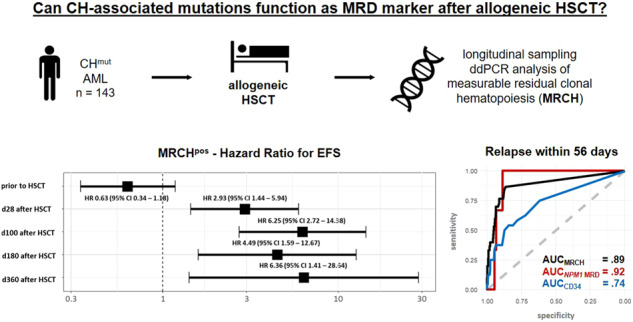
Graphical Abstract
Subject terms: Translational research, Acute myeloid leukaemia
To the Editor:
Some of the gene mutations detected during the development of myeloid neoplasm are also frequently found in elderly individuals without diagnosed hematologic malignancy and here referred to as clonal hematopoiesis (CH) of indeterminate potential (CHIP) [1]. CHIP associates with an elevated risk of developing a myeloid neoplasm [1], as compared to non-CHIP conductors [1]. Acute myeloid leukemia (AML) arises from the clonal expansion of aberrant hematopoietic stem cells, for which early events like CH-associated mutations together with later events like FLT3-ITD, NRAS or NPM1 mutations initiate the disease. In AML, the most frequently described CH-associated mutations affect the genes DNMT3A, TET2, and ASXL1 (‘DTA’) [2, 3]. However, also non-DTA mutations were detected in healthy individuals as well as in patients with myeloid neoplasm and described as CH, including aberrations in splicing factors like SRSF2, SF3B1, and U2AF1, in JAK2, or IDH2 R140Q [1, 2, 4–6] At AML diagnosis, CH-associated mutations are often found at high variant allele frequencies (VAF) and frequently persist at high VAFs in remission after chemotherapy [2, 6]. Whether CH-associated mutations detected in complete remission (CR) in AML patients reflect persistent AML or are part of the physiologically aging stem cell pool is not clearly differentiated. Their persistence in CR after chemotherapy has not been linked to adverse outcomes and has very limited value for measurable residual disease (MRD) evaluation [2, 7]. However, the MRD status is an important prognostic tool when based on reliable markers, with NPM1 mutations being the best evaluated molecular MRD marker [2, 8]. As NPM1 mutations are only present in 30% of younger, and 20% of older AML patients [2], additional molecular MRD markers, especially in older patients, are needed for improved relapse prediction. An allogeneic hematopoietic stem cell transplantation (HSCT) results in the total replacement of a patient’s hematopoiesis. Subsequently, CH-associated mutations might function as alternative MRD markers in the post-HSCT period.
We analyzed 143 AML patients with at least one known CH-associated mutation at diagnosis receiving an allogeneic HSCT after myeloablative (mac, 13%), reduced-intensity (ric, 26%), or non-myeloablative (nma, 61%) conditioning. Median age at HSCT was 62.4 (range 31.6–76.4) years. For further patients’ characteristics see Supplementary Tables S1 and S2. Written informed consent was obtained in accordance with the Declaration of Helsinki. Median follow up after HSCT was 3.1 years.
All patients had bone marrow or peripheral blood samples for assessment of CH-associated mutations available during follow up, i.e. up to 28 days prior to HSCT (in CR/CRi, n = 101) and/or following HSCT (n = 88). Analyzed CH-associated mutations were in the genes DNMT3A (n = 58), SRSF2 (n = 27), IDH2 (n = 27), ASXL1 (n = 16), TET2 (n = 17), JAK2 (n = 16), SF3B1 (n = 11), and U2AF1 (n = 7). Mutation-specific digital droplet (dd)PCR assays were developed using a competitive probe-approach (for details see Supplementary Information and Supplementary Tables S3, S4). In patients with multiple CH-associated mutations, measurable residual clonal hematopoiesis (MRCH) positivity was defined as positivity for at least one analyzed mutation.
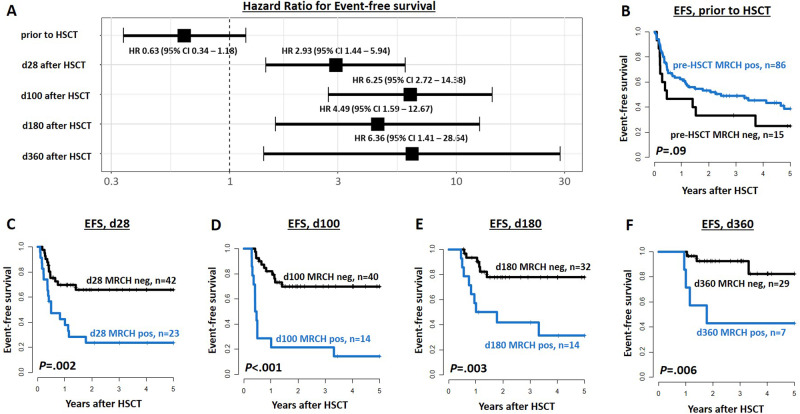
In concordance with previous studies [2, 6, 7, 9], CH-associated mutations frequently persisted in CR prior to allogeneic HSCT and did not indicate adverse outcomes (Fig. 1A, Supplementary Figs. S1A and S2A). In contrast, the majority of AML patients cleared their MRCH in CR following allogeneic HSCT. Also other reports described MRCH clearance after allogeneic HSCT in 20/21 patients [9] and DNMT3A mutation clearance in all patients achieving a full donor chimerism [6]. Diagnostic CH-associated mutations are usually also present at relapse as they often affect the majority of AML cells [6, 7], which was true for all patients in our study with relapse material available. In addition, relapse was preceded by at least one MRCHpos sample at a median time of 53 days in 90% of relapsing patients (Supplementary Information and Supplementary Fig. S3). Interestingly, Nakamura et al. also indicated in a subset of patients (n = 12) an association of DTA persistence and relapse after allogeneic HSCT [10].
Fig. 1. Event-free survival (EFS) of AML patients undergoing allogeneic HSCT according to the persistence of measurable residual clonal hematopoiesis (MRCH) at different time points in CR/CRi.
A Comparison of Hazard ratios at the time points prior to HSCT, and at days 28, 100, 180, and 360 after HSCT. B–F EFS according to MRCH status at (B) up to 28 days prior to HSCT (n = 101), (C) 28 days after HSCT (n = 55), (D) 100 days after HSCT (n = 54), (E) 180 days after HSCT (n = 46), and (F) 360 days after HSCT (n = 36).
In cumulative outcome analyses regarding the MRCH status 28, 100, 180, and 360 days after HSCT, MRCHpos status associated with a significantly higher cumulative incidence of relapse (CIR, Supplementary Fig. S1C–F) and shorter event-free survival (EFS, Fig. 1C–F) at all analyzed time points, and shorter overall survival (OS) 28 and 100 days after HSCT (Supplementary Fig. S2C–F). While relapse was frequent in MRCHpos patients irrespective of the analyzed time point early after HSCT (43–65%), relapse rates in MRCHneg patients continuously decreased with advancing time after HSCT (from 24% at day 28 after HSCT to 12% at day 100, 6% at day 180, and 0% at day 360, respectively, Supplementary Fig. S4).
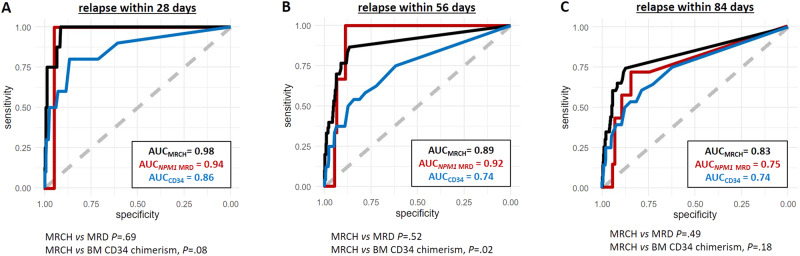
When we analyzed the relapse risk regarding the MRCH burden after HSCT, receiver operating characteristics curves showed a high efficacy of relapse prediction within 28 days (AUCMRCH = 0.98), 56 days (AUCMRCH = 0.89), and 84 days (AUCMRCH = 0.83, Fig. 2). Of note, these predictions performed better than the bone marrow CD34 chimerism in predicting relapse within 28 and 56 days, and were not inferior to that of established NPM1 MRD analysis (Supplementary Information and Fig. 2). For patient examples of individual MRCH and NPM1 MRD comparisons, see Supplementary Information and Supplementary Fig. S5. A previous report has suggested NPM1 MRD after HSCT to be the superior method compared to chimerism analysis [11], which also seems to be true for the here evaluated MRCH. Although being the best evaluated MRD marker in AML, NPM1 mutations are lost at relapse in 10–14% of patients [8, 12]. A higher mutation stability was reported for IDH2 und DNMT3A mutations (95–100%) [13], indicating that persisting CH can contribute to relapse in both NPM1 mutated and wildtype patients [6, 12, 13]. Together with the high frequency of CH-associated mutations their use for MRD assessment can provide valuable risk evaluation in NPM1-negative patients and complement NPM1 MRD in NPM1 mutated patients after allogeneic HSCT.
Fig. 2. ROC curves for relapse prediction in AML patients after allogeneic HSCT according to the MRCH burden (black), NPM1 MRD burden (red), and the bone marrow CD34 chimerism (blue) as continuous variables, measured in morphologic remission.
A relapse within 28 days after measurement, (B) relapse within day 56 days after measurement, and (C) relapse within 84 days after measurement.
The term CH-associated mutation is used inconsistently in literature. While over 30 mutated genes have been shown to behave like CHIP in healthy individuals, the DTA genes are most frequently evaluated and recognized in this context [2, 3]. To exclude distinct prognostic effects, we performed separate analyses for DTA and non-DTA MRCH. Here, we observed a similar significance for relapse prediction within 28, 56, and 84 days, as well as – although restricted by low patient numbers – in cumulative analyses at defined time points after allogeneic HSCT (Supplementary Figs. S6 and S7). One previous study analyzed DTA and non-DTA mutations (utilizing a 42 gene panel, and separating the three DTA mutations from the remaining 39 non-DTA mutations) for their value as MRD markers on days 90 and 180 post-HSCT [3]. While non-DTA MRDpos was associated with a higher CIR, RFS, and OS, DTA MRDpos only showed a trend towards adverse outcomes. However, sample numbers for DTA MRDpos patients were low (n = 9), only analyzed at two time points after HSCT and—in contrast to our study—CH-associated mutations as well as not-CH-associated mutations were grouped together as non-DTA mutations [3]. Nevertheless, DTA MRD and non-DTA MRD VAFs were increasing similarly prior to relapse when performing monthly sample analyses [3], which matches our observations.
Finally, we analyzed the capability of relapse prediction for different CH-associated genes. Here, all genes with adequate sample numbers available (i.e., DNMT3A, IDH2 R140, SRSF2, and U2AF1) were able to predict relapse within 28, 56, and 84 days. However, SRSF2 MRCH showed a relatively low sensitivity for predicting relapse longer than 28 days after sampling (Supplementary Fig. S8). In fact, all three patients in our cohort for whom relapse was not preceded by an MRCHpos sample were measured by SRSF2 MRCH. Recently, Fabre et al. published data on longitudinal dynamics of CH in a cohort without hematologic malignancies. Here, SRSF2 clones had the highest growth rate [14], matching our observations of a very short time from SRSF2 MRCHpos status to open relapse. In contrast, we observed the highest rate of MRCHpos without consecutive relapse for JAK2 mutations. JAK2 MRCHpos was often found within two months after nma or ric HSCT and turned MRCHneg with reduction of immunosuppression or development of a chronic GvHD. JAK2 mutated clones were also described to show irregular growth trajectories [14] and JAK2 activation to contribute to PD-L1 expression [15], which may explain the response of JAK2 MRCH to graft-versus-leukemia effects and their relevance after HSCT without mac conditionings.
Our study has some limitations. While we were able to analyze MRCH mutations cumulatively, we lack sufficient patient and sample numbers to analyze all distinct genes separately. As our data indicates different dynamics and subsequent value for relapse prediction of separate genes, larger cohorts are needed to address this point. Additionally, longer follow up is needed to evaluate how sufficiently MRCH predicts late relapse after HSCT.
In conclusion, our ddPCR assays allow for replicable sensitivity with low hands-on time and costs, which is ideal for repetitive monitoring in a clinical setting. Our data suggests MRCH as a feasible marker for MRD assessment in AML patients after allogeneic HSCT, with comparable clinical value as the standard MRD marker NPM1. Subsequently, evaluation of MRCH may be a valuable clinical tool to detect relapse early after allogeneic HSCT, especially in patients lacking or loosing conventional MRD markers such as NPM1. When confirmed, MRCH can aid in guiding preemptive treatment decisions to improve outcomes after HSCT.
Supplementary information
Acknowledgements
The current and past member of Sebastian Schwind’s lab would like to express their deepest gratitude for his guidance, mentorship, patience, and friendship over the past 10 years. Although he left us much too early, he left a lasting impact on us as physicians and researchers. This study was supported by the Deutsche Gesellschaft für Innere Medizin (Clinician Scientist Program, MJ), Ein Herz für Kinder e.V., the Verein Zusammen gegen den Krebs e.V. [SSch, MJ], the Deutsche Jose-Carreras-Stiftung (04 R/2016 [SSch]), and an intramural scholarship of the University Leipzig (LB). The authors thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Sabine Leiblein, Martina Pleß, Ulrike Bergmann, Janet Bogardt, Annette Jilo, and Dagmar Cron for their help in determining cytogenetic, morphologic, and immunological analyses; Karoline Goldmann, and Julia Schulz for their help in molecular analyses, and Christine Günther, Claudia Diener, Manuela Quandt, Scarlett Schwabe, Ines Kovacs, and Kathrin Wildenberger for their help in sample processing. Presented in part at the 2022 Annual Meeting of the American Society of Hematology in New Orleans/USA.
Author contributions
LB, SSch, and MJ contributed to the design and analysis of this study and the writing of the manuscript, and all authors agreed on the final version. MJ, LB, JG, MB, JU, DBr, and DBa carried out the laboratory-based research; MJ and SSch performed statistical analyses; and MM, KHM, MH, GNF, VV, and UP provided administrative support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-023-02072-y.
References
- 1.Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl J Med. 2014;371:2477–87. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N. Engl J Med. 2018;378:1189–99. doi: 10.1056/NEJMoa1716863. [DOI] [PubMed] [Google Scholar]
- 3.Heuser M, Heida B, Büttner K, Wienecke CP, Teich K, Funke C, et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv. 2021;5:2294–304. doi: 10.1182/bloodadvances.2021004367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corces-Zimmerman MR, Majeti R. Pre-leukemic evolution of hematopoietic stem cells: the importance of early mutations in leukemogenesis. Leukemia. 2014;28:2276–82. doi: 10.1038/leu.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaidzik VI, Weber D, Paschka P, Kaumanns A, Krieger S, Corbacioglu A, et al. DNMT3A mutant transcript levels persist in remission and do not predict outcome in patients with acute myeloid leukemia. Leukemia. 2018;32:30–37. doi: 10.1038/leu.2017.200. [DOI] [PubMed] [Google Scholar]
- 8.Krönke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29:2709–16. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Morita K, Loghavi S, Wang F, Furudate K, Sasaki Y, et al. Clonal dynamics and clinical implications of postremission clonal hematopoiesis in acute myeloid leukemia. Blood. 2021;138:1733–9. doi: 10.1182/blood.2020010483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura S, Yokoyama K, Shimizu E, Yusa N, Kondoh K, Ogawa M, et al. Prognostic impact of circulating tumor DNA status post–allogeneic hematopoietic stem cell transplantation in AML and MDS. Blood. 2019;133:2682–95. doi: 10.1182/blood-2018-10-880690. [DOI] [PubMed] [Google Scholar]
- 11.Klyuchnikov E, Badbaran A, Massoud R, Fritzsche-Friedland U, Freiberger P, Ayuk F, et al. Post-Transplantation Day +100 Minimal Residual Disease Detection Rather Than Mixed Chimerism Predicts Relapses after Allogeneic Stem Cell Transplantation for Intermediate-Risk Acute Myelogenous Leukemia Patients Undergoing Transplantation in Complete Remi. Transpl Cell Ther. 2022;28:374.e1–e9. doi: 10.1016/j.jtct.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Höllein A, Meggendorfer M, Dicker F, Jeromin S, Nadarajah N, Kern W, et al. NPM1 mutated AML can relapse with wild-type NPM1: persistent clonal hematopoiesis can drive relapse. Blood Adv. 2018;2:3118–25. doi: 10.1182/bloodadvances.2018023432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou H-A, Kuo Y-Y, Liu C-Y, Chou W-C, Lee MC, Chen C-Y, et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood. 2012;119:559–68. doi: 10.1182/blood-2011-07-369934. [DOI] [PubMed] [Google Scholar]
- 14.Fabre MA, de Almeida JG, Fiorillo E, Mitchell E, Damaskou A, Rak J, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606:335–42. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prestipino A, Emhardt AJ, Aumann K, Sullivan DO, Gorantla SP, Duquesne S, et al. HHS Public Access immune escape in myeloproliferative neoplasms. Sci Transl Med. 2019;10. 10.1126/scitranslmed.aam7729.Oncogenic. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.