Abstract
The Ph1 oncology trial landscape is evolving in response to advances in understanding of cancer biology, novel drug discovery platforms, and therapeutic modalities. To uncover emerging trends in oncology drug development, we identified 7,061 solid tumour Ph1 trials (2009–2021) from clinicaltrials.gov to determine the numbers of trials commenced, therapeutic classes, combinations, tumour streams, and geographical distribution. Ph1 oncology trials increased by an average of 5.2 %/year. There was a significant relative increase in the number of immunotherapy studies and a significant relative decrease in trials containing chemotherapy. Between 2009 and 2021, multi-agent combination trials outnumbered single-agent trials and single-class trials outnumbered multimodal combination trials. The proportion conducted in the Asia-Pacific significantly increased. Multiregional trials decreased during the COVID-19 pandemic, reducing projected trial numbers in Asia-Pacific and Europe whilst increasing single-region trials in North America. Further study is required to track recovery post-pandemic, and the emergence of novel modalities (e.g. ADCs and cellular therapies).
Investment in research and development of novel cancer therapeutics has rapidly increased in the past decade, due to successive waves of new drug approvals in precision oncology and immuno-oncology approaches [1]. Consequently, the number, design, complexity, and geographic distribution of phase I (Ph1) oncology trials has evolved [1,2]. Improved understanding of trends in the development of novel therapeutics in Ph1 oncology trials would enable resource investment and infrastructure planning to be targeted appropriately. Evaluation of international clinical trials registries provides a more comprehensive representation of the Ph1 clinical trial landscape than literature review by minimising publication bias and lag [3].
We characterised the changing trends in the Ph1 oncology clinical trials in patients with solid tumours registered on clinicaltrials.gov over between 2009 and 2021. Ph1 clinical trials registered on ClinicalTrials.gov scheduled to start between 1 January 2009–31 December 2021 were extracted using the search parameters: cancer, ≥18 years old, active, recruiting, completed, early Ph1, Ph1, and interventional. Exclusion criteria: not conducted in patients with solid tumours, testing a device or procedure, solely involving supportive care or radiotherapy, or if the therapeutic mechanism was unknown. Eligible Ph1 trials were categorized by: (1) start date, (2) therapeutic class: chemotherapy, targeted therapy, immunotherapy, radiopharmaceuticals, antibody-drug conjugates (ADCs), cellular, viral, hormonal, or radiotherapy, (3) tumour stream: breast, lung, nervous system, gastrointestinal, genitourinary, head and neck, skin, gynaecological, sarcoma, neuroendocrine or multiple, (4) geography, (5) single-agent or multi-agent combination trials, the latter including 2 drugs from the any class and (6) single-class or multimodal combination trials, defined as using 2 therapeutic classes (Table 1). Expected trends were calculated using the least squares method. Groups were compared using an unpaired, two-sided, t-tests.
Table 1.
Classification of trial type according to number of therapeutic agents and therapeutic classes involved. Trials with both single and combination arms were classified as using ≥2 drugs.
| Trial type | 1 drug | ≥2 drugs |
|---|---|---|
| 1 therapeutic class | Single-class, single-agent trial | Single-class, multi-agent trial |
| ≥ 2 therapeutic classes | Not applicable | Multi-modal combination trial |
11,715 trials were identified and classified by two independent reviewers (RK, CG), with a third reviewer (BT) arbitrated when consensus was not reached. 4,654 trials were excluded, leaving 7,061 trials for analysis. The studies were excluded sequentially according to the following criteria: haematological malignancy only (2,659 trials), non-malignant condition or healthy subjects (438 trials), paediatric population only (155 trials), testing a device or procedure only (335 trials), diagnostic or supportive only (959 trials), radiotherapy alone (75 trials) or if the therapeutic mechanism was unknown (33).
In instances of overlapping therapeutic class of a particular agent, it was classified according to the class of predominant anti-cancer effect. For example, TT-00420 (Tinengotinib) is a spectrum-selective multi-kinase inhibitor that targets cell proliferation, angiogenesis, and immune-oncology pathways by inhibiting Aurora kinases A/B and Janus kinases (JAK) involved in cytokine signalling and receptor tyrosine kinases (FGFRs and VEGFRs) involved in the tumour microenvironment [4]. Although there is an immune-modulatory component to the mechanism of action, this is not the predominant mechanism of action, and it is therefore classified as a targeted therapy.
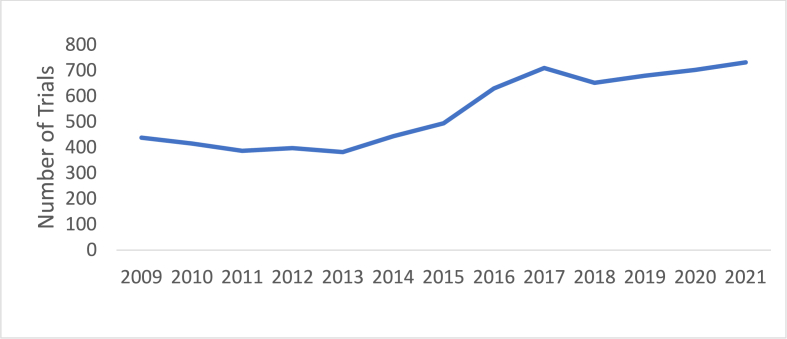
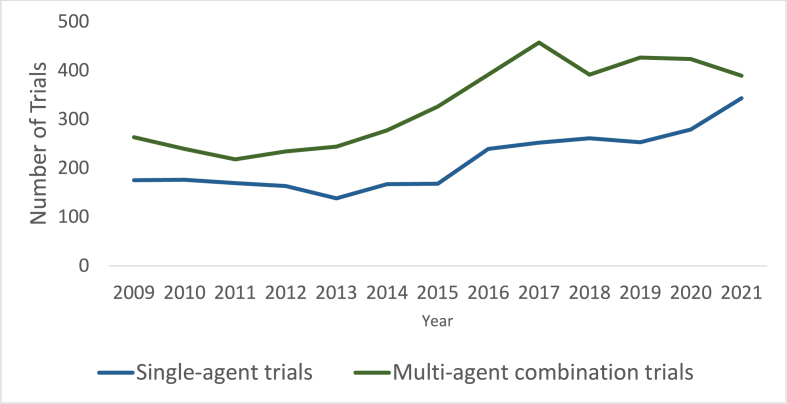
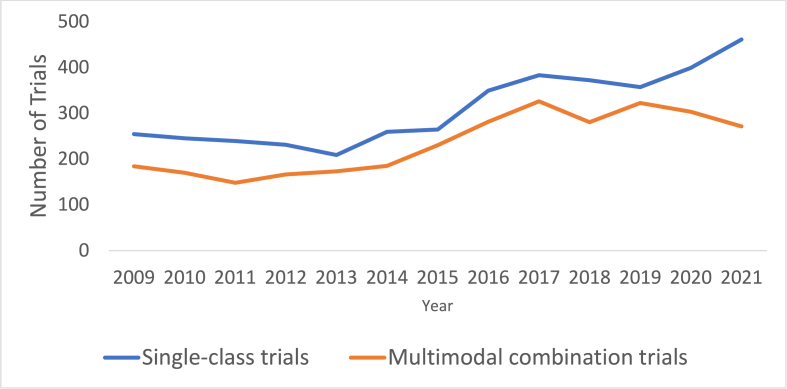
The number of Ph1 trials registered on ClinicalTrials.gov increased by a mean of 5.2 % per year between 2009 and 2021, with the most rapid increase from 2015 to 2016 by 27.5 % (Fig. 1). Multi-agent combination Ph1 trials were consistently more frequent than single-agent Ph1 trials (4,278 (40.6 %) vs 2,783 trials (39.4 %)) (Fig. 2). Single-class trials were consistently more frequent than multimodal combination trials (4,022 (60.0 %) vs 3,039 trials (40.0 %)) (Fig. 3). When examining the regional differences in this regard, it was notable that between 2019 and 2021, average numbers of multi-agent and single-agent trials annually were approximately even in Asia Pacific (100 vs 105 trials) and Europe (29 vs 31 trials) while in North America, multi-agent trials accounted for a much larger proportion (192 vs 100 trials). There were no significant regional differences with regard to increase in therapeutic class over time.
Fig. 1.
Number of newly registered phase 1 oncology trials over time.
Fig. 2.
Single-agent versus multi-agent combination phase 1 trials over time.
Fig. 3.
Single-class versus multimodal combination phase 1 trials over time.
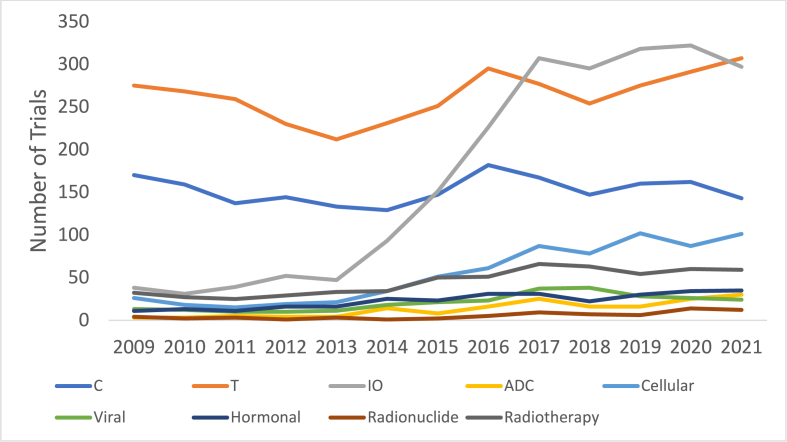
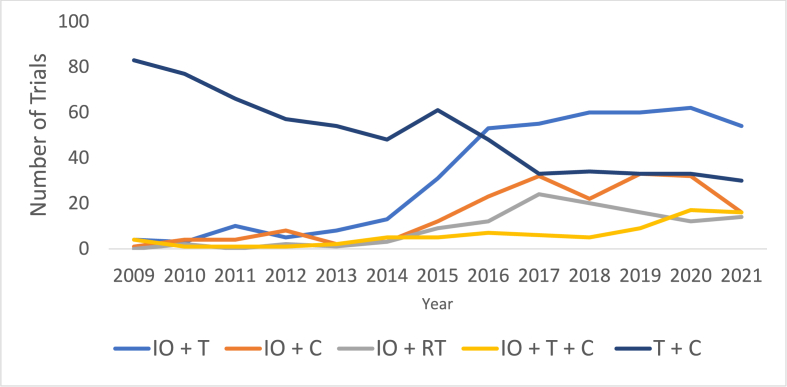
Ph1 trials containing chemotherapy declined by an average 0.8 % annually, a significant proportional mean difference of 5.6 % from the overall increasing Ph1 trials (95 % CI:0.2–11.1 %, p = 0.043) (Fig. 4). The number of trials which used immunotherapy agents proportionally increased at a significantly higher mean annual rate of 22.8 % than the overall number of trials at 4.8 % annually (mean annual difference 18.0 %, 95 % CI:0.02–36.0 %, p = 0.049). Cellular therapies increased by an average of 15.5 % annually, not significantly different to overall trial increases (p = 0.140). Immunotherapy plus targeted combinations saw the highest average annual increase of 41.1 % (Fig. 5). Targeted plus chemotherapy trials decreased most in absolute terms, from 83 trials in 2009 to 30 trials in 2021, an average decline of 7.17 % annually. This was not significant when compared to changes in overall trial numbers (mean annual difference: 12.0 %, 95 % CI: 0.5 to 24.5, p = 0.058).
Fig. 4.
Inclusion of therapeutic modalities in Phase I trials over time. C = chemotherapy, T = Targeted therapy, IO = Immunotherapy, ADC = Antibody-drug conjugate. All trials including each therapeutic class were included in this figure, irrespective of their combination with other therapeutic classes within the same trial.
Fig. 5.
Phase 1 trials by combination of therapeutic classes over time. C = chemotherapy, T = Targeted therapy, IO = Immunotherapy, RT = Radiotherapy.
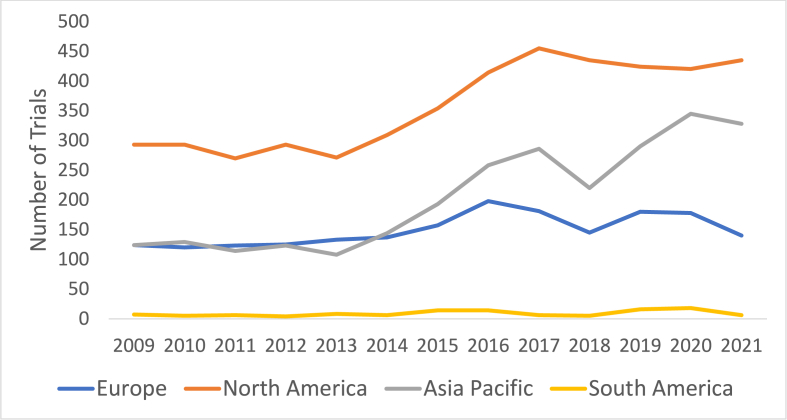
Single tumour stream-specific Ph1 trials (gastrointestinal: 14.0 %, lung: 8.4 %, genitourinary: 7.6 %, breast 6.7 %) were more common than multiple tumour-types trials (43.3 %). Of the 7,061 total Ph1 trials, 1,638 (23.2 %) were held across multiple regions. Of note, there has been a significant increase in the proportion of Ph1 clinical trials which included an Asia-Pacific site, from a mean of 30.1 % (2009–2014) to 41.8 % (2015–2020) (p = 0.003) (Fig. 6).
Fig. 6.
Phase 1 trials by geographical region over time. Note: those trials which included multiple regions were counted towards the total of both regions.
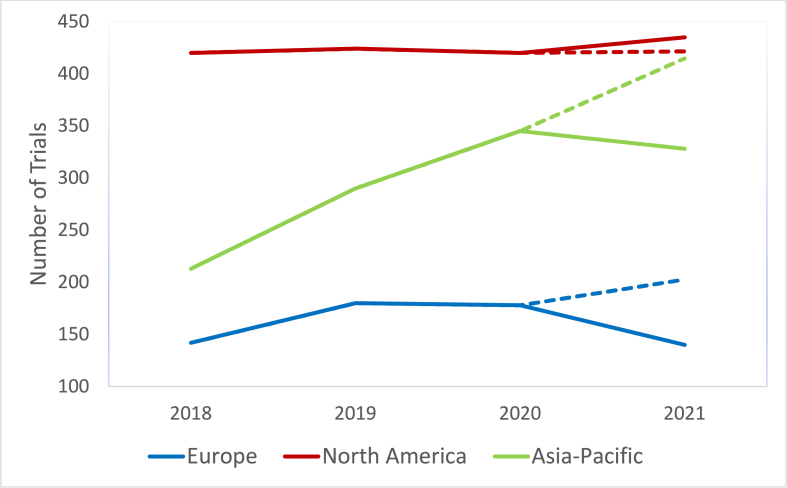
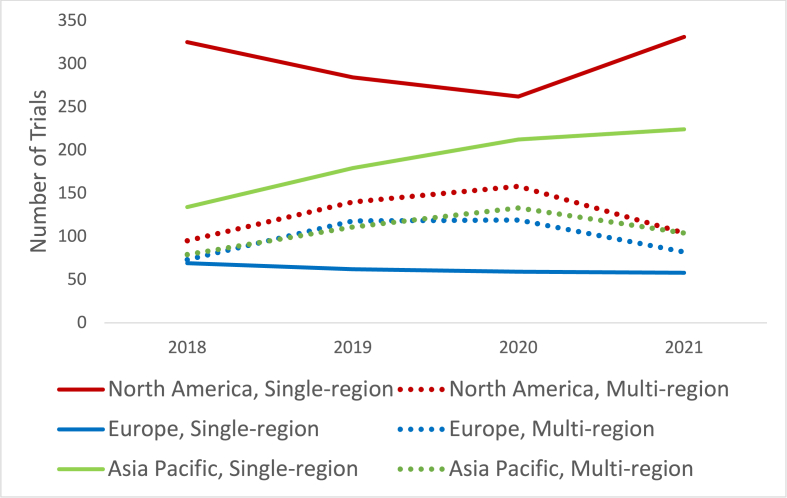
To assess the impact of the COVID-19 pandemic on the registration of Ph1 trials in each region, we calculated an expected number of trials for each region, projected from the trend of the preceding 3 years, and compared that with the actual number of registered trials. Trial numbers in Asia-Pacific and Europe were lower than expected by 21.0 % (415 vs 328 trials) and 31.0 % (203 vs 140 trials) respectively (Fig. 7). Total trial registration numbers were as expected in North America, however from the 2020 to 2021 calendar years, there was a 29.6 % decline in multi-region trials (from 169 to 119 trials) and a 46.5 % increase in single-region trials (from 226 to 331 trials) in North America (Fig. 8).
Fig. 7.
Impact of the COVID-19 pandemic on projected number of phase 1 trials by geographical region. Dotted lines indicate expected phase 1 trial numbers for 2021, calculated using the least squares method, examining data from the preceding three years.
Fig. 8.
Single versus multi-region phase 1 trial registrations by region over time.
Consistent with previous studies [5,6], we showed increase in the use of immunotherapy in Ph1 trials across the study period. The Food and Drug Association (FDA) approved the use of the CTLA-4 inhibitor ipilimumab for melanoma in March of 2011, but it was not until 2014, when the PD-1 inhibitor pembrolizumab was approved, that rates of immunotherapy use in Ph1 trials increased dramatically (Fig. 4). A predominance of multi-agent combination trials and of single-class trials was consistent with previous studies [6]. Upadhaya et al. [5] concluded in November of 2020 that impacts of COVID-19 on Ph1 trials had peaked in April 2020, and was recovering. Our data instead suggests the impacts of the COVID-19 pandemic on Ph1 trials were ongoing in 2021. Marked shifts away from multi-regional collaboration were seen, increasing single-region trials in North America, while single-region trials registered in other regions remained steady (Fig. 8). This may impact negatively on the capacity for recruitment of large patient populations, reduce ethnic diversity in trial populations and limit capacity to study rare tumours or specific molecular subtypes.
This study provides important insights into trends in phase I trial activity but has some limitations. Not all trials are registered on clinicaltrials.gov. Tumour stream-specific analysis was limited to trials recruiting only one tumour stream; trials recruiting 2 or more tumour streams were not included in this analysis. Small absolute numbers of some therapeutic classes in Ph1 trials (i.e. cellular therapies) limited the significance of emerging proportional changes when comparing with other modalities. When examining trial registrations by region, we did not discriminate between leading and participating regions. Overlapping drug mechanisms were not accounted for, limiting classification according to the predominant mechanism of action.
In summary, our data showed that Ph1 trials are increasing in number and heterogeneity in terms of types of drugs used and combinations. There was significant increase in Ph1 trials using immunotherapy, whilst studies using chemotherapy decreased. COVID-19 negatively impacted on multi-region participation, reinforcing a landscape of North America-led single-region trials, without significant impact on the absolute number of Ph1 trials registered in other regions. More research is required to examine the permanence of Covid-19 pandemic related changes, and track the emergence of less common therapeutic classes, including ADCs, cellular and viral therapies.
Funding
Not applicable.
Role of the funder
Not applicable.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Richard Kelly
•No disclosures
Ben Tran
•Research Funding: Amgen, Astellas, AstraZeneca, Bayer, BMS, Genentech, Ipsen, Janssen, Pfizer, Movember, MSD
•Honorarium: Amgen, Astellas, AstraZeneca, Bayer, BMS, Ipsen, Janssen, Merck, MSD, Pfizer, Sanofi, Tolmar
•Consulting/Advisory: Amgen, Astellas, AstraZeneca, Bayer, BMS, Ipsen, IQVIA, Janssen, Merck, MSD, Novartis, Pfizer, Roche, Sanofi, Tolmar
Jayesh Desai
•Consulting or Advisory Role: BeiGene, Pierre Fabre, Bayer, GlaxoSmithKline, KGaA, Boehringer Ingelheim, Roche/Genentech, Daiichi Sankyo Europe GmbH, Novartis, Pfizer, Ellipses Pharma, Axelia Oncology, Amgen
•Research Funding: Roche, GlaxoSmithKline, Novartis, BeiGene, Lilly, Bristol-Myers Squibb, AstraZeneca/MedImmune
Christina Guo
•Research Funding: US DoD, Wellcome Trust
•Current employee of Genentech Inc. The work presented herein is not affiliated with her current employment.
Data availability
Data will be made available on request.
References
- 1.Kather J.N., Berghoff A.S., Ferber D., Suarez-Carmona M., Reyes-Aldasoro C.C., Valous N.A., et al. Large-scale database mining reveals hidden trends and future directions for cancer immunotherapy. OncoImmunology. 2018;7(7) doi: 10.1080/2162402X.2018.1444412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakiba C., Grellety T., Bellera C., Italiano A. Encouraging trends in modern phase 1 oncology trials. N. Engl. J. Med. 2018;378(23):2242–2243. doi: 10.1056/NEJMc1803837. [DOI] [PubMed] [Google Scholar]
- 3.Zarin D.A., Tse T., Williams R.J., Califf R.M., Ide N.C. The ClinicalTrials.gov results database--update and key issues. N. Engl. J. Med. 2011;364(9):852–860. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piha-Paul S.A., Xu B., Raghav K.P.S., Meric-Bernstam F., Janku F., Dumbrava E.E., et al. First-in-human, phase I study of TT-00420, a multiple kinase inhibitor, as a single agent in advanced solid tumors. J. Clin. Oncol. 2022;40(16_suppl):3013. doi: 10.1093/oncolo/oyad338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upadhaya S., Hubbard-Lucey V.M., Yu J.X. Immuno-oncology drug development forges on despite COVID-19. Nat. Rev. Drug Discov. 2020;19(11):751–752. doi: 10.1038/d41573-020-00166-1. [DOI] [PubMed] [Google Scholar]
- 6.Chihara D., Lin R., Flowers C.R., Finnigan S.R., Cordes L.M., Fukuda Y., et al. Early drug development in solid tumours: analysis of National Cancer Institute-sponsored phase 1 trials. Lancet. 2022;400(10351):512–521. doi: 10.1016/S0140-6736(22)01390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.