Abstract
Objectives
Tissue engineering approaches via repopulation of acellular biological grafts provide an exciting opportunity to generate lung grafts for transplantation. Alveolar type 2 (AT2) cells are a promising cell source for re-epithelialization. There are however inherent limitations with respect to their survival and growth, thus impeding their usability for tissue engineering applications. This study investigates the use of mesenchymal stromal cells to support primary AT2 cells for recellularization of mouse lung scaffolds.
Methods
AT2 cells and bone marrow-derived mesenchymal cells (BMC) were co-delivered to decellularized mouse lung scaffolds. Recellularized lungs were evaluated for cell surface coverage, viability, and differentiation at 1 and 4 days after cell seeding. Recellularization was evaluated via histological analysis and immunofluorescence.
Results
Simultaneous delivery of AT2 and BMC into acellular lung scaffolds resulted in enhanced cell surface coverage and reduced AT2 cell apoptosis in the recellularized scaffolds at Day 1 but not Day 4. AT2 cell number decreased after 4 days in both of AT2 only and codelivery groups suggesting limited expansion potential in the scaffold. After retention in the scaffold, AT2 cells differentiated into Aqp5-expressing cells.
Conclusions
Our results indicate that BMC support AT2 cell survival during the initial attachment and engraftment phase of recellularization. While our findings suggest only a short-term beneficial effect of BMC, our study demonstrates that AT2 cells can be delivered and retained in acellular lung scaffolds; thus with preconditioning and supporting cells, may be used for re-epithelialization. Selection and characterization of appropriate cell sources for use in recellularization, will be critical for ultimate clinical application.
Keywords: Alveolar type 2 cells, Bone marrow-derived mesenchymal cells, Decellularized lung scaffold, Recellularization, Lung regeneration
Highlights
-
•
Repopulation of biological scaffolds is an exciting approach to generate bioengineered grafts.
-
•
Alveolar type 2 cells are a promising cell source for re-epithelialization.
-
•
Mesenchymal cells can support early retention of alveolar type 2 cells on scaffolds.
1. Introduction
Lung transplantation remains the only definitive treatment for end-stage lung disease [1,2]. However, supply of donor lungs is limited and allograft rejection and infection remain unavoidable problems for transplant recipients who must remain on immunosuppression for life [2,3]. Thus, alternative therapies are needed.
One exciting field of research is tissue engineering, specifically using decellularization and recellularization [[4], [5], [6], [7]] approaches for the generation of bioengineered grafts. The premise of this technology is that cellular components are removed from donor scaffolds while extracellular matrix components are maintained. The process thus results in an acellular scaffold which can be repopulated with recipient-derived or exogenous cells [6,8]. While a promising approach, there are several steps that need to be optimized. Rodent-scale whole-organ scaffolds allow for testing and optimization of recellularization conditions in a cost-effective and reproducible fashion [9]. In this study, we focus specifically on one of the key challenges for lung scaffold recellularization, which is the lack of an appropriate cell source to reconsitute the epithelium [10].
Alveolar type 2 (AT2) cells have been shown to be a promising cell type for lung regeneration and repair [11]. They are also believed to be distal lung progenitor cells, thus undergoing self-renewal to generate AT2 cells and differentiating to alveolar type 1 (AT1) cells [12]. There are however, inherent limitations with respect to their usability for tissue engineering applications including limited survival and growth in 2D ex vivo culture conditions, slow mitotic rate [13], rapid loss of phenotype, and an incomplete understanding of the mechanisms controlling AT2 cell function [7,11]. Therefore, to use AT2 cells for tissue engineering application and especially for recellularization of lung scaffolds, these limitations have to be addressed.
One strategy is to use a supporting cell population to enhance survival and growth during the recellularization process. Stromal cell populations have been shown to support epithelium [[14], [15], [16]]. In the lung, fibroblast-epithelial cell interactions are important in alveologenesis and AT2 maintenance [14]. And the mesenchymal alveolar niche has been shown to be critical for alveolar epithelial cell growth and self-renewal [15,16].
Here, we investigate the effect of using a stromal cell population with AT2 cells during recellularization. Specifically, we co-delivered AT2 cells and bone-marrow derived mesenchymal stromal cells (BMC) to acellular mouse lung scaffolds and evaluated the extent of recellularization, the viability and growth of cells in the scaffold. This study aims to evaluate whether support from mesenchymal cells will support the survival and growth of primary AT2 cells on the decellularized lung scaffolds.
2. Materials and methods
2.1. Animal husbandry
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University Health Network (Approval number: AUP2798.13). Adult C57/BL6 mice (Jackson Laboratory, ME, USA) were maintained under specific pathogen-free conditions.
2.2. Mouse lung decellularization
Decellularization was completed as previously described [9,17,18]. Briefly, 8-12-week C57BL/6 male mice were euthanized and heart-lung blocks were extracted. Lungs were sequentially perfused and incubated with distilled water, 0.1 % Triton X100, 2 % sodium deoxycholate, sodium chloride solution, and DNase I (0.1 mg/mL, ThermoFisher Scientific, CA, USA) solution (Supplemental Figure 1a). Three to five mice per group were used in this study.
Quality of decellularization was evaluated by DNA assay and histology. DNA quantification was done using the Quant-iT PicoGreen dsDNA assay kit (Thermo Fisher Scientific, MA, USA) as per manufacturer instructions.
2.3. AT2 cell isolation
AT2 cells were isolated using a previously described protocol with minor modifications [19,20]. Briefly, mice were sacrificed and the lungs were flushed. The trachea was canulated and instilled with Dispase II solution (50 U/mL; Roche Applied Science, Germany) to release cells. After digestion, single cells were re-suspended firstly in red blood cell lysis buffer (Sigma, ON, Canada) and in 2 % vol/vol FBS–PBS for all subsequent procedures. For purification of epithelial cells, freshly isolated cells were suspended with a mixture of antibodies (anti-CD45, anti-CD31, anti-EpCAM) for approximately 20 min in 4 °C. All antibody details are listed in Table 1. Cell viability was accessed by propidium iodide (1 μg/mL) staining. Sorting was performed to isolate EpCAM+, CD31−, CD45− cells using a MoFlo BRU cell sorter (Becton Dickinson) (Supplemental Fig. 2a-i). A purity check for sorted cells was conducted with every isolation.
Table 1.
Antibodies for flow cytometry and immunofluorescence (IF) staining.
| Name (Fluorophore) | Host (Clone) | Dilution | Company (Product#) |
|---|---|---|---|
| CD31 (PE) | Rat (MEC 13.3) | 1:200 | BD Bioscience (553,373) |
| CD45 (PE) | Rat (30-F11) | 1:300 | BD Bioscience (553,081) |
| EpCAM (APC) | Rat (G8.8) | 1:200 | Abcam (ab95641) |
| CD16/CD32 | Rat (2.4G2) | 1:50 | BD Bioscience (553,142) |
| CD73 (APC) | Rat (REA778) | 1:50 | Miltenyi Biotech (130-111-518) |
| CD90.2 (PE/Cy7) | Rat (53-2.1) | 1:300 | Biolegend (140,309) |
| CD29 (PE) | Armenian Hamster (HMβ1-1) | 1:80 | Biolegend (102,207) |
| CD105 (Alexa Fluor 488) | Rat (MJ7/18) | 1:25 | Biolegend (120,405) |
| CD44 (APC) | Rat (IM7) | 1:300 | BD Bioscience (561,862) |
| CD34 (FITC) | Rat (RAM34) | 1:50 | BD Bioscience (560,238) |
| Sca-1 (PE/Cy7) | Rat (D7) | 1:100 | BD Bioscience (558,162) |
| SPC | Rabbit (Polyclonal) | 1:500 | MilliporeSigma (AB3786) |
| Aquaporin 5 | Rabbit (Polyclonal) | 1:200 | Abcam (ab78486) |
| Collagen Type IV | Goat (Polyclonal) | 1:10 | MilliporeSigma (AB769) |
2.4. Isolation of BMC
Mesenchymal stromal cells were procured from mouse bone marrow as previously described [21,22]. Isolated BMC were cultured in a Dulbecco's modified Eagle's medium (DMEM) and plastic-adherent stromal cells were cultivated and used within up 5 passages.
We evaluated differentiation of BMC into adipocytes (MesenCult™ Adipogenic Differentiation Kit (Mouse): STEMCELL technologies, BC, Canada), osteocytes (MesenCult™ Osteogenic Stimulatory Kit (Mouse); STEMCELL technologies), and chondrocytes (Human/Mouse StemXVivoTM Chondrogenic Media; R&D systems, MN, USA) according to manufacturer's instructions.
Flow cytometry was used to confirm the BMC population via surface marker expression as per established protocols [21,22]. All antibody details are listed in Table 1.
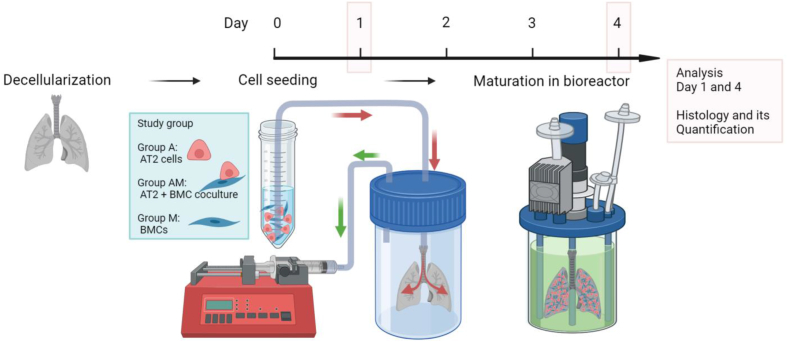
2.5. Cell delivery and maturation
Cell delivery was conducted using our previously reported negative pressure cell seeding approach [8,18]. Briefly, a cell suspension (2 million cells per 1.5 mL of medium) was prepared and PneumaCultEx (STEMCELL technologies, Vancouver, BC, Canada) was used as growth media for cell delivery and bioreactor culture. The cell suspension was connected to the tracheal catheter in the main reservoir which was also connected to the syringe pump. The pressure gradient in the reservoir facilitated delivery of the cell suspension. Lungs were recellularized with an AT2 cells only group (Group A: 2 × 106 AT2 cells), BMC only group (Group M: 2 × 106 Mesenchymal cells) and AT2 and BMC group (Group AM: 1.0 × 106 cells each of AT2 and BMC). Seeded lungs were maintained in static conditions for 18 h, then transferred to the bioreactor and vascular perfusion system allowing perfusion through the PA at the rate of 1.5 mL/min for 3 days. The total number of cells for recellularization was selected based on our previously described studies investigating lung re-epithelialization with lung cell lines [8,17,18]. For consistency, the total number of cells was maintained constant across groups.
2.6. Histological analysis quantification
Samples were fixed with 10 % formalin, solution for 1 day, embedded in paraffin blocks and sectioned at 5 μm.
Three sectioned slides were prepared for each lung. H&E (Hematoxylin-eosin) and staining was performed according to established protocols [8,18]. Scanned images were processed with HALOTM software (Indica labs; NM, USA) for image quantification. Cell surface and scaffold areas were used in the formula below to obtain cell surface coverage percentage = (Cell area/(Cell area + ECM area)) X 100 (%) [8].
2.7. Immunofluorescence (IF) staining
Sections were deparaffinized, treated with blocking solution (Protein Block Serum-Free, Agilent Dako, CA, USA) followed by incubation with a primary antibody at 4 °C overnight. All antibody details are listed in Table 1. Sections were then treated with appropriate secondary antibodies. Counterstain for nuclei with 4’, 6-diamino2-phenylindole (DAPI) or Nuclear Green LCS1TM (Abcam) was performed. Stained slides were scanned by the whole-slide scanner (AxioScanTM, Zeiss, Germany). To evaluate positive cells, manual counting of images including the SPC/Aquaporin 5 (Aqp5)/DAPI were performed. Three fields of view in each stain were counted for 3 independent samples per group.
2.8. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling (TUNEL) staining
TUNEL assay (In Situ Cell Death Detection Kit; Roche Diagnostics, Germany) was conducted according to the manufacturer instructions. To quantify the numbers of apoptotic cells, images of 3 randomly chosen fields/slides were taken for each repopulated lung and TUNEL-positive cells were manually counted.
2.9. Statistical analysis
Data are reported as mean ± standard deviation (SD). All statistical analyses were performed using JMP Pro software (version 14.2.0; SAS Institute, NC). Comparisons amongst groups were performed by analysis of variance with Tukey's honestly significant difference test and 2-way ANOVA. Values of p < 0.05 were considered significant.
3. Results
3.1. Simultaneous delivery of AT2 and BMC into decellularized lung scaffolds results in enhanced cell surface coverage
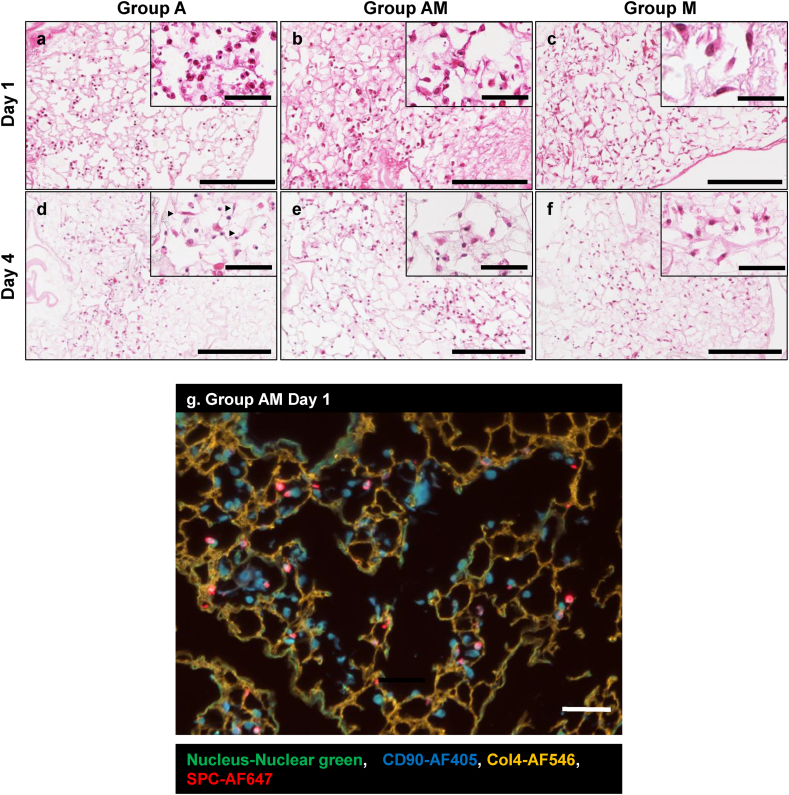
The DNA content of decellularized scaffolds was significantly lower than that of native lungs (Supplemental Figure 1b). H&E staining and IF staining with DAPI revealed that the decellularized lungs contain no nuclei (Supplemental Fig. 1c-f). Recellularization and evaluation of recellularized grafts was completed as depicted in Fig. 1. The AT2 population was confirmed by expression of prosurfactant protein C (SPC) using flow cytometry with a purity of 87.5 ± 4.7 % (Supplemental Figure 2a-ii). BMC showed the ability to differentiate into 3 lineages, Adipocytes (Oil red O staining, Supplemental Fig. 2b-i), Osteocytes (Alizarin red staining, Supplemental Figure 2b-ii) and Chondrocytes (Alcian blue staining, Supplemental Figure 2b-iii). Additionally, BMC were positive for CD90.2, CD29, CD44, Sca-1, CD45, and negative for CD34, CD73, CD105 (Supplemental Figure 2b-iv). Retention of cells in the scaffolds was confirmed by histological and IF staining. We observed cuboidal AT2 cells (Group A) and spindle-like BMC (Group M), as well as the combination of both cell types (Group AM) in the scaffolds on Day 1 (Fig. 2a–c) after recellularization. On Day 4, we identified cells with spindle morphology in scaffolds recellularized with AT2 cells only (Group A) (Fig. 2d). We did not find any significant changes in morphology in the BMC only (Group M) and co-culture groups (Group AM). Fig. 2g shows AT2 cells, labelled by SPC, and BMC, labelled by CD90 within the decellularized lung extracellular matrix labelled by collagen 4 (Col4).
Fig. 1.
Schematic overview of experimental design. Experimental design included 3 groups: (1) Decellularized mouse lungs recellularized by alveolar type 2 (AT2) cells (Group A), AT2 and bone-marrow derived mesenchymal cells (BMC) (Group AM), and BMC (Group M). Recellularized samples were cultured for up to 4 days in a bioreactor.
Fig. 2.
Recellularization of acellular lung scaffolds with AT2 and BMC. Hematoxylin-eosin (H&E) staining and immunofluorescence (IF) staining of lung scaffolds repopulated with AT2, BMC or AT2 co-delivered with BMC (a–f). Histology images depicting retention of (a) cuboidal AT2 cells (Group A), (c) spindle-like shaped BMC, (b) mixture or AT2 and BMC, after 1 day of culture. Histology images showing AT2 cells with (d) mixed cuboidal and spindle shaped (arrowhead) morphology (Group A), (f) retained spindle-like shaped BMC, (e) mixture or AT2 and BMC with both spindle and cuboidal morphology, after 4 days of culture. Scale bar = 200 μm in all images. (g) Representative IF stained image for coculture sample (Group AM, Day 1) showing SPC-positive cells and CD90-positive cells, were present within the extracellular matrix (stained with Collagen 4). Scale bar = 50 μm.
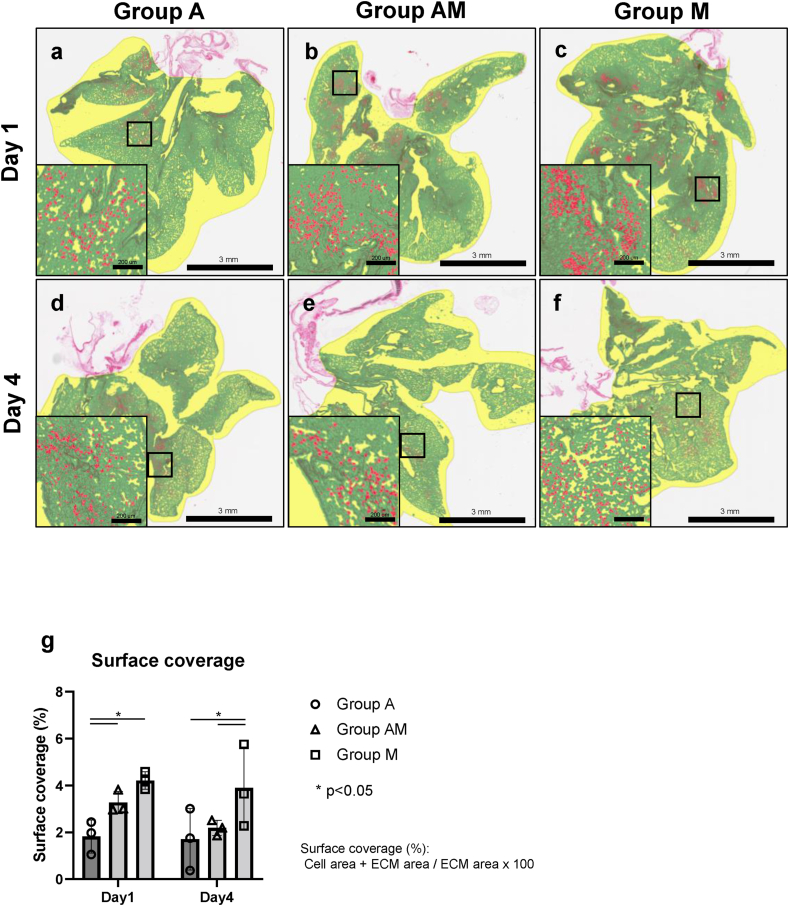
Surface coverage quantification on H&E-stained sections was conducted by HALOTM software which differentiates cell area (red), extracellular matrix (green), and background (yellow) (Fig. 3a–g). As expected, mesenchymal cells showed better cell surface coverage compared to AT2 cells on Day 1 and 4. On Day 1, Group A showed significantly less surface coverage than the coculture Group AM. However, this difference was not observed on Day 4. Overall, the number of cells onto the lung scaffold decreased from Day 1 to Day 4 (Fig. 2a–f).
Fig. 3.
Quantification of recellularized lung scaffolds. (a–d) Surface coverage quantification with red depicting cell area, green showing extracellular matrix area and yellow capturing the background. (a) Group A, (b) Group AM, (c) Group M, at Day 1, (d) Group A, (e) Group AM, (f) Group M, at Day 4. Scale bar = 3 mm. (e) Surface coverage quantification resulting from HALO image analysis (HALOTM) showing mean ± SD.
3.2. Simultaneous delivery of AT2 and BMC reduces cell apoptosis in recellularized scaffolds
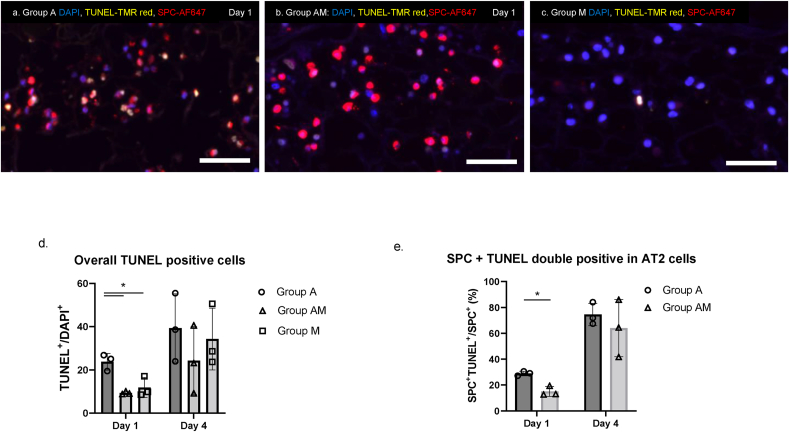
We evaluated the viability of the cells in the scaffold using TUNEL staining for apoptosis (Fig. 4a–c). A greater number of TUNEL-positive cells was observed in the scaffolds seeded with AT2 cells only (Group A) compared to other groups as shown in lung scaffolds sections stained with SPC marking AT2 cells. Quantification confirmed that Group A had a significantly higher amount of TUNEL-positive apoptotic cells on Day 1 (Fig. 4d). Furthermore, the proportion of apoptotic AT2 cells (Fig. 4e) is significantly less in the group co-delivered with the BMC (Group AM) on Day 1.
Fig. 4.
Evaluation of cell viability in recellularized lungs. Recellularized lung scaffolds were evaluated via Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling (TUNEL) staining and immunofluorescence (IF) staining for SPC in (a) Group A, (b) Group AM, and (c) Group M. (Scale bar = 50 μm). (d) Quantification of apoptotic cells of stained slides with Group A showing a significantly higher amount of TUNEL-positive apoptotic cells on Day 1. (e) Quantification of the proportion of TUNEL+SPC+ double-positive cells in the SPC+ AT2 cell population at Day 1 showing less apoptotic SPC+ in Group AM. Results are shown as group mean ± SD and analyzed via a Tukey HSD test, ∗:p < 0.05.
3.3. No significant changes in AT2 cell fate after 4 days of recellularization in the bioreactor
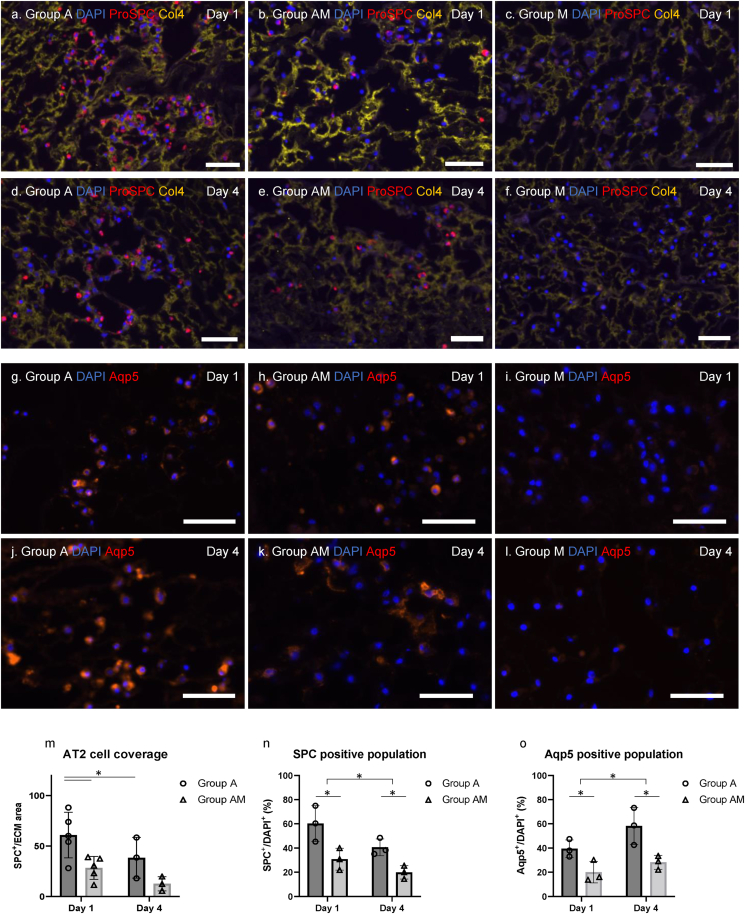
AT2 cells (SPC-positive cells) were still observed within the lung scaffolds after 4 days of bioreactor culture. However, the overall AT2 cells number was decreased in both Group A and Group AM from numbers initially observed at Day 1 (Fig. 5a, c, d, f). Quantification of SPC-positive cells is shown in Fig. 5m and was measured as the number of SPC-positive cell area per area of scaffold (Col4 staining). We performed Aqp5 staining for potential differentiation to AT1 (Fig. 5g–l) and found that the Aqp5-positive cell population increased over time in both Group A and Group AM. There were no SPC/Aqp5-positive cells in Group M (Fig. 5b, e, h, k). Furthermore, SPC/Aqp5-positive cell populations were manually counted and evaluated (Fig. 5n and o). The population of SPC-positive AT2 cells decreased over time while the population of Aqp5-positive AT1 cells increased from Day 1 to Day 4. This trend was observed in both groups. Taken together, our results show that AT2 cells can be retained in lung scaffolds but their expansion is limited. Of those that remain, a subset continued to express SPC however, the majority express Aqp5 suggesting possible differentiation to AT1 cells.
Fig. 5.
AT2 cell fate within lung scaffolds following 4 days of bioreactor culture. Immunofluorescence (IF) staining of recellularized lung slices for Surfactant protein C (SPC; red), and Collagen 4 (Col4; yellow) with (4′, 6-diamino2-phenylindole (DAPI); blue) counterstain for nuclei for (a) Groups A, (b) AM and (c) M, at Day 1. IF staining of recellularized lung slices SPC (red), Col4 (yellow) and DAPI (blue) for nuclei for (d) Groups A, (e) AM and (f) M, at Day 4 after bioreactor culture. IF staining for Aquaporin 5 (Aqp5; red) and DAPI (blue) for (g) Groups A, (h) AM and (i) M, at Day 1 after cell seeding. IF staining for Aquaporin 5 (Aqp5; red) and DAPI (blue) for Groups (j) A, (k) AM and (l) M, at Day 4 after cell seeding. Scale bar = 50 μm. (m) Quantification of AT2 cells (SPC-positive cells) using the image analyzing software (m). Results are expressed as mean ± SD (Tukey HSD test, ∗: p < 0.05). Quantification of (n) SPC-positive and (o) Aqp5-positive cell populations of stained sections at Days 1 and 4. Results are expressed as mean ± SD (2-way ANOVA, ∗: p < 0.05).
4. Discussion
Simultaneous delivery of AT2 and BMC into acellular lung scaffolds resulted in enhanced initial cell surface coverage and reduced AT2 cell apoptosis in recellularized scaffolds, yet this effect did not persist at 4 days. Further, the number of AT2 cells in the lungs decreased after 4 days in both groups suggesting that AT2 cells can initially survive and be retained but have limited growth potential in the scaffold. A marked number of those AT2 cells that do remain in the scaffold, differentiate into Aqp5-expressing cells. Moreover, while there is an initial supportive effect of BMC, no beneficial effects are observed after 4 days.
Many studies have reported the beneficial effects of mesenchymal stromal cell (MSC) populations on epithelial cells in vitro [[14], [15], [16],23]. While the precise mechanism of support has not been elucidated, MSC regulation of miR-30b-3p has been shown to play a role. Delivery of miR-30b-3p via MSCs-derived exosomes acts to decrease the expression of Serum amyloid A3 in recipient airway epithelial cells, which promotes proliferation while inhibiting the apoptosis of epithelial cells, thereby conferring protection against acute lung injury [21]. Beneficial effects are also believed to require spatial proximity to stromal cells. For instance, AT2 and AT1 cells are thought to receive signals, important for epithelial cell viability and function, from the adjoining lung mesenchyme [16]. Indeed, previous work has suggested that AT2 cells are located near Pdgfra+ mesenchymal cells, which promote AT2 self-renewal and differentiation into AT1 cells [24]. Moreover, paracrine signaling from mesenchymal populations can also provide therapeutic effects [21,25]. For instance, MSCs enhanced the barrier function in lung epithelial cells in a transwell culture model in which epithelial cells were grown in both direct and indirect contact conditions. Authors concluded that both direct adhesion and indirect paracrine effects strengthened the barrier function of lung alveolar epithelium in vitro [25]. Thus, it is likely that in this study, he initial support provided by BMC was can be attributed to potential direct cell to cell contact with AT2 cells as well as via paracrine signaling of neighbouring MSCs retained in the scaffold.
We utilized freshly isolated primary mouse AT2 cells together with bone-marrow-derived mesenchymal cells for re-epithelialization of lung scaffolds. AT2 cells are critical for alveolar regeneration in both mice and humans, serving as regional progenitors in the alveoli, capable of differentiation into AT1 cells, and self-renewal to replenish the alveolar epithelium [11,19,26]. While theoretically an ideal source, AT2 cells are difficult to work with ex vivo due to their limited capacity for survival and expansion [11,27]. Calle et al., used primary AT2 cells isolated from rats and humans for recellularization of decellularized rat lung scaffolds, and found that AT2 cells lost their epithelial phenotype within 7 days of bioreactor culture. They further found that the seeded AT2 population showed increased expression of mesenchymal markers, perhaps as part of a survival or wound-healing program [7]. In our study, the majority of the scaffold-retained AT2 cells, gained expression of Aqp5 suggesting possible differentiation to AT1 cells. This may in part be due to local differentiation cues provided by the scaffold itself. In support of our findings, Lwebuga-Mukasa et al., reported that the basement membrane can direct AT2 to adopt a differentiated AT1 phenotype [28]. It is important to note that while unlikely, we can not rule out that some of the Aqp5 expressing cells can be a result of existing AT1 cells in the originally seeded population. These observations were true for the AT2 cell only group as well as the AT2 cells simultaneously delivered with BMC. Thus, we do not believe that, with the current recellularization parameters, the BMC had a role in the potential differentiation of AT2 cells in the scaffold.
Culture conditions within the bioreactor system are essential for successful recellularization. And the cultivation media selected has a key role in both maintaining the viability of the cells within the scaffold as well as promoting cell growth and differentiation. We selected PneumaCultEx the growth media during the recellularization process in order to provide the appropriate growth factors and molecules required for cultivating AT2 cells. Some literature suggests that the usage of this medium can potentially result in the differentiation of mesenchymal stem cells towards an epithelial phonotype including AT2 cells [29,30]. Other literature suggests that the use of PneumacultEx for MSC culture in a mouse recellularization model showed transient expression of the early lung developmental marker TTF-1 [17]. In our study, the duration of recellularization is not sufficient to induce MSC differentiation into epithelial cells.
Our study has a number of limitations. Firstly, the purity of our primary AT2 cell population was approximately 90 %. Thus 10 % of the Epcam+ epithelial cells were not AT2 cells and could include AT1 cells as well as other cell types such as club and basal cells. While inclusion of other airway epithelial cells in the scaffold is not detrimental to recellularization and is likely beneficial for the overall re-epithelialization process, heterogeneity in the cell population can confound AT2-specific findings. Secondly, BMC cell delivery conditions may not have been ideal to support long-term growth of AT2 cells. Perhaps a sequential delivery of the cell populations rather than simultaneous delivery could have resulted in greater AT2 support. Sequential cell delivery for recellularization has been done in the lung with stromal and endothelial populations introduced into the scaffold prior to epithelial cells [31]. This may allow for more ideal conditions for epithelial cell engraftment and growth. In addition, BMC are a heterogeneous population and specific sub-populations which are enriched for Pdgfra+ cells may have a more pronounced supportive effect on AT2 cells [24]. Moreover, optimization of bioreactor settings such as the addition of ventilation to maintain AT2 cell phenotype [18,32], adjustment in cell delivery sequence of epithelial cells and mesenchymal populations, preconditioning of scaffold prior to recellularization [33] and the growth media composition (i.e., with higher concentrations of FGF2 and FGF10 or survival enhancing factors such as Rho-associated protein kinase inhibitor (ROCK inhibitor), could further enhance AT2 cell growth in the scaffold [7,18,27]. Thirdly, the source from which the mesenchymal cells were isolated from may not be the most appropriate and perhaps improved results would have been seen with lung-specific mesenchyme.
5. Conclusion
Our results indicate that BMC support AT2 cell survival during the initial attachment and engraftment phase of recellularization. And while we do not see a beneficial effect of BMC after 4 days of bioreactor culture, optimization of co-culture conditions may result in beneficial longer-term effects. Despite the fact that our findings suggest BMC have a short-term beneficial effect on AT2 cells in recellularized lung scaffolds, our study does show that AT2 cells can potentially be used as a cell source for recellularization. With advances in techniques for isolation of AT2 cells and further optimization of recellularization parameters, it is possible to achieve re-epithelialization of lung scaffolds using primary AT2 cells. Recellularization of whole-organ acellular scaffolds is an attractive approach to create bioengineered grafts for eventual lung transplantation and finding the right source of cells for use in recellularization, will be key in making significant progress in the field.
Data availability statement
Data will be provided upon request.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2023.11.011.
Contributor Information
Golnaz Karoubi, Email: golnaz.karoubi@uhn.ca.
Thomas K. Waddell, Email: Tom.Waddell@uhn.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Salvi S., Kumar G.A., Dhaliwal R., Paulson K., Agrawal A., Koul P.A., et al. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health. 2018;6(12):e1363–e1374. doi: 10.1016/S2214-109X(18)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi R., Tsuchiya T., Mitsutake N., Nishimura S., Matsuu-Matsuyama M., Nakazawa Y., et al. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci Rep. 2017 Aug 16;7(1):8447. doi: 10.1038/s41598-017-09115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khush K.K., Potena L., Cherikh W.S., Chambers D.C., Harhay M.O., Hayes D., Jr., et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: 37th adult heart transplantation report—2020; focus on deceased donor characteristics. J Heart Lung Transplant. 2020 Oct;39(10) doi: 10.1016/j.healun.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uriartea J.J., Franziska E., Rolandsson S.E., Pouliota R.A., Weiss D.J. Lung bioengineering: advances and challenges in lung decellularization and recellularization. Curr Opin Organ Transplant. 2018 Dec;23(6):673–678. doi: 10.1097/MOT.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuchiya T., Doi R., Obata T., Hatachi G., Nagayasu T. Lung microvascular niche, repair, and engineering. Front Bioeng Biotechnol. 2020 Feb 21;8:105. doi: 10.3389/fbioe.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calle E.A., Mendez J.J., Ghaedi M., Leiby K.L., Bove P.F., Herzog E.L., et al. Fate of distal lung epithelium cultured in a decellularized lung extracellular matrix. Tissue Eng. 2015 Jun;21(11–12):1916–1928. doi: 10.1089/ten.tea.2014.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadipour M., Duchesneau P., Taniguchi D., Waddell T.K., Karoubi G. Negative pressure cell delivery augments recellularization of decellularized lungs. Tissue Eng C Methods. 2021 Jan;27(1):1–11. doi: 10.1089/ten.TEC.2020.0251. [DOI] [PubMed] [Google Scholar]
- 9.Gilpin S.E., Ren X., Okamoto T., Guyette J.P., Mou H., Rajagopal J., et al. Enhanced lung epithelial specification of human iPSCs on decellularized lung matrix. Ann Thorac Surg. 2014 November;98(5):1721–1729. doi: 10.1016/j.athoracsur.2014.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilpin S.E., Charest J.M., Ren X., Ott H.C. Bioengineering lungs for transplantation. Thorac Surg Clin. 2016;26(2):163–171. doi: 10.1016/j.thorsurg.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Olajuyin A.M., Zhang X., Ji H.L. Alveolar type 2 progenitor cells for lung injury repair Cell Death Discovery. 2019;5:63. doi: 10.1038/s41420-019-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoshiba K., Yokohori N., Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol. 2003;28:555–562. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- 13.Uhal B.D. Cell cycle kinetics in the alveolar epithelium. Am J Physiol. 1997 Jun;272:L1031–L1045. doi: 10.1152/ajplung.1997.272.6.L1031. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi K., Shichino S., Ueha S., Nakajima T., Hashimoto S., Yamazaki S., et al. Mesenchymal-epithelial interactome analysis reveals essential factors required for fibroblast-free alveolosphere formation. iScience. 2019;11:318–333. doi: 10.1016/j.isci.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zepp J.A., Zacharias W.J., Frank D.B., Cavanaugh C.A., Zhou S., Morley M.P., et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017 Sep 7;170(6):1134–1148. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly A.B., Wallis J.M., Borg Z.D., Bonvillain R.W., Deng B., Ballif B.A., et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng. 2012 Jan;18(1–2):1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadipour M., Taniguchi D., Duchesneau P., Aoki F.G., Phillips G., Sinderby C., et al. Use of high-rate ventilation results in enhanced recellularization of bioengineered lung scaffolds. Tissue Eng C Methods. 2021 Dec;27(12):661–671. doi: 10.1089/ten.TEC.2021.0182. [DOI] [PubMed] [Google Scholar]
- 19.Guo L., Karoubi G., Duchesneau P., Aoki F.G., Shutova M.V., Roger I., et al. Interrupted reprogramming of alveolar type II cells induces progenitor-like cells that ameliorate pulmonary fibrosis. NPJ Regen Med. 2018;3:14. doi: 10.1038/s41536-018-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha M., Lowell C.A. Isolation of highly pure primary mouse alveolar epithelial type II cells by flow cytometric cell sorting. Bio Protoc. 2016 November 20;6(22) doi: 10.21769/BioProtoc.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi X., Wei X., Lv H., An Y., Li L., Lu P., et al. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019 Oct 15;383(2) doi: 10.1016/j.yexcr.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Maridas D.E., Redina-Ruedy E., Le P.T., Rosen C.J., et al. Isolation, culture, and differentiation of bone marrow stromal cells and osteoclast progenitors from mouse. J Vis Exp. 2018;(131) doi: 10.3791/56750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez J.J., Ghaedi M., Steinbacher D., Niklason L.E. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng. 2014 Jun 1;20(11–12):1735–1746. doi: 10.1089/ten.tea.2013.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miwa H., Era T. Tracing the destiny of mesenchymal stem cells from embryo to adult bone marrow and white adipose tissue via Pdgfrα expression. Development. 2018;145(2) doi: 10.1242/dev.155879. [DOI] [PubMed] [Google Scholar]
- 25.Ishii M., Tsuchiya T., Doi R., Morofuji Y., Fujimoto T., Muto H., et al. Increased in vitro intercellular barrier function of lung epithelial cells using adipose-derived mesenchymal stem/stromal cells. Pharmaceutics. 2021 Aug 16;13(8):1264. doi: 10.3390/pharmaceutics13081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basil M.C., Morrisey E.E. Lung regeneration: a tale of mice and men. Semin Cell Dev Biol. 2020 Apr;100:88–100. doi: 10.1016/j.semcdb.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner A.I., Jackson S.R., Zhao G., Quansah K.K., Farshchian J.N., Neupauer K.M., et al. Mesenchyme-free expansion and transplantation of adult alveolar progenitor cells: steps toward cell-based regenerative therapies. Npj Regenerative Medicine. 2019;4:17. doi: 10.1038/s41536-019-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lwebuga-Mukasa J.S., Ingbar D.H., Madri J.A. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162(2):423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 29.Ma N., Gai H., Mei J., Ding F.B., Bao C.R., Nguyen D.M., et al. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol Int. 2011 Dec;35(12):1261–1266. doi: 10.1042/CBI20110026. [DOI] [PubMed] [Google Scholar]
- 30.Liu A.R., Liu L., Chen S., Yang Y., Zhao H.J., Liu L., et al. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol. 2013 Jun;228(6):1270–1283. doi: 10.1002/jcp.24282. [DOI] [PubMed] [Google Scholar]
- 31.Nichols J.E., Francesca S.L., Niles J.A., Vega S.P., Argueta L.B., Frank L., et al. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med. 2018 Aug 1;10(452) doi: 10.1126/scitranslmed.aao3926. [DOI] [PubMed] [Google Scholar]
- 32.Poon J.C.H., Liao Z., Suzuki T., Carleton M.M., Soleas J.P., Aitchison J.S., et al. Design of biomimetic substrates for long-term maintenance of alveolar epithelial cells. Biomater Sci. 2018 Jan 30;6(2):292–303. doi: 10.1039/c7bm00647k. [DOI] [PubMed] [Google Scholar]
- 33.Gilpin S.E., Li Q., Evangelista-Leite D., Ren X., Reinhardt D.P., Frey B.L., et al. Fibrillin-2 and Tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials. 2017 Sep;140:212–219. doi: 10.1016/j.biomaterials.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be provided upon request.