Abstract
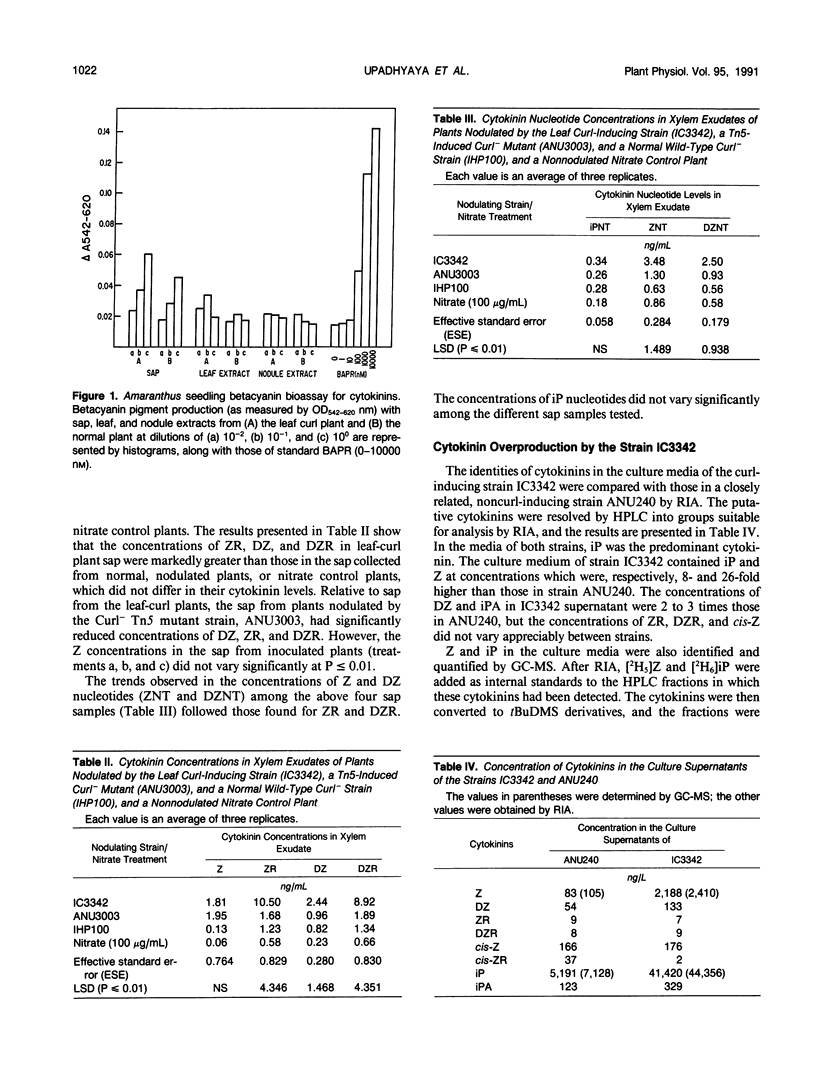
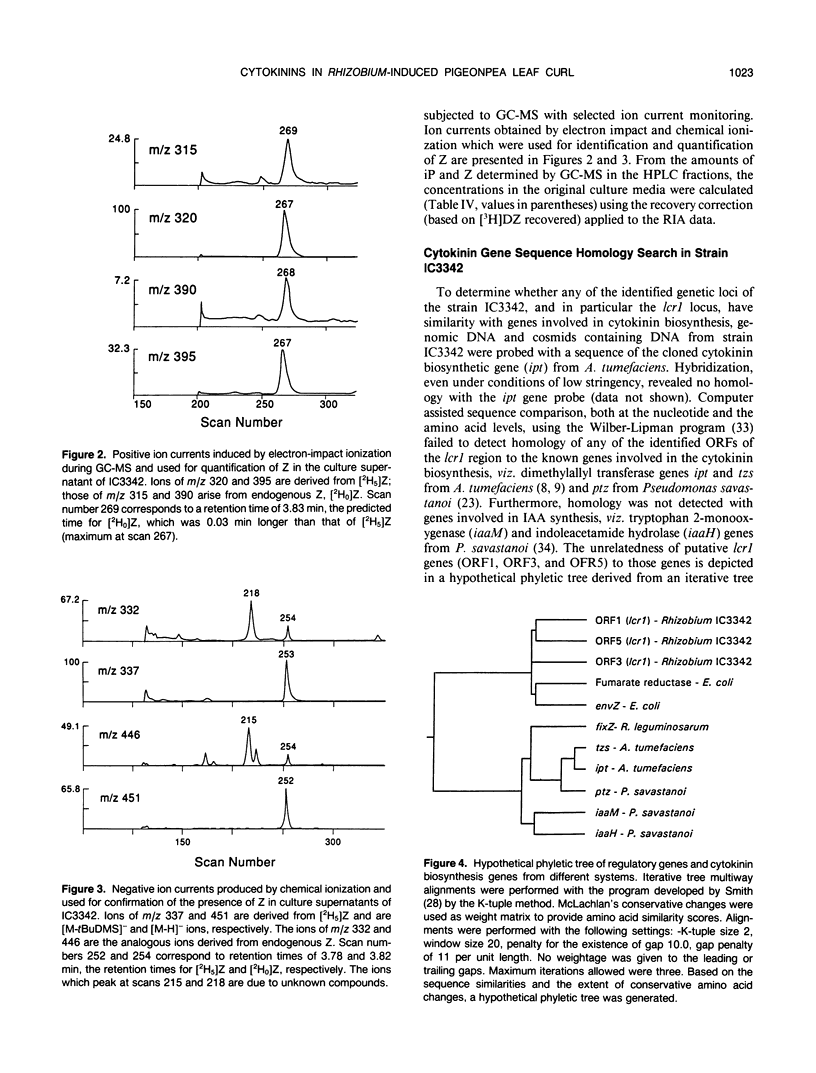
A uniquely abnormal shoot development (shoot tip-bending, leaf curling, release from apical dominance, and stunted growth) in pigeonpea (Cajanus cajan Millsp) induced by a nodulating Rhizobium strain, IC3342, is thought to be due to a hormonal imbalance. Amaranthus betacyanin bioassay indicated that xylem exudate and leaf extracts from pigeonpea plants with Rhizobium-induced leaf curl symptoms contained high concentrations of cytokinin relative to those in normal plants. Radioimmunoassay (RIA) of samples purified with high performance liquid chromatography revealed that zeatin riboside (ZR) and dihydrozeatin riboside (DZR) concentrations in xylem sap from plants with leaf curl symptoms were 7 to 9 times higher than those in the sap from symptomless, nodulated plants. The sap from symptomless plants nodulated by a Curl− mutant had ZR and DZR concentrations comparable to those in the normal plant sap. RIA indicated that the respective concentrations of zeatin and N6-isopenteny-ladenine in culture filtrates of the curl-inducing strain IC3342 were 26 and 8 times higher than those in filtrates of a related normal nodulating strain (ANU240). Gas chromatographic-mass spectrometric analyses revealed similar differences. Gene-specific hybridization and sequence comparisons failed to detect any homology of IC3342 DNA to Agrobacterium tumefaciens or Pseudomonas savastanoi genetic loci encoding enzymes involved in cytokinin biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Regier D. A., Gordon M. P. Cytokinin production by Agrobacterium and Pseudomonas spp. J Bacteriol. 1987 Sep;169(9):4242–4248. doi: 10.1128/jb.169.9.4242-4248.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenoch-Jones J., Letham D. S., Parker C. W., Rolfe B. G. Quantitation of cytokinins in biological samples using antibodies against zeatin riboside. Plant Physiol. 1984 Aug;75(4):1117–1125. doi: 10.1104/pp.75.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenoch-Jones J., Summons R. E., Djordjevic M. A., Shine J., Letham D. S., Rolfe B. G. Mass spectrometric quantification of indole-3-acetic Acid in Rhizobium culture supernatants: relation to root hair curling and nodule initiation. Appl Environ Microbiol. 1982 Aug;44(2):275–280. doi: 10.1128/aem.44.2.275-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G. F., Rogers S. G., Fraley R. T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Gibson M. M., Ellis E. M., Graeme-Cook K. A., Higgins C. F. OmpR and EnvZ are pleiotropic regulatory proteins: positive regulation of the tripeptide permease (tppB) of Salmonella typhimurium. Mol Gen Genet. 1987 Apr;207(1):120–129. doi: 10.1007/BF00331499. [DOI] [PubMed] [Google Scholar]
- Hocart C. H., Wong O. C., Letham D. S., Tay S. A., MacLeod J. K. Mass spectrometry and chromatography of t-butyldimethylsilyl derivatives of cytokinin bases. Anal Biochem. 1986 Feb 15;153(1):85–96. doi: 10.1016/0003-2697(86)90065-5. [DOI] [PubMed] [Google Scholar]
- Miller C. O. Ribosyl-trans-Zeatin, A Major Cytokinin Produced by Crown Gall Tumor Tissue. Proc Natl Acad Sci U S A. 1974 Feb;71(2):334–338. doi: 10.1073/pnas.71.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. A., Torrey J. G. Studies on cytokinin production by Rhizobium. Plant Physiol. 1972 Jan;49(1):11–15. doi: 10.1104/pp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. K., Morris R. O. Nucleotide sequence and expression of a Pseudomonas savastanoi cytokinin biosynthetic gene: homology with Agrobacterium tumefaciens tmr and tzs loci. Nucleic Acids Res. 1986 Mar 25;14(6):2555–2565. doi: 10.1093/nar/14.6.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramina A. Aspects of 8-[C]Benzylaminopurine Metabolism in Phaseolus vulgaris. Plant Physiol. 1979 Feb;63(2):298–300. doi: 10.1104/pp.63.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine M., Watanabe K., Syono K. Molecular cloning of a gene for indole-3-acetamide hydrolase from Bradyrhizobium japonicum. J Bacteriol. 1989 Mar;171(3):1718–1724. doi: 10.1128/jb.171.3.1718-1724.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Nucleotide sequence of the fnr gene and primary structure of the Enr protein of Escherichia coli. Nucleic Acids Res. 1982 Oct 11;10(19):6119–6130. doi: 10.1093/nar/10.19.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Sturtevant D. B., Taller B. J. Cytokinin Production by Bradyrhizobium japonicum. Plant Physiol. 1989 Apr;89(4):1247–1252. doi: 10.1104/pp.89.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P. Rhizobium infection and nodulation: a beneficial plant disease? Annu Rev Microbiol. 1983;37:399–424. doi: 10.1146/annurev.mi.37.100183.002151. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Palm C. J., Brooks B., Kosuge T. Nucleotide sequences of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6522–6526. doi: 10.1073/pnas.82.19.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]


