Abstract
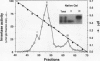
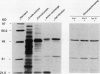
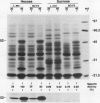
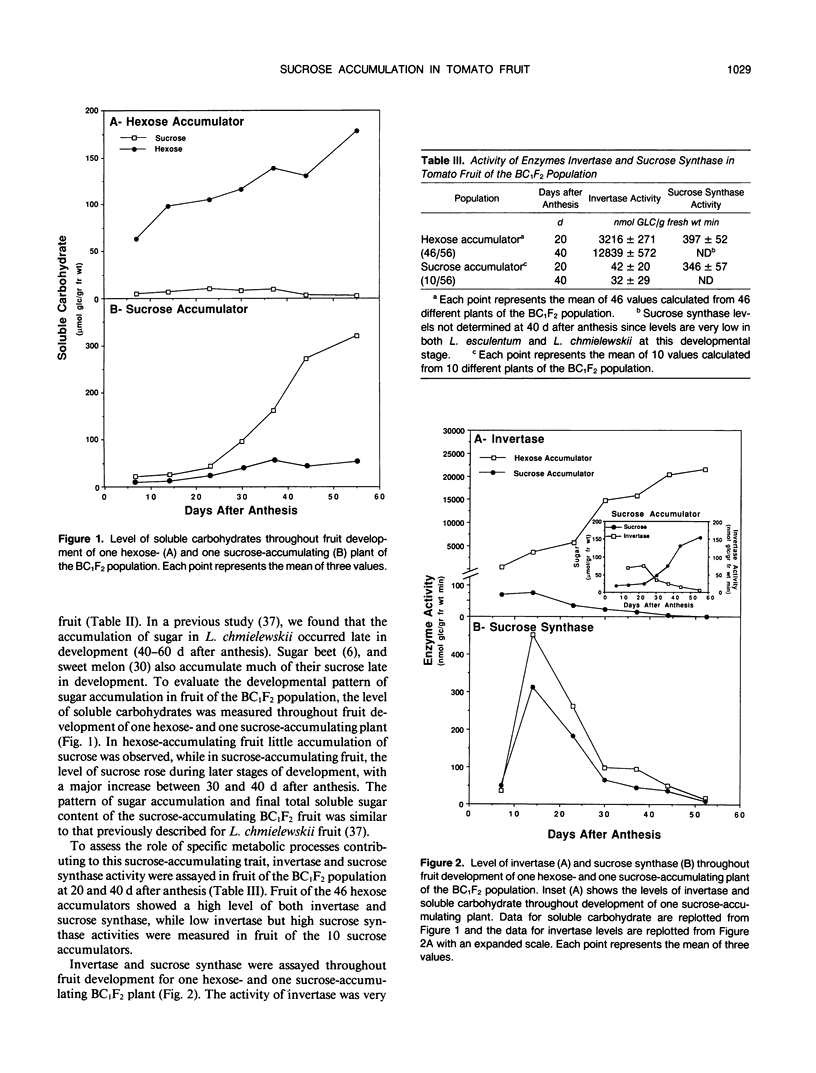
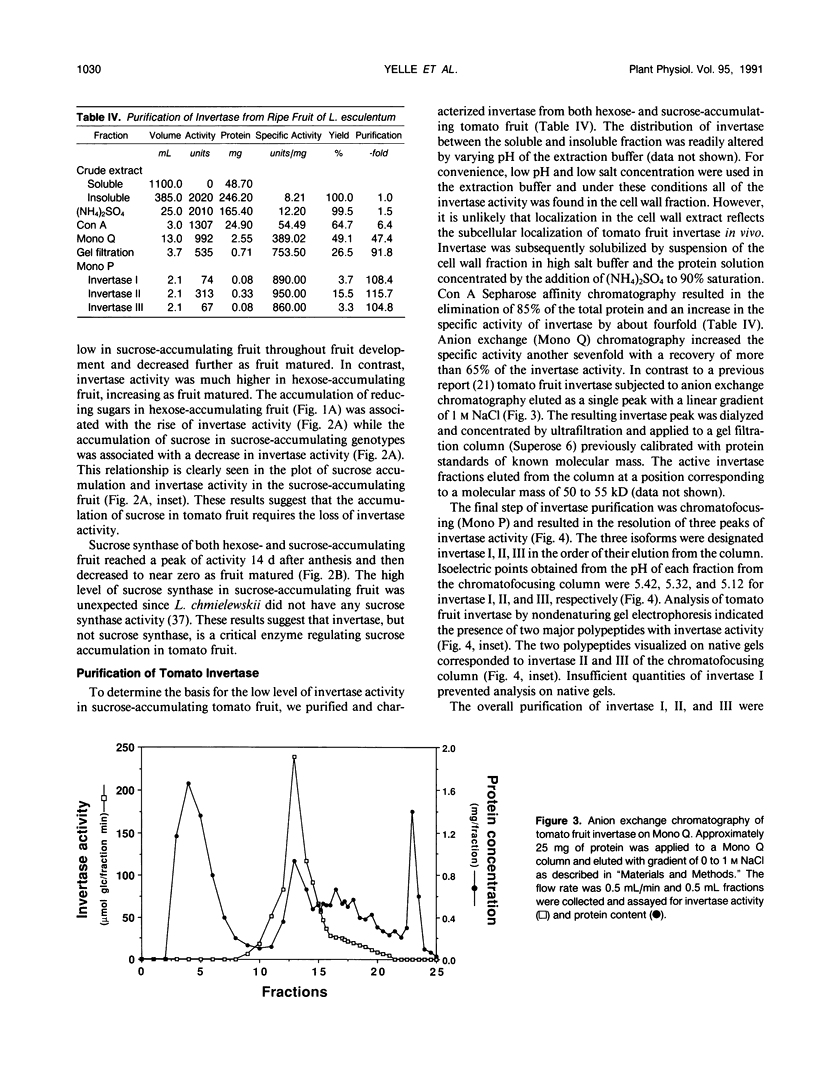
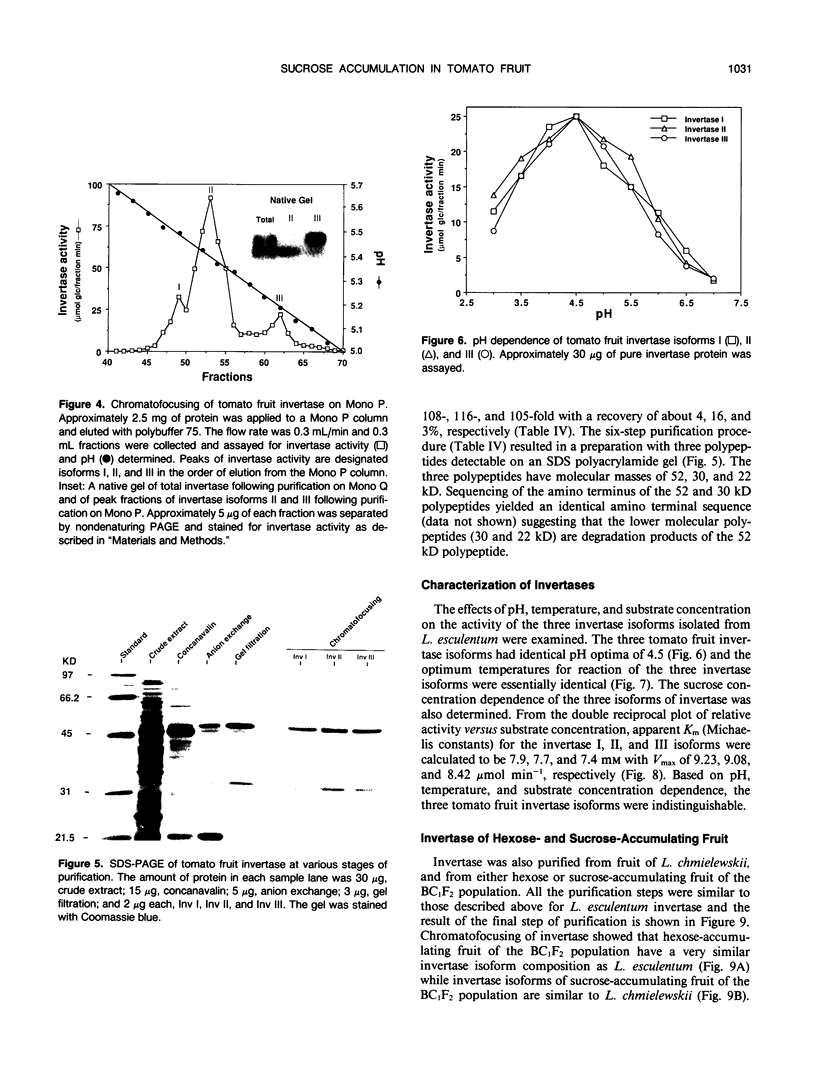
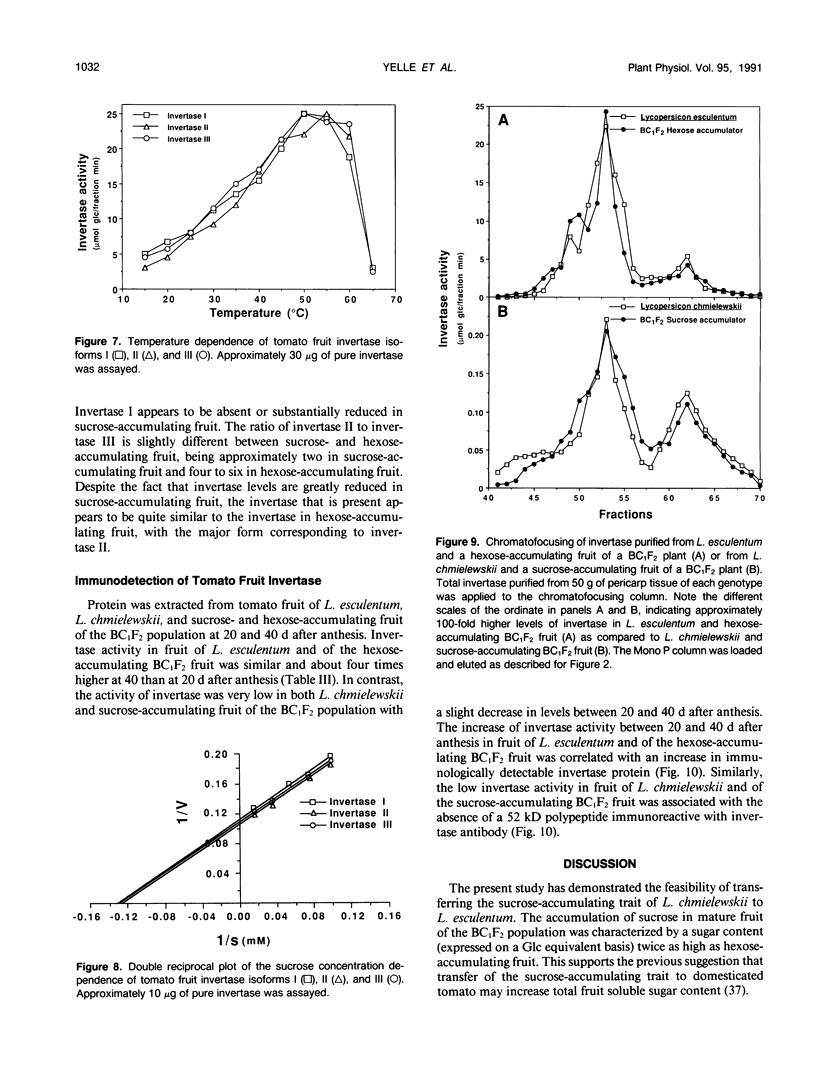
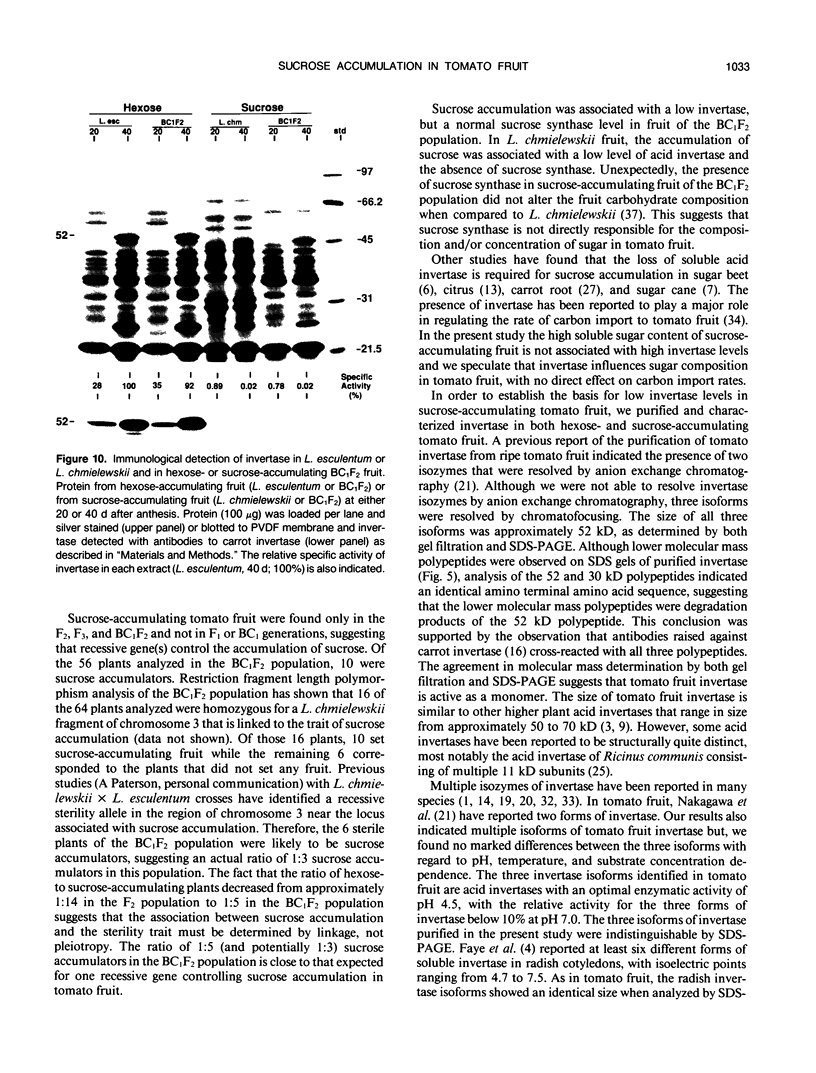
Fruit of domesticated tomato (Lycopersicon esculentum) accumulate primarily glucose and fructose, whereas some wild tomato species, including Lycopersicon chmielewskii, accumulate sucrose. Genetic analysis of progeny resulting from a cross between L. chmielewskii and L. esculentum indicated that the sucrose-accumulating trait could be stably transferred and that the trait was controlled by the action of one or two recessive genes. Biochemical analysis of progeny resulting from this cross indicated that the sucrose-accumulating trait was associated with greatly reduced levels of acid invertase, but normal levels of sucrose synthase. Invertase from hexose-accumulating fruit was purified and could be resolved into three isoforms by chromatofocusing, each with isoelectric points between 5.1 and 5.5. The invertase isoforms showed identical polypeptide profiles on sodium dodecyl sulfate polyacrylamide gel electrophoresis, consisting of a primary 52 kilodalton polypeptide and two lower molecular mass polypeptides that appear to be degradation products of the 52 kilodalton polypeptide. The three invertase isoforms were indistinguishable based on pH, temperature, and substrate concentration dependence. Immunological detection of invertase indicated that the low level of invertase in sucrose-accumulating fruit was due to low levels of invertase protein rather than the presence of an invertase inhibitor. Based on comparison of genetic and biochemical data we speculate that a gene either encoding tomato fruit acid invertase or one required for its expression, plays an important role in determining sucrose accumulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davies S. C., McWilliam A. C., Hewitt P. E., Devenish A., Brozovic M. Red cell alloimmunization in sickle cell disease. Br J Haematol. 1986 Jun;63(2):241–245. doi: 10.1111/j.1365-2141.1986.tb05546.x. [DOI] [PubMed] [Google Scholar]
- Faye L., Mouatassim B., Ghorbel A. Cell Wall and Cytoplasmic Isozymes of Radish beta-Fructosidase Have Different N-Linked Oligosaccharides. Plant Physiol. 1986 Jan;80(1):27–33. doi: 10.1104/pp.80.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel O., Wang S. F. Determination of enzymatic activity in polyacrylamide gels. I. Enzymes catalyzing the conversion of nonreducing substrates to reducing products. Anal Biochem. 1969 Mar;27(3):545–554. doi: 10.1016/0003-2697(69)90068-2. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T. Sucrose translocation and storage in the sugar beet. Plant Physiol. 1979 May;63(5):828–832. doi: 10.1104/pp.63.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. II. Relationship of Invertase Activity to Sugar Content & Growth Rate in Storage Tissue of Plants Grown in Controlled Environments. Plant Physiol. 1963 May;38(3):344–348. doi: 10.1104/pp.38.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Blanchette J. T., Okita T. W. Wheat invertases : characterization of cell wall-bound and soluble forms. Plant Physiol. 1985 Jun;78(2):241–245. doi: 10.1104/pp.78.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurière C., Laurière M., Sturm A., Faye L., Chrispeels M. J. Characterization of beta-fructosidase, an extracellular glycoprotein of carrot cells. Biochimie. 1988 Nov;70(11):1483–1491. doi: 10.1016/0300-9084(88)90285-4. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Rees T., Fuller W. A., Banfield J. The location of acid invertase activity and sucrose in the vacuoles of storage roots of beetroot (Beta vulgaris). Biochem J. 1979 Mar 15;178(3):539–547. doi: 10.1042/bj1780539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Sugawara S. Purification and Some Properties of Cell Wall-bound Invertases from Sugar Beet Seedlings and Aged Slices of Mature Roots. Plant Physiol. 1980 Jul;66(1):93–96. doi: 10.1104/pp.66.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Takahashi T., Sugawara S. Acid and alkaline invertases in suspension cultures of sugar beet cells. Plant Physiol. 1988 Jan;86(1):312–317. doi: 10.1104/pp.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F. E., Vattuone M. A., Fleischmacher O. L., Sampietro A. R. Purification and characterization of Ricinus communis invertase. J Biol Chem. 1985 Apr 25;260(8):4952–4957. [PubMed] [Google Scholar]
- Robinson N. L., Hewitt J. D., Bennett A. B. Sink metabolism in tomato fruit : I. Developmental changes in carbohydrate metabolizing enzymes. Plant Physiol. 1988 Jul;87(3):727–730. doi: 10.1104/pp.87.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno G. L., Gamundi S. S., Pontis H. G. A procedure for the assay of sucrose synthetase and sucrose phosphate synthetase in plant homogenates. Anal Biochem. 1979 Feb;93(1):196–199. [PubMed] [Google Scholar]
- Silvius J. E., Snyder F. W. Comparative Enzymic Studies of Sucrose Metabolism in the Taproots and Fibrous Roots of Beta vulgaris L. Plant Physiol. 1979 Dec;64(6):1070–1073. doi: 10.1104/pp.64.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. B., Knox R. B. Invertases of Lilium Pollen : Characterization and Activity during In Vitro Germination. Plant Physiol. 1984 Mar;74(3):510–515. doi: 10.1104/pp.74.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Zamski E., Tomos A. D. Turgor regulation of sucrose transport in sugar beet taproot tissue. Plant Physiol. 1986 Jun;81(2):478–481. doi: 10.1104/pp.81.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle S., Hewitt J. D., Robinson N. L., Damon S., Bennett A. B. Sink Metabolism in Tomato Fruit : III. Analysis of Carbohydrate Assimilation in a Wild Species. Plant Physiol. 1988 Jul;87(3):737–740. doi: 10.1104/pp.87.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]