Abstract
Hsc66, a stress-70 protein, and Hsc20, a J-type accessory protein, comprise a newly described Hsp70-type chaperone system in addition to DnaK-DnaJ-GrpE in Escherichia coli. Because endogenous substrates for the Hsc66-Hsc20 system have not yet been identified, we investigated chaperone-like activities of Hsc66 and Hsc20 by their ability to suppress aggregation of denatured model substrate proteins, such as rhodanese, citrate synthase, and luciferase. Hsc66 suppressed aggregation of rhodanese and citrate synthase, and ATP caused effects consistent with complex destabilization typical of other Hsp70-type chaperones. Differences in the activities of Hsc66 and DnaK, however, suggest that these chaperones have dissimilar substrate specificity profiles. Hsc20, unlike DnaJ, did not exhibit intrinsic chaperone activity and appears to function solely as a regulatory cochaperone protein for Hsc66. Possible interactions between the Hsc66-Hsc20 and DnaK-DnaJ-GrpE chaperone systems were also investigated by measuring the effects of cochaperone proteins on Hsp70 ATPase activities. The nucleotide exchange factor GrpE did not stimulate the ATPase activity of Hsc66 and thus appears to function specifically with DnaK. Cross-stimulation by the cochaperones Hsc20 and DnaJ was observed, but the requirement for supraphysiological concentrations makes it unlikely that these interactions occur significantly in vivo. Together these results suggest that Hsc66-Hsc20 and DnaK-DnaJ-GrpE comprise separate molecular chaperone systems with distinct, nonoverlapping cellular functions.
The Hsp70, or stress-70, protein family is a ubiquitous class of proteins of ∼70 kDa, which function as ATP-dependent molecular chaperones (reviewed in references 5, 16, 17, 20, 21, 34, and 36). Hsp70 proteins have been shown to play roles in de novo protein folding, degradation of misfolded proteins, membrane trafficking, regulatory processes, and maintaining cell viability upon stress. A number of different Hsp70 isoforms have been identified in eukaryotes, but only a single Hsp70, DnaK, has been characterized in prokaryotes. Recently, a second prokaryotic family member, encoded by the hscA gene and designated Hsc66 (for heat shock cognate Mr of ∼66 kDa), was identified in Escherichia coli (26, 54). DNA sequence data from a number of other bacteria, including Actinobacillus actinomycetemcomitans (47), Azotobacter vinelandii (68), Buchnera aphidicola (8), Haemophilus influenzae (14), Neisseria gonorrhoeae (48), Neisseria meningitidis (39), Pseudomonas aeruginosa (44), and Salmonella typhimurium (63) indicate that Hsc66 in addition to DnaK occurs widely.
The cellular function of Hsc66 has not been determined. In E. coli, Hsc66 is constitutively expressed at a level similar to that of DnaK, comprising ∼1% of the total cellular protein, but unlike DnaK, Hsc66 levels do not increase significantly upon heat shock (62). The high constitutive expression of Hsc66 and lack of induction by thermal stress suggest an important cellular role under normal growth conditions. Disruption of the hscA gene in E. coli, however, does not result in any gross phenotypic changes (26, 63) and has not as yet provided insight into the function of Hsc66. In contrast, dnaK null mutants have major growth defects (6, 41), suggesting that Hsc66 has function(s) separate from those of DnaK. ATPase activity consistent with its role as an ATP hydrolysis-coupled chaperone has been demonstrated for Hsc66 (62), but chaperone-like activities (prevention of protein aggregation and assisted protein folding) and coupling of ATP binding and hydrolysis with polypeptide binding affinity have not been shown.
The chaperone activities of DnaK and other Hsp70 chaperones are regulated by DnaJ and Hsp40 accessory proteins (∼40 kDa) which stimulate the ATPase activity of the chaperone (31), and this interaction is mediated by an N-terminal J-domain segment (27, 64). The ATPase activity of Hsc66 is regulated by Hsc20 (62), a 20-kDa protein encoded by the hscB gene (27). The N-terminal 70-residue sequence of Hsc20 exhibits similarities to the N-terminal J-domain sequence of DnaJ and Hsp40 proteins, including the His-Pro-Asp J-motif signature sequence (3) and hydrophobic core residues observed in J-domain nuclear magnetic resonance structures (43, 45, 59). The remainder of Hsc20 (residues 71 to 171), on the other hand, is not homologous to the C-terminal region of DnaJ or other Hsp40 proteins and lacks the Gly- and Phe-rich, Cys-rich zinc finger, and C-terminal segments shown to be important for both Hsp70 interactions and J-protein chaperone activity (57, 64). Homologs of the hscB (Hsc20) gene are also found adjacent to the hscA (Hsc66) gene in each of the organisms listed above. Hsc20 thus appears to represent a new subfamily of J-type cochaperones. These “small Jac’s” (for J-type accessory chaperones) (∼20 kDa) each contain a N-terminal J-domain presumed to mediate interactions with Hsc66 and a unique C-terminal domain whose function is unknown. The similarity of the J-domain of Hsc20 to that of DnaJ raises the question of whether “cross-talk” between the two chaperone systems might occur, i.e., interaction of Hsc20 with DnaK as well as with Hsc66 and interaction of DnaJ with Hsc66 as well as with DnaK. DnaK is additionally subject to regulation by GrpE, which facilitates exchange between ADP and ATP (31), but possible interactions between GrpE and Hsc66 have not been investigated.
To investigate the chaperone activity of Hsc66, we have studied its ability to prevent aggregation of three model substrate proteins (rhodanese, citrate synthase, and luciferase) as well as nucleotide effects on this activity. We have also investigated possible interactions between the Hsc66-Hsc20 and DnaK-DnaJ-GrpE chaperone systems by measuring cross-stimulation of chaperone ATPase activities.
MATERIALS AND METHODS
Materials.
The DnaK expression plasmid pJM2 was provided by G. C. Walker. E. coli W3110 was from the American Type Culture Collection (ATCC 27325), DH5αF′IQ cells were from Gibco-BRL, and BL21(DE3)pLysS cells were from Novagen. Enzymes for DNA manipulation were obtained from Boehringer-Mannheim Corp., New England Biolabs, Inc., or U.S. Biochemical Corp. Synthetic oligonucleotides were obtained from Operon Technologies. Bacterial growth medium components were from Difco, and other reagents were from Sigma Chemical Co.
Overexpression and purification of Hsc66, Hsc20, DnaK, DnaJ, and GrpE.
Hsc66, Hsc20, and DnaK were expressed and purified as previously described (62). The DnaJ and GrpE expression vectors, pTrcDnaJ and pTrcGrpE, respectively, were constructed by PCR amplification of their genes from genomic DNA isolated from E. coli K-12 strain W3110 and cloning them into pTrc99a (Pharmacia).
BL21 cells transformed with pTrcDnaJ were grown in Terrific broth (51) at 37°C. Protein expression was induced with 0.5 mM isopropylthio-β-d-galactoside (IPTG) at an A600 of ∼1. After ∼16 h, cells were harvested by centrifugation, frozen, thawed, and lysed in a French pressure cell in a solution containing 50 mM HEPES (pH 8.0), 0.5 mM EDTA, 1 mM dithiothreitol (DTT) with 0.1% Triton X-100, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF) to inhibit proteolysis. The supernatant fluid following centrifugation at 29,000 × g for 30 min was combined and diluted with an equal volume of a solution containing 100 mM HEPES (pH 6.5), 1 mM DTT, and 0.5 mM EDTA. This solution was passed over a DEAE-cellulose column (DE-52; Whatman), and the unbound material was loaded on a cation-exchange column (Bio-Rex 70; Bio-Rad). DnaJ was eluted from this column by using a 2-liter linear gradient from 200 to 700 mM NaCl. Those fractions appearing homogeneous by gel electrophoresis were combined and dialyzed against buffer containing 50 mM HEPES (pH 7.2), 0.5 mM EDTA, 1 mM DTT, 100 mM NaCl, and 0.02% Triton X-100. The final preparation, which did not exhibit any detectable ATPase activity, was centrifuged for 20 min at 24,000 × g, concentrated to ∼35 mg of protein/ml by ultrafiltration, frozen in liquid nitrogen, and stored at −70°C.
DH5αF′IQ cells transformed with pTrcGrpE were grown in Terrific broth (51), induced with 0.5 mM IPTG at A600 of ∼1.4, and grown for ∼16 h to allow expression. Cells were harvested by centrifugation, frozen, thawed, and lysed in TED buffer (50 mM Tris-HCl [pH 8.1], 0.5 mM EDTA, 1 mM DTT, 0.1 mM PMSF) in a French pressure cell. The soluble supernatant fluid following centrifugation at 39,000 × g for 30 min was diluted to a 1-liter total volume with TED buffer and loaded on a DEAE-cellulose column (DE-52; Whatman). GrpE was eluted from this column by using a 1-liter linear gradient from 0 to 400 mM NaCl. Fractions shown to contain GrpE by gel electrophoresis were combined, diluted to ∼1 liter with TED buffer, and loaded on a DEAE-Sepharose column (Q-Sepharose; Pharmacia). GrpE was eluted from this column by using a 1-liter linear gradient from 0 to 200 mM NaCl. Fractions containing GrpE were combined, concentrated, applied to a Sephadex G-100 column, and eluted in TED buffer containing 200 mM NaCl. Fractions appearing homogeneous by gel electrophoresis were combined and dialyzed against TED buffer. The preparation did not exhibit any detectable ATPase activity and was concentrated to ∼47 mg of protein/ml by ultrafiltration, frozen in liquid nitrogen, and stored at −70°C.
Analytical methods.
Spectrophotometric measurements were performed with a Cary 1 spectrophotometer. The extinction coefficients of all proteins at 280 nm were calculated by using average absorptivities for tryptophan and tyrosine of 5,600 and 1,400 (M · cm)−1, respectively (18, 33, 40). The extinction coefficients at 280 nm (M · cm)−1 were as follows: Hsc20, 16,800; Hsc66, 19,600; DnaK, 15,400; DnaJ, 14,000; GrpE, 1,400; rhodanese, 60,200; citrate synthase, 77,000; luciferase, 37,800. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed by the method of Laemmli (28). Determination of GrpE binding to prokaryotic Hsp70 proteins was accomplished with a Superdex 200 HR 10/31 column on a fast protein liquid chromatography system (Pharmacia) by the method of Schonfeld et al. (52).
Rhodanese aggregation assays.
Rhodanese from bovine liver (Sigma Chemical Co.) was stored at −70°C in aliquots of 10 mg/ml in 100 mM Tris-HCl (pH 7.8)–5 mM DTT. Denaturation was accomplished by diluting samples sevenfold into denaturation solution (6 M guanidine hydrochloride, 100 mM HEPES [pH 7.5, adjusted with NaOH], 150 mM KCl, 24 mM NaCl, 10 mM DTT) and incubating this solution at 25°C for 1 h. Aggregation assays were performed in a cuvette with a 1-cm path length containing a reaction volume of 1 ml, and the solution contained assay buffer (100 mM HEPES [pH 7.5] [adjusted with NaOH], 150 mM KCl, 24 mM NaCl, 10 mM MgCl2), and nucleotides and phosphate as indicated, as well as various concentrations of chaperones. Nucleotide concentrations were 400 μM in all assays unless indicated otherwise. Because commercial samples of ADP (Sigma catalog no. A2754) were found to contain ∼2.5% ATP, 1 mM phosphate was added to increase the affinity for ADP (50) and minimize effects due to the contamination. The aggregation assay solution was preequilibrated for 5 min at 25°C prior to addition of denatured rhodanese. Rhodanese was then diluted 20-fold into the aggregation assay solution and mixed rapidly for 15 s, and its aggregation was monitored continuously for 20 min by measuring turbidity changes at 320 nm.
Citrate synthase thermal aggregation assays.
Citrate synthase from porcine liver (Sigma Chemical Co.) was stored as a 158 μM (8.2-mg/ml) crystalline suspension at 4°C. Thermal aggregation was performed by incubating a solution containing assay buffer, nucleotides, and indicated amounts of chaperones at 43°C for 10 min. Crystalline citrate synthase was diluted 100-fold into this mixture and mixed rapidly for 15 s, and aggregation was monitored by measured changes at 320 nm as described by Lee (29). Assays were carried out at 43°C in a 1-ml cuvette with a 1-cm path length without stirring for 30 min.
Luciferase aggregation assays.
Luciferase (Sigma Chemical Co.) was stored at 10 mg/ml in 1 M glycylglycine (pH 7.4). Luciferase was denatured by diluting it 10-fold into luciferase denaturation solution (25 mM HEPES [pH 7.5], 50 mM KCl, 5 mM MgCl2, 5 mM DTT, 6 M guanidine hydrochloride), and this mixture was incubated at 25°C for 1 h. Aggregation was performed by diluting denatured luciferase 100-fold into a solution containing assay buffer and indicated amounts of chaperones. Aggregation was measured by monitoring changes in turbidity at 320 nm. Assays were carried out in a 1-ml cuvette with a 1-cm path length for 10 min.
Luciferase refolding assays.
Luciferase was denatured by diluting it 10-fold into luciferase denaturation solution, and this mixture was incubated at 30°C for 30 min. Denatured luciferase was diluted 100-fold into refolding buffer (25 mM HEPES [pH 7.5], 50 mM KCl, 5 mM MgCl2, 1 mM DTT, 1 mM ATP) and various combinations of chaperones. The concentrations of chaperones used were 4 μM DnaK, 4 μM Hsc66, 0.5 μM DnaJ, 1 μM GrpE, and 0.5 to 10 μM Hsc20. The refolding reaction was incubated at 30°C and assayed for activity 40 min after addition of denatured luciferase. Luciferase activity assays were performed by diluting 10 μl of the refolded reaction mixture into 200 μl of luciferase assay solution (50 mM HEPES [pH 7.5], 50 mM KCl, 5 mM magnesium acetate, 5 mM DTT, 0.3 mM coenzyme A, 0.5 mM luciferin, 1 mM ATP). Activities were determined at 23°C by direct counting of photons from a cuvette (0.3 by 0.3 mm) for 1 min with a SLM-Aminco 8100 fluorometer. Activities are expressed as percentages of the activity measured for a sample not subjected to denaturation.
ATPase assays.
ATPase activities were determined as described previously (62) by measuring phosphate release, using a coupled enzyme assay with the EnzChek phosphate assay kit as recommended by the manufacturer (Molecular Probes) (66). Assays were carried out in assay buffer using 1 mM DTT; reaction mixtures including DnaJ additionally contained 0.02% Triton X-100 to maintain solubility of DnaJ (this detergent had no effect on the ATPase activity of Hsc66 or DnaK in the absence of DnaJ or on standard curves). All components except the ATP were mixed together in a 1-ml reaction mixture and incubated at 25°C in the spectrophotometer for 5 min prior to starting the reaction with the addition of ATP. First-order ATPase rates were corrected for the degradation of the coupled enzyme assay substrate, 2-amino-6-mercapto-7-methylpurine ribonucleoside (MESG), and for all assay conditions, reported reaction rates were directly proportional to enzyme concentration.
RESULTS
Effects on protein aggregation.
Because no endogenous substrate proteins have been identified for Hsc66, we investigated its chaperone activity by the ability to suppress aggregation of denatured bovine rhodanese, porcine citrate synthase, and firefly luciferase. These proteins have been used previously as model substrates for other chaperones, including members of the Hsp25 (13, 24), Hsp40 (10, 12, 32, 57) Hsp70 (30, 37, 53, 58), and Hsp90 (4, 25, 67) families. For studies utilizing rhodanese and luciferase, proteins were denatured using 6 M guanidine hydrochloride prior to incubation with Hsc66. Aggregation was initiated by diluting this solution into a reaction mixture containing Hsc66 at 25°C and monitored by measuring increases in absorbance due to changes in turbidity. In the case of citrate synthase, the native protein was diluted into a reaction mixture containing Hsc66 at 43°C to mimic thermal denaturation conditions, and aggregation was monitored by measuring absorbance increases.
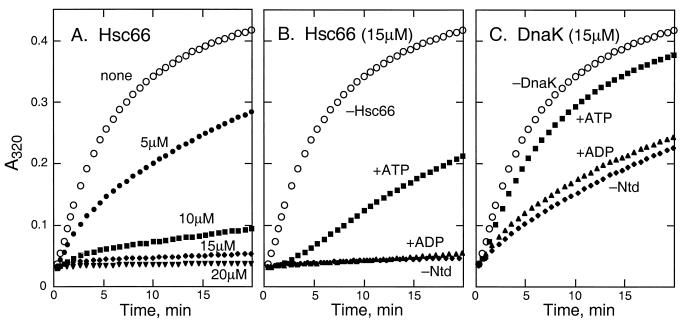
The effects of various concentrations of Hsc66 on the aggregation of denatured rhodanese are shown in Fig. 1A. Partial suppression of aggregation was observed at a molar ratio of Hsc66 to rhodanese of 2:1, but complete (>90%) suppression over the time course of the experiment required ratios of >8:1. The high concentration of Hsc66 relative to that of rhodanese required for complete protection is similar to that observed for other chaperones (67) and likely reflects both the affinity of the chaperone for the unfolded polypeptide and the presence of multiple binding sites on the unfolded protein. The effects of nucleotides on the activity of Hsc66 are shown in Fig. 1B. Under the conditions used, ADP has little or no effect on activity, whereas ATP reduces the ability of Hsc66 to suppress aggregation by about half. These findings are consistent with models for Hsp70 regulation in which ADP stabilizes a chaperone conformation having high substrate affinity, thereby affording protection from aggregation, whereas ATP favors a more rapidly exchanging state which allows for refolding and/or aggregation (35, 42, 58). Thus, the cellular ratio of ADP to ATP and their exchange rates will determine the activity of Hsc66. For comparison, a similar experiment on the effect of DnaK on rhodanese aggregation is shown in Fig. 1C. At the concentration shown, DnaK was somewhat less effective than Hsc66 in suppressing aggregation, although complete suppression was obtained with molar ratios of DnaK to rhodanese greater than 10:1 (data not shown). Nucleotides have similar effects on DnaK activity as for Hsc66, with ATP reducing the aggregation suppression and ADP having little effect.
FIG. 1.
Effects of Hsc66 and DnaK on rhodanese aggregation. (A) Aggregation of 2 μM rhodanese alone (none) and in the presence of 5, 10, 15, or 20 μM Hsc66. (B) Aggregation of 2 μM rhodanese alone (−Hsc66) and in the presence of 15 μM Hsc66 without nucleotide (−Ntd), with 400 μM ADP and 1 mM phosphate (+ADP), or with 400 μM ATP (+ATP). (C) Aggregation of 2 μM rhodanese alone (−DnaK) and in the presence of 15 μM DnaK without nucleotide (−Ntd), with 400 μM ADP and 1 mM phosphate (+ADP), or with 400 μM ATP (+ATP). Denatured rhodanese in 6 M guanidine hydrochloride was diluted into reaction mixtures, and absorbance changes at 320 nm were used to monitor aggregation at 25°C.
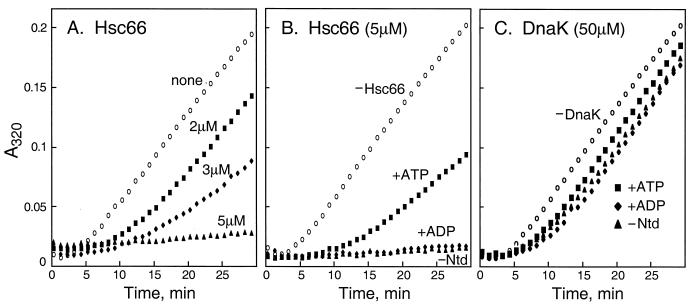
The effect of Hsc66 on the aggregation of citrate synthase is shown in Fig. 2. Figure 2A shows that aggregation is effectively suppressed at a ratio of Hsc66 to citrate synthase of >3:1. The lower molar ratio of Hsc66 required for suppression of aggregation of citrate synthase compared to that required for rhodanese may reflect the time-dependent unfolding of citrate synthase under these conditions such that the ratio of Hsc66 to denatured citrate synthase is probably higher than 3:1. The effects of nucleotides on Hsc66 chaperone activity with citrate synthase (Fig. 2B) are similar to those observed with rhodanese. Under these conditions, ADP has no effect, whereas ATP appears to favor release of polypeptide, allowing aggregation to occur more readily. In contrast to Hsc66, DnaK exhibited very little activity with citrate synthase (Fig. 2C). Even at a concentration ratio of 30:1, only a small decrease in aggregation was observed in the absence or presence of nucleotides. This difference in activity between Hsc66 and DnaK suggests that the chaperones differ in their polypeptide binding specificity profiles.
FIG. 2.
Effects of Hsc66 and DnaK on citrate synthase aggregation. (A) Aggregation of 1.6 μM citrate synthase alone (none) and in the presence of 2, 3, or 5 μM Hsc66. (B) Aggregation of 1.6 μM citrate synthase alone (−Hsc66) and in the presence of 5 μM Hsc66 without nucleotide (−Ntd), with 400 μM ADP (+ADP), or with 400 μM ATP (+ATP). (C) Aggregation of 1.6 μM citrate synthase alone (−DnaK) and in the presence of 50 μM DnaK without nucleotide (−Ntd), with 400 μM ADP (+ADP), or with 400 μM ATP (+ATP). Native citrate synthase was diluted into reaction mixtures at 43°C, and absorbance changes at 320 nm were used to monitor aggregation resulting from thermal denaturation.
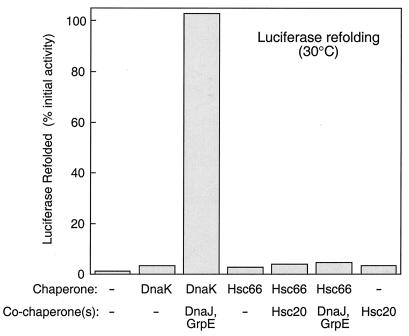
In contrast to the effects of Hsc66 on aggregation of rhodanese and citrate synthase, no aggregation suppression activity was observed with denatured firefly luciferase even using ratios of Hsc66 to luciferase of up to 100:1 (data not shown). Under similar conditions, DnaK was able to suppress luciferase aggregation by ∼30% (data not shown). As shown in Fig. 3, Hsc66 was also unable to assist refolding of denatured luciferase, whether alone or in the presence of Hsc20 or DnaJ and GrpE. DnaK, in contrast, was able to fully restore the activity of luciferase in the presence of DnaJ and GrpE (Fig. 3). These results, similar to those with citrate synthase, suggest that Hsc66 and DnaK exhibit different specificities for recognition of unfolded polypeptides.
FIG. 3.
Effects of Hsc66 and DnaK on refolding of denatured luciferase. Denatured luciferase in 6 M guanidine hydrochloride was diluted into reaction mixtures containing 1 mM ATP and incubated for 40 min at 30°C. Chaperone concentrations used were as follows: DnaK and Hsc66 (4 μM), DnaJ (0.5 μM), GrpE (1 μM), and Hsc20 (4 μM). Luciferase activities were determined at 23°C and are expressed as a percentage of the activity of a sample not subjected to denaturation. −, no chaperone or cochaperone.
Possible chaperone activity of the cochaperone Hsc20 was also investigated using the rhodanese, citrate synthase, and luciferase aggregation and refolding assays. Hsc20 alone had no effect (<10% reduction) on aggregation rates of any of these proteins when tested at concentration ratios up to a 25-fold molar excess (data not shown). This can be contrasted with findings for DnaJ and Hsp40 proteins which have been found to prevent rhodanese aggregation when present at stoichiometric levels (57). Recent studies with yeast Ydj1 have implicated the C-terminal region of Hsp40 proteins in interactions with unfolded proteins (32), and Hsc20 lacks a similar region. Possible effects of Hsc20 on Hsc66 chaperone activity in the rhodanese and citrate synthase aggregation suppression assays were also studied. No effect of Hsc20 was observed in the absence of nucleotide or in the presence of ADP or 400 μM ATP (data not shown). At low levels of ATP (<80 μM), Hsc20 enhanced the activity of Hsc66. This effect, however, appears to result from stimulation of the ATPase activity of Hsc66, rather than a direct effect on peptide binding. At ATP concentrations of <80 μM, ATP is depleted during the course of the assay, and the resulting ADP complex is expected to exhibit greater aggregation suppression. Thus, Hsc20 appears to stimulate chaperone activity solely by elevating the ATPase activity of Hsc66. This can be contrasted with DnaJ, which not only stimulates the ATPase activity of DnaK but also acts to target peptide substrates to the chaperone (58).
ATPase activities of the Hsc66 and DnaK chaperone systems.
Because the chaperone activities of Hsp70 proteins are regulated by nucleotides, it was of interest to compare the ATPase activities of the Hsc66-Hsc20 and DnaK-DnaJ-GrpE systems. For this purpose, assays were performed with chaperone and cochaperone concentrations approximating those which occur in vivo. The cellular concentrations of Hsc66 and Hsc20 are estimated to be ∼20 and ∼10 μM, respectively (62), and those of DnaK, DnaJ, and GrpE are ∼20, ∼1, and ∼10 μM, respectively, under nonstress conditions (1, 23, 38). Table 1 shows turnover numbers (in moles of Pi produced per mole of chaperone per minute) for Hsc66 and DnaK in the presence and absence of their respective cochaperones. Assays were performed with 400 μM ATP, which should yield maximal activities, assuming that Hsc66 has high affinity for ATP as found for DnaK (Km of ∼1 nM) (50). Under these conditions, the intrinsic ATPase activity of Hsc66 (in the absence of Hsc20) is approximately threefold higher than that for DnaK alone. In the presence of a physiological level of Hsc20, the activity of Hsc66 was stimulated ca. twofold, whereas a physiological level of DnaJ stimulated DnaK to a greater extent, ∼5.5-fold. Because of the higher intrinsic ATPase activity of Hsc66, however, the Hsc66-Hsc20 and DnaK-DnaJ reaction mixtures exhibit similar total activities. Addition of GrpE, which catalyzes nucleotide exchange with DnaK, increased the overall ATPase rate of the DnaK-DnaJ-GrpE system to a value ca. twofold greater than that observed for the Hsc66-Hsc20 system. These results suggest that the Hsc66-Hsc20 and DnaK-DnaJ-GrpE systems have roughly similar ATPase activities, but it should be emphasized that actual physiological ATPase activities of the two chaperone systems will depend critically on the exact cellular concentrations of the cochaperone proteins (see below) as well as any additional regulatory factors that remain to be identified.
TABLE 1.
ATPase activities of Hsc66-Hsc20 and DnaK-DnaJ-GrpE chaperone systemsa
| Chaperone(s) | Turnover (min−1) |
|---|---|
| 20 μM Hsc66 | 0.12 |
| + 10 μM Hsc20 | 0.24 |
| 20 μM DnaK | 0.04 |
| + 1 μM DnaJ | 0.22 |
| + 1 μM DnaJ + 10 μM GrpE | 0.47 |
Assays were carried out using 400 μM ATP in 100 mM HEPES (pH 7.5)–150 mM KCl–24 mM NaCl–1 mM DTT; reaction mixtures including DnaJ additionally contained 0.02% Triton X-100.
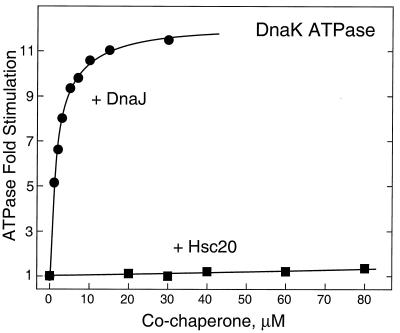
We next investigated the possibility of interactions between the Hsc66-Hsc20 and DnaK-DnaJ-GrpE systems by assaying for cross-stimulation of the ATPase activity of Hsc66 and DnaK by Hsc20, DnaJ, and/or GrpE. Figure 4 compares the effects of Hsc20 and DnaJ on the ATPase activity of Hsc66. The results are plotted as the increase in ATPase activity relative to the activity in the absence of cochaperone. Assuming 1:1 stoichiometry, hyperbolic saturation curves were obtained when the data were corrected for bound cochaperone. Extrapolation to saturating levels of Hsc20 indicate a maximal stimulation of approximately sixfold, and the concentration of Hsc20 required for half-maximal stimulation (∼12 μM) is in the same range as the estimated cellular concentration of the cochaperone. DnaJ stimulated Hsc66 to a similar maximal extent (ca. fivefold), but the concentration required for half-maximal stimulation was ∼110 μM. This value is ∼100-fold higher than the cellular level of DnaJ under nonstress conditions and ∼10-fold greater than under heat shock conditions (1), suggesting that DnaJ is not likely to affect the activity of Hsc66 to a significant extent in vivo.
FIG. 4.
Effects of Hsc20 and DnaJ on ATPase activity of Hsc66. Increases in basal ATPase activities at 25°C are plotted as a function of free cochaperone concentration. The concentration of Hsc66 was 5 μM. The ATP concentration used was 400 μM, and the buffer contained 0.1 M HEPES (pH 7.5), 0.15 M KCl, 24 mM NaCl, and 10 mM MgCl2. Concentrations of free cochaperone were calculated assuming 1:1 binding stoichiometry with Hsc66 by using the equation [cochaperone]free = [cochaperone]total − E × ΔV/Vmax, where [cochaperone]total is the concentration of added cochaperone, ΔV is the observed rate change at [cochaperone]total, E is the total concentration of Hsc66, and ΔVmax is the rate increase extrapolated to infinite cochaperone concentration. Curves represent calculated hyperbolic saturation functions, assuming maximal stimulation of 6.3-fold for Hsc20 and 5.4-fold for DnaJ and half-maximal stimulation at 12 μM for Hsc20 and 100 μM for DnaJ.
Figure 5 shows a comparison of the effects of Hsc20 and DnaJ on the ATPase activity of DnaK. Hsc20 stimulated DnaK only very weakly at the concentrations tested. Maximal stimulation was estimated to be ca. twofold with half-maximal stimulation requiring >200 μM Hsc20, a value ∼20-fold higher than normal cellular levels of Hsc20 (62). DnaJ, in contrast, stimulated DnaK ATPase activity ∼12-fold, and half-maximal stimulation occurred at ∼1.8 μM DnaJ, near the cellular concentration for DnaJ (1). The high, nonphysiological concentrations of Hsc20 and DnaJ required for cross-stimulation of DnaK and Hsc66, respectively, suggest Hsc66 and Hsc20 comprise a chaperone pair that functions separately from DnaK and DnaJ in vivo.
FIG. 5.
Effects of Hsc20 and DnaJ on ATPase activity of DnaK. Increases in basal ATPase activity at 25°C are plotted as a function of total cochaperone concentration. The concentration of DnaK was 20 μM; other conditions are as given in the legend to Fig. 3. Curves represent hyperbolic saturation functions, assuming a maximal stimulation of 2.3-fold and half-maximal stimulation at 280 μM for Hsc20 and 12.2-fold maximal stimulation and half-maximal stimulation at 1.8 μM for DnaJ.
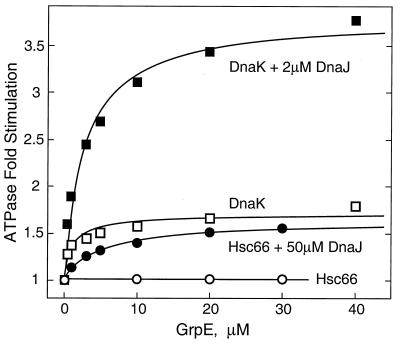
GrpE, which functions as a nucleotide exchange factor and stimulates the ATPase activity of DnaK (31), was also assayed for effects on the ATPase activity of Hsc66 (Fig. 6). No stimulation was observed at GrpE concentrations of up to 30 μM either in the presence or absence of Hsc20. When assays were performed in the presence of high concentrations of DnaJ, however, a small degree of stimulation was observed, and the effect of GrpE on Hsc66 ATPase activity in the presence of 50 μM DnaJ is shown. Extrapolation to saturating concentrations of GrpE indicates a maximal stimulation of ∼1.6-fold with half-maximal stimulation at ∼5 μM GrpE. This effect can be contrasted with the effects of GrpE on the ATPase activity of DnaK (Fig. 6; see also reference 31). GrpE stimulates DnaK in the absence of DnaJ (maximal stimulation ∼1.7-fold, half-maximal stimulation ∼1.2 μM) and with physiological concentrations of DnaJ causes even greater stimulation (3.8-fold above the DnaJ-stimulated activity and ∼53-fold above DnaK alone). Previous studies have also shown that GrpE is able to form a stable 2:1 complex with DnaK in the absence of nucleotides (52), and we used size exclusion chromatography to investigate the possibility of interactions of GrpE with Hsc66 which may not be manifested as effects on ATPase activity. No complex formation could be detected between Hsc66 and GrpE, whereas a GrpE-DnaK complex was observable under identical conditions (data not shown). These findings suggest that while GrpE is able to stimulate the ATPase activity of Hsc66 to a small degree in the presence of supraphysiological levels of DnaJ, GrpE is not likely to function as a cochaperone with Hsc66 under normal cellular conditions.
FIG. 6.
Effects of GrpE concentration on the ATPase activity of Hsc66 and DnaK. Increases in basal ATPase activity at 25°C are plotted as a function of total GrpE concentration. Five micromolar Hsc66 or 10 μM DnaK was used where indicated. Other conditions are as described in the legend to Fig. 3. The curves shown represent hyperbolic saturation functions, assuming a maximal stimulation of 1.6-fold for Hsc66 with DnaJ, 1.7-fold for DnaK in the absence of DnaJ, and 3.8-fold for DnaK with DnaJ, with half-maximal stimulation at GrpE concentrations of 5.0, 1.2, and 2.8 μM, respectively.
DISCUSSION
The bacterial DnaK, DnaJ, and GrpE proteins comprise one of the first chaperone systems described and remain the prototypical Hsp70 model. It had been generally assumed that in prokaryotes this single Hsp70 system was sufficient for cellular functioning, and the discovery of genes encoding a second Hsp70-type chaperone (hscA) and J-type cochaperone (hscB) in E. coli was surprising (26, 54). (Other genes encoding proteins exhibiting sequence similarities to regions of DnaK and Hsp70 proteins and DnaJ and Hsp40 proteins have subsequently been identified in E. coli [60].) The amino acid sequence of the hscA gene product, Hsc66, suggests that its overall structure is similar to those of DnaK and other Hsp70 proteins, but the relatively low overall sequence identity (∼40%) suggests that important functional differences may exist between the two chaperones. In addition, the hscB gene product, Hsc20, differs significantly from DnaJ and other J-type cochaperones, exhibiting low (<15%) amino acid sequence identity in the N-terminal J-domain and having a short C-terminal domain in place of other segments commonly found in Hsp40 proteins. These differences between Hsc66 and Hsc20 compared to other Hsp70 systems raise questions regarding the function and regulation of the Hsc66-Hsc20 system.
In previous studies, we found that Hsc66 possesses a low basal ATPase activity typical of Hsp70 proteins and that this intrinsic activity is stimulated by Hsc20 (62). The results described herein show that when assayed using concentrations approximating those found in vivo, the Hsc66-Hsc20 and DnaK-DnaJ-GrpE systems exhibit roughly similar ATP hydrolysis activities, suggesting that the two systems may possess similar chaperone capacities in vivo. Furthermore, studies on suppression of aggregation of denatured model substrate proteins establish that Hsc66 exhibits chaperone activity in a nucleotide-dependent manner, as expected for an ATP hydrolysis-coupled system. The results are consistent with the generally accepted model for Hsp70 action (42) in which ATP destabilizes Hsc66-peptide complexes, and peptide binding and release are coupled to ATP hydrolysis and ADP-ATP exchange rates. Hsc20, on the other hand, does not exhibit intrinsic chaperone activity and appears to act strictly as a cochaperone to regulate the ATPase activity of Hsc66. This can be contrasted with the role of DnaJ, which can associate with substrate proteins and exhibits intrinsic chaperone activity in addition to its regulatory effects on DnaK ATPase activity (22, 57). Hsc20 lacks domains commonly found in DnaJ and other Hsp40 proteins, and the function of the small C-terminal domain present in Hsc20 is not known.
Whereas none of the model substrate proteins tested are found in E. coli, the different chaperone activities of Hsc66 and DnaK imply important differences between the two chaperones. With rhodanese, both Hsc66 and DnaK were effective in suppressing aggregation, although slightly lower molar ratios of Hsc66 were required for complete protection. With citrate synthase and luciferase, Hsc66 was effective in suppressing aggregation only with the former, whereas DnaK was effective only with the latter. These differences in substrate specificity profiles presumably arise from structural differences in the peptide binding domains of the two chaperones. Alignment of the amino acid sequence of Hsc66 with the β-sandwich subdomain of DnaK shown to bind peptide (69) reveals that only ∼50% of the residues are conserved, and 7 of 16 residues directly contacting bound peptide in DnaK are replaced with other amino acids in Hsc66. Peptide binding preferences have not been established for Hsc66, but based on these sequence dissimilarities, they are likely to differ from those determined for DnaK (15, 49). In the case of citrate synthase, it is noteworthy that Hsc66 is active in suppressing aggregation at temperatures causing heat shock in E. coli (42°C). Our previous studies demonstrated that Hsc66 ATPase activity increases with temperature up to 50°C (62), and the chaperone and ATPase activities of Hsc66 at elevated temperatures suggest that Hsc66 maintains function under thermal stress as well as under normal growth conditions.
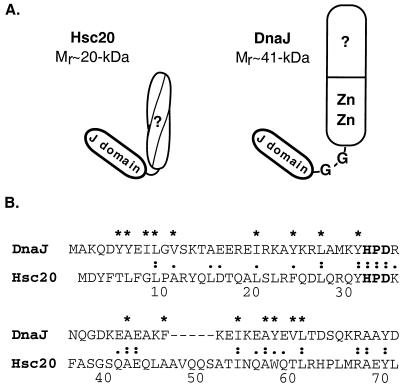
Despite differences in substrate specificity profiles, the overall similarities between Hsc66 and DnaK and between Hsc20 and DnaJ raised the question of whether the proteins function independently or whether heterologous interactions might result in “cross-talk” between the two systems. Based on the results obtained here using ATPase stimulation as a measure of cross-reactivity, the requirement for supraphysiological cochaperone concentrations for cross-stimulation make it appear unlikely that these interactions occur to any significant extent in vivo. The finding that DnaJ can stimulate Hsc66 ATPase activity when present at sufficiently high concentrations to favor binding, however, suggests that some key structural features are shared by DnaJ and Hsc20. Figure 7A shows schematic representations of Hsc20 and DnaJ. Both proteins have N-terminal J-domains of ∼70 residues, but DnaJ has a large C-terminal segment (∼33 kDa) containing a glycine-rich linker region, a cysteine-rich zinc finger-like region, and a peptide binding region (7, 11, 46, 56), whereas Hsc20 has a smaller C-terminal domain (∼12 kDa) that is predicted to fold as a coiled-coil structure (9). Studies with truncated forms of DnaJ have suggested that both the J-domain and glycine-rich linker are required for interaction with DnaK (27, 64), and the lack of a glycine-rich linker region coupled with differences in the J-domain in Hsc20 may explain the very low activity of Hsc20 with DnaK. Other studies in which the glycine-rich region was deleted, however, have shown that this did not affect the ability of DnaJ to stimulate the ATPase activity of DnaK (65). This points to differences in J-domain sequences as the primary cause of the weak interaction of Hsc20 with DnaK. In contrast, DnaJ is able to substitute for Hsc20 in stimulating the ATPase activity of Hsc66, although higher concentrations are required. The activity of DnaJ with Hsc66 implies that the major interactions with Hsc66 are mediated by the J-domain common to both DnaJ and Hsc20 and that the C-terminal domain of Hsc20 is not essential for ATPase stimulation. An alignment of the amino acid sequences of the J-domain regions of Hsc20 and DnaJ provides insight into residues which might be critical for this interaction (Fig. 7B). Residues conserved in the two proteins include the J-motif signature sequence, HPD, and adjacent amino acids as well as several residues observed to be buried in the hydrophobic cores of the DnaJ (43, 60) and Hsp40 (45) J-domains. Only residues exposed on the surface would be available for binding to Hsc66, suggesting that the YHPDK sequence at positions 31 to 35 of Hsc20 is likely to be sufficient to specify the interaction. The corresponding sequence is observed to occur in a surface-exposed loop in nuclear magnetic resonance structures of fragments of DnaJ (43) and Hsp40 (45). Mutation of the histidine residue of the HPD sequence in DnaJ (64) and Ydj1 (61) has been shown to lead to inactivation, establishing the importance of this region, and we have found that a His32→Cys mutant of Hsc20 is also inactive (55).
FIG. 7.
Comparison of Hsc20 and DnaJ proteins. (A) Schematic representations of Hsc20 and DnaJ domain structures (see text). (B) Comparison of the J-domain sequences of Hsc20 and DnaJ. The J-motif signature sequence, HPD, is shown in bold type. Identical (:) and similar (.) amino acids are indicated. Core hydrophobic residues in DnaJ are indicated with an asterisk (43, 59).
The activity of the DnaK system is also subject to regulation by GrpE, which increases rates of peptide binding and release by facilitating exchange between ADP and ATP. Analysis of the complete sequence of the E. coli K-12 genome (2) reveals a single GrpE-type protein, suggesting the possibility that GrpE could function as a nucleotide exchange factor for Hsc66 in addition to DnaK. However, no interactions between GrpE and Hsc66 were detectable by using either ATPase stimulation or size exclusion chromatography. Analysis of the crystal structure of the DnaK-GrpE complex reveals a large number of contacts between the two proteins that span multiple regions of the ATPase domain (19). Alignment of the sequence of Hsc66 with DnaK reveals that only 4 of 21 amino acids in DnaK in contact with GrpE are identical, and while the relative importance of individual residues has not been determined, the numerous differences present in Hsc66 may preclude binding of GrpE. In this regard, Hsc66 behaves like eukaryotic cytosolic Hsp70 proteins which do not appear to utilize a GrpE-like cochaperone (37). In these cases, nucleotide exchange may not be a rate-limiting step in the chaperone cycle as it is for DnaK (50). The finding that GrpE stimulated Hsc66 ATPase activity in the presence of high levels of DnaJ is surprising and suggests the possibility that GrpE and DnaJ may directly interact when modulating Hsp70 ATPase activity.
The specific cellular function(s) of Hsc66 and Hsc20 is not known, but their relatively high expression levels under nonstress conditions imply important general housekeeping roles. Some inferences regarding their function(s) can be drawn from analysis of recent genome sequence data. Sequences of the genomes of several bacteria, including E. coli (2), H. influenzae (14), B. aphidicola (8), N. gonorrhoeae (48) and P. aeruginosa (44), reveal that each of these organisms has a gene cluster containing hscA and hscB homologs together with genes homologous to Fe-S cluster maturation genes (nif genes) of nitrogen-fixing bacteria. An analogous gene cluster separate from the nif genes was also recently identified in the nitrogen-fixing bacterium A. vinelandii (68). The occurrence of nif-like genes in non-nitrogen-fixing organisms and their counterparts in A. vinelandii led Zheng and coworkers to propose that these genes might play a role in formation or repair of iron sulfur proteins and to designate them as isc (iron sulfur cluster) genes (68). The sequential gene arrangement in the cluster, iscSUA-hscBA-fdx, is similar in each of the above organisms, and it appears likely that the genes are cotranscribed and encode proteins with coupled functions. Thus, the role of the Hsc66-Hsc20 chaperone system may be to function together with the iscS, iscU, iscA, and fdx gene products to assist in protein folding steps involved in assembly and/or repair of iron sulfur clusters in Fe-S proteins.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant GM54624 and Training grant GM07311.
We thank Dennis Ta for expert technical assistance and Mark Brandt for stimulating discussions.
REFERENCES
- 1.Bardwell J C A, Tilly K, Craig E A, King J, Zylicz M, Georgopoulos C. The nucleotide sequence of the Escherichia coli K12 dnaJ+ gene. A gene that encodes a heat shock protein. J Biol Chem. 1986;261:1782–1785. [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides M, Glasner J D, Rode C K, Mayhew G F, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Bork P, Sander C, Valencia A, Bukau B. A module of the DnaJ heat shock proteins found in malaria parasites. Trends Biochem Sci. 1992;17:129. doi: 10.1016/0968-0004(92)90319-5. [DOI] [PubMed] [Google Scholar]
- 4.Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715. [DOI] [PubMed] [Google Scholar]
- 5.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 6.Bukau B, Walker G C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate role for heat shock protein in normal metabolism. J Bacteriol. 1989;171:2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caplan A J, Cyr D M, Douglas M G. Eukaryotic homologs of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins. Mol Biol Cell. 1993;4:555–563. doi: 10.1091/mbc.4.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark M A, Baumann L, Baumann P. Sequence analysis of a 34.7 kb DNA segment from the genome of Buchnera aphidicola containing groEL, dnaA, the atp operon, gidA and rho. Curr Microbiol. 1998;36:158–163. doi: 10.1007/pl00006760. [DOI] [PubMed] [Google Scholar]
- 9.Cupp-Vickery J R, Vickery L E. Crystallization and preliminary X-ray crystallographic properties of Hsc20, a J-motif co-chaperone protein from Escherichia coli. Protein Sci. 1997;6:2028–2030. doi: 10.1002/pro.5560060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cyr D. Cooperation of the molecular chaperone Ydj1 with specific Hsp70 homologs to suppress protein aggregation. FEBS Lett. 1995;359:129–132. doi: 10.1016/0014-5793(95)00024-4. [DOI] [PubMed] [Google Scholar]
- 11.Cyr D M, Langer T, Douglas M G. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 12.Deloche O, Liberek K, Zylicz M, Georgopoulos C. Purification and biochemical properties of Saccharomyces cerevisiae Mdj1p, the mitochondrial DnaJ homologue. J Biol Chem. 1997;272:28539–28544. doi: 10.1074/jbc.272.45.28539. [DOI] [PubMed] [Google Scholar]
- 13.Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann R D, Adams M D, White O, Clayton R A, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 15.Fourie A M, Sambrook J F, Gething M J. Common and divergent peptide binding specificities of hsp70 molecular chaperones. J Biol Chem. 1994;269:30470–30478. [PubMed] [Google Scholar]
- 16.Georgopoulos C, Welch W J. Role of major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–635. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 17.Gething M J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 18.Gill S C, VonHippel P H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 19.Harrison C J, Hayer-Hartle M, Liberto M D, Hartl F U, Kurlyan J. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science. 1997;276:431–435. doi: 10.1126/science.276.5311.431. [DOI] [PubMed] [Google Scholar]
- 20.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;38:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 21.Hendrick J P, Hartl F U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 22.Hendrick J P, Langer T, Davis T A, Hartl F U, Wiedmann M. Control of folding and membrane translocation by binding of the chaperone DnaJ to nascent polypeptides. Proc Natl Acad Sci USA. 1993;90:10216–10220. doi: 10.1073/pnas.90.21.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herendeen S L, VanBogelen R A, Neidhardt F C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979;139:185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 25.Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- 26.Kawula T H, Lelivelt M J. Mutations in a gene encoding a new Hsp70 suppress rapid DNA inversion and bgl activation, but not proU deprepression, in hns-1 mutant Escherichia coli. J Bacteriol. 1994;176:610–619. doi: 10.1128/jb.176.3.610-619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karzal A W, McMacken R. A bipartite signaling mechanism involved in DnaJ-mediated activation of the Escherichia coli DnaK protein. J Biol Chem. 1996;271:11236–11246. doi: 10.1074/jbc.271.19.11236. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lee G J. Assaying proteins for molecular chaperone activity. Methods Cell Biol. 1995;50:325–334. doi: 10.1016/s0091-679x(08)61040-7. [DOI] [PubMed] [Google Scholar]
- 30.Levy E J, McCarty J, Bukau B, Chirico W J. Conserved ATPase and luciferase refolding activities between bacteria and yeast Hsp70 chaperones and modulators. FEBS Lett. 1995;368:435–440. doi: 10.1016/0014-5793(95)00704-d. [DOI] [PubMed] [Google Scholar]
- 31.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Z, Cyr D M. The conserved carboxyl terminus and zinc finger-like domain of the co-chaperone Ydj1 assist Hsp70 in protein folding. J Biol Chem. 1998;273:5970–5978. doi: 10.1074/jbc.273.10.5970. [DOI] [PubMed] [Google Scholar]
- 33.Mach H, Middaugh C R, Lewis R V. Statistical determination of the average values of the extinction coefficient of tyrosine and tryptophan in native proteins. Anal Biochem. 1992;200:74–80. doi: 10.1016/0003-2697(92)90279-g. [DOI] [PubMed] [Google Scholar]
- 34.Martin J, Hartl F U. Chaperone-assisted protein folding. Curr Opin Struct Biol. 1997;7:41–52. doi: 10.1016/s0959-440x(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 35.McCarty J S, Buchberger A, Reinstein J, Bukau B. The role of ATP in the functional cycle of the DnaK chaperone system. J Mol Biol. 1995;249:126–137. doi: 10.1006/jmbi.1995.0284. [DOI] [PubMed] [Google Scholar]
- 36.McKay D B. Structure and mechanism of 70kDa heat-shock-related proteins. Adv Prot Chem. 1993;44:67–98. doi: 10.1016/s0065-3233(08)60564-1. [DOI] [PubMed] [Google Scholar]
- 37.Minami Y, Hohfeld J, Ohtsuka K, Hartl F U. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 38.Neidhardt F C, VanBogelen R A. Heat shock response. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1334–1345. [Google Scholar]
- 39.Neisseria meningitidis Sequencing Group at the Sanger Centre.ftp://ftp.sanger.ac.uk/pub/pathogens/nm.
- 40.Pace C N, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of proteins. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paek K-H, Walker G C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987;168:283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palleros D P, Reid K L, Shi L, Welch W J, Fink A L. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365:664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- 43.Pellecchia M, Szyperski T, Wall D, Georgopoulos C, Wuthrich K. NMR structure of the J-domain and the Gly/Phe-rich region of the Escherichia coli DnaJ chaperone. J Mol Biol. 1996;260:236–250. doi: 10.1006/jmbi.1996.0395. [DOI] [PubMed] [Google Scholar]
- 44.Pseudomonas Genome Project.http://www.pseudomonas.com.
- 45.Qian Y Q, Patel D, Hartl F U, McColl D J. Nuclear magnetic resonance solution structure of the human Hsp40 (HDJ-1) J-domain. J Mol Biol. 1996;260:224–235. doi: 10.1006/jmbi.1996.0394. [DOI] [PubMed] [Google Scholar]
- 46.Rassow J, Voos W, Pfanner N. Partner proteins determine multiple functions of Hsp70. Trends Cell Biol. 1995;5:207–212. doi: 10.1016/s0962-8924(00)89001-7. [DOI] [PubMed] [Google Scholar]
- 47.Roe, B. A., F. Z. Najar, S. Clifton, and D. W. Dyer. Actinobacillus Genome Sequencing Project. http://www.genome.ou.edu/act.html.
- 48.Roe, B. A., S. P. Lin, L. Song, X. Yuan, S. Clifton, and D. W. Dyer. Gonococcal Genome Sequencing Project. http://www.genome.ou.edu/gono.html.
- 49.Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell R, Jordan R, McMacken R. Kinetic characterization of the ATPase cycle of the DnaK molecular chaperone. Biochemistry. 1998;37:596–607. doi: 10.1021/bi972025p. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 52.Schonfeld H J, Schmidt D, Schroder H, Bukau B. The DnaK chaperone system of E. coli: quaternary structures and interactions of the DnaK and GrpE components. J Biol Chem. 1995;270:2183–2189. doi: 10.1074/jbc.270.5.2183. [DOI] [PubMed] [Google Scholar]
- 53.Schroder H, Langer T, Hartl F U, Bukau B. DnaK, DnaJ, and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 1993;12:4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seaton B L, Vickery L E. A gene encoding a DnaK/hsp70 homolog in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:2066–2070. doi: 10.1073/pnas.91.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silberg, J. J., and L. E. Vickery. Unpublished results.
- 56.Silver P A, Way J C. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993;74:5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- 57.Szabo A, Korszun R, Hartl F U, Flanagan J. A Zn finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- 58.Szabo A, Langer T, Schroder H, Flanagan J, Bukau B, Hartl F U. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system—DnaK, DnaJ and GrpE. Proc Natl Acad Sci USA. 1994;91:10345–10349. doi: 10.1073/pnas.91.22.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szyperski T, Pellecchia M, Wall D, Georgopoulos C, Wuthrich K. NMR structure determination of the Escherichia coli DnaJ molecular chaperone: secondary structure and backbone fold of the N-terminal region (residues 2-108) containing the highly conserved J domain. Proc Natl Acad Sci USA. 1994;91:11343–11347. doi: 10.1073/pnas.91.24.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tatusov R L, Koonin E V, Lipman D J. A genomic perspective on protein families. Science. 1997;278:631–637. doi: 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- 61.Tsai J, Douglas M G. A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem. 1996;271:9347–9354. doi: 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- 62.Vickery L E, Silberg J J, Ta D T. Hsc66 and Hsc20, a new heat shock cognate molecular chaperone system from Escherichia coli. Protein Sci. 1997;6:1047–1056. doi: 10.1002/pro.5560060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vickery, L. E., and D. T. Ta. Unpublished data.
- 64.Wall D, Zylicz M, Georgopoulos C. The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for λ replication. J Biol Chem. 1994;269:5446–5451. [PubMed] [Google Scholar]
- 65.Wall D, Zylicz M, Georgopoulos C. The conserved G/F motif of the DnaJ chaperone is necessary for the activation of the substrate binding properties of the DnaK chaperone. J Biol Chem. 1995;270:2139–2144. doi: 10.1074/jbc.270.5.2139. [DOI] [PubMed] [Google Scholar]
- 66.Webb M R. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc Natl Acad Sci USA. 1992;89:4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young J C, Schneider C, Hartl F U. In vitro evidence that hsp90 contains two independent chaperone sites. FEBS Lett. 1997;418:139–143. doi: 10.1016/s0014-5793(97)01363-x. [DOI] [PubMed] [Google Scholar]
- 68.Zheng L, Cash V L, Flint D H, Dean D R. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 69.Zhu, X., X. Zhao, W. F. Burkholder, A. Gragerov, C. M. Ogata, M. E. Gottesman, and W. A. Hendrickson. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272:1606–1614. [DOI] [PMC free article] [PubMed]