Abstract
Introduction
This study aimed to compare the accuracy of seven implantable collamer lens (ICL) implantation vault prediction formulae.
Methods
We retrospectively analyzed 328 patients (328 eyes) who underwent ICL implantation and the prediction accuracy of seven formulae: NK, KS, WH, Luo, Zhu, Hun, and ZZ were compared. Moreover, the accuracy of the seven formulae for different ICL sizes was compared. The formulae were tested using mean absolute prediction error (MAE), median absolute prediction error (MedAE), prediction error (PE) percentages at ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm, and Bland-Altman analysis.
Results
The PE of the seven formulae were statistically significant (P < 0.001). The KS (101.00 µm) and WH formulae (116.65 µm) had the smallest MedAE, followed by the Luo (123.62 µm), NK (141.50 µm), Hun (152.68 µm), ZZ (196.00 µm) and Zhu formula (225.98 µm). The highest percentage of PE in the range of ± 300µm was 94.3% and 93% for the KS and WH formulae, respectively. Among the different ICL size groupings, the KS formula predicted the smallest MedAE for 12.1 mm and 12.6 mm, whereas the Luo and WH formulae predicted the smallest MedAE for 13.2 mm and 13.7 mm, respectively.
Conclusions
The KS and WH formulae provided better outcomes by predicting the vault with higher accuracy than of the NK, Hun, Luo, ZZ, and Zhu formulae.
Trial Registration
ChiCTR2200065501.
Keywords: Formula comparison, Implantable collamer lens, Implantable collamer lens vault prediction, Prediction error, Vault
Key Summary Points
| Why carry out this study? |
| Vault prediction is a popular research topic in the field of implantable collamer lens (ICL) implantation. Recently, many researchers have derived vault prediction formulae; however, comparative studies validating these formulae are lacking. |
| Our study aimed to compare the accuracy of the NK, KS, WH, Luo, Zhu, Hun, and ZZ formulae in predicting the vault and to provide guidance for clinical applications. |
| What was learned from the study? |
| We found that KS and WH formulae have the lowest median absolute prediction error (MedAE) and highest percentage of prediction error (PE) range of ± 300 µm, and the difference between predicted and actual vault is smaller. |
| The KS and WH formulae were more accurate than the NK, Zhu, Luo, Hun, and ZZ formulae and provided good vault predictions. |
Introduction
Implantable collamer lens (ICL) (STAAR Surgical, Monrovia, CA, USA) implantation involves the implantation of an artificial lens into the posterior chamber while preserving one's own lens to correct a patient's refractive error. The advantages of ICL include a wide range of refractive error corrections, good postoperative visual quality, safety, and stability [1–3]. ICL has been extensively used to correct high myopia and has also recently been used for treating patients with low-to-moderate myopia [4].
The most widely used clinical model is the ICL V4c, which is able to avoid laser peripheral iridotomy because of its 360-µm central hole design that promotes intraocular aqueous circulation. Although ICL V4c reduces the risk of postoperative complications associated with this procedure [5], postoperative safety cannot be ignored. The vault is the central vertical distance between the posterior surface of the ICL and the anterior surface of its own lens and is the most important indicator of the safety of the procedure, with a vault that is either too high or too low causing serious complications. If the vault is too low, anterior subcapsular cataracts and ICL rotation can occur [6]. If the vault is too high, it can lead to closed-angle glaucoma, corneal endothelial decompensation, and Urrets-Zavalia syndrome [7, 8]. Inappropriate postoperative vault is the primary cause of secondary ICL implantation [9]. Secondary ICL implantation means that ICL replacement or adjustment of ICL position in the eye is required to avoid serious consequences. Secondary ICL implantation increases the burden on the patient, necessitating the importance of preoperative vault prediction for ICL implantation.
Recently, many researchers have published vault prediction formulae based on preoperative ocular and ICL-related parameters, such as ICL size, angle-to-angle diameter (ATA), crystalline lens rise (CLR), and ciliary sulcus angle (CSA) [10–13]. The predicted vault values were derived by substituting the relevant parameters for the clinician’s preoperative reference. However, relevant studies that further validate and compare the formulae predictability and accuracy of these formulae are lacking. Therefore, clinicians may lack references when using these formulae.
Therefore, this study aimed to compare the accuracy of various vault prediction formulae in predicting postoperative vaults. The following seven prediction formulae were evaluated: NK, KS, WH, ZZ, Luo, Hun, and Zhu.
Methods
Participants
This retrospective study included 338 eyes from 338 individuals who underwent ICL implantation (EVO ICL Model V4c) at the Department of Optometry and Ophthalmology, Hunan Provincial People’s Hospital, between March 2022 and May 2023. Only the right eye was analyzed. The study was approved by the Ethics Committee of Hunan Provincial People's Hospital (approval no. 2022–141) and was conducted in accordance with the Declaration of Helsinki. This study was registered with the Chinese Clinical Trial Registry (https://www.chictr.org.cn/; registration number: ChiCTR2200065501). Informed consent was obtained from all the patients. Patients who met the following criteria were included: (1) aged between 18 and 45 years; (2) corneal endothelial cell count ≥ 2000 cells/mm2; (3) anterior chamber depth ≥ 2.80 mm; (4) horizontal placement of all non-toric ICL and horizontal rotation axis of the toric ICL (TICL) of ≤ 10°. Patients with a history of ocular diseases, such as glaucoma, keratoconus, history of intraocular surgery, and ciliary body cysts, were excluded.
All the patients underwent a comprehensive preoperative eye examination, including visual acuity, subjective and objective optometry, slit-lamp microscopy, intraocular pressure, and retinal examination. To avoid the influence of accommodation on the vault [14], all the measurements were recorded in a natural light environment with a stable light source. The axial length (AL) and horizontal white-to-white diameter (WTW), photopic pupil diameter (PPD), ATA, anterior chamber width (ACW), CLR, lens thickness (LT), and anterior chamber depth (ACD) were measured using the AL-Scan (NIDEK, Japan), Sirius (CSO, Italy), and AS-OCT (CASIA2, Tomey, Japan), respectively. Horizontal sulcus-to-sulcus distance (HSTS), vertical sulcus-to-sulcus distance (VSTS), and CSA were measured using ultrasound biomicroscopy (UBM) (Suoer, Tianjin). The actual vault at 3 months postoperatively was measured using AS-OCT. All the data were averaged over three measurements by an experienced physician.
We used the WTW and ACD measurements to select the ICL V4c size based on the manufacturer's nomogram. ICL V4c power was obtained from an online calculator provided by the manufacturer (https://evo-ocos.staarag.ch). A single surgeon (Hua Wang) performed the entire surgery by creating a 3.0-mm clear corneal incision, implanting the ICL, and adjusting the four footplates of the ICL to the posterior chamber with a special adjustment hook, as described in our previous study [10].
Vault Prediction Formula Calculation
Using the collected data, the predicted vault of the seven formulae were back-calculated. The vault prediction equations used for comparison in this study are as follows:
The NK formula is based on ACW and CLR for the prediction of vault; the vault prediction values were automatically obtained from CASIA2 AS-OCT [13].
The KS formula is based on ICL size and ATA for the prediction of vault; the predicted vault values were automatically obtained from CASIA2 AS-OCT [12].
The ZZ formula, based on HSTS, VSTS, and LT for the prediction of vault; predicted vault values were obtained by utilizing data from the website (http://www.zzcal.com) [15].
The WH formula is based on the ICL size, ATA, CLR, and CSA for vault prediction. Vault (μm) = 414.98 × ICL size (mm) − 111.78 × ATA (mm) − 0.59 × CLR (μm) − 3.12 × CSA (°) − 3,119.43 [10]. The predicted vault was obtained by inputting the actual implanted ICL size, ATA, CLR, and CSA into Excel (2021, Microsoft Corp.).
The Zhu formula, based on ICL size, HSTS, VSTS, and LT, was used to predict the vault. Vault (μm) = -1369.05 + 657.121 × ICL size (mm)—287.408 × HSTS (mm)—432.497 × LT (mm)—137.33 × VSTS (mm) [11]. The predicted vault was obtained by inputting the actual implanted ICL size, HSTS, VSTS, and LT into Excel.
Luo’s formula is based on ICL size, ACD, and ATA for the prediction of the vault. Vault (μm) = – 1279 + 291 × ACD (mm) + 210 × ICL V4c size (mm ) — 144 × ATA (mm) [16]. The predicted vault was obtained by inputting the actual implanted ICL size, ACD, and ATA into Excel.
The Hun formula is based on ACD, PPD, and AL for the prediction of the vault. Vault (μm) = -784 + 0.171 × ACD (mm) + 38 × PPD (mm) + 17 × AL (mm) [17]. The predicted vault was obtained by inputting ACD, PPD, and AL into Excel.
Statistical Analysis
The actual vault at 3 months postoperatively minus the formula-predicted vault was considered the prediction error (PE). Indicators for evaluating formulae also include standard deviation (SD), absolute prediction error (AE), mean absolute prediction error (MAE), median absolute prediction error (MedAE), and percentage of PE within ± 50 μm, ± 100 μm, ± 200 μm, and ± 300 μm.
A subgroup analysis was performed by categorizing the patients into four groups based on the ICL size (12.1 mm, 12.6 mm, 13.2 mm, and 13.7 mm).
All data were analyzed and processed using SPSS software version 25.0 (IBM, Armonk, NY, USA). Normally distributed data were tested using the Shapiro-Wilk test. The Friedman test was used to compare the PE differences between the seven formulae, and post-hoc comparisons were performed using the Wilcoxon signed-rank test with Bonferroni correction. The Cochran’s Q test was used to compare the percentage of eyes with a PE within ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm. A Bland-Altman plot was used to evaluate the agreement between the actual and predicted vaults. Bilateral P < 0.05 was considered statistically significant.
Results
Demographics and Biometry Data
This study included 338 individuals (338 eyes), comprising 98 men and 240 women (mean age: 26.26 ± 5.93 years). Of them, 245 and 93 patients had ICL and TICL, respectively. Table 1 summarizes the preoperative data of the patients and the intraoperative sizes used.
Table 1.
Patient demographics and characteristics
| Characteristics | Mean ± SD | 95% CI | Range |
|---|---|---|---|
| Age (years) | 26.26 ± 5.93 | 25.45–27.08 | 18,45 |
| Sex (male/female) | 98/240 | ||
| WTW (mm) | 11.75 ± 0.36 | 11.71–11.79 | 10.69,12.86 |
| ACD (mm) | 3.25 ± 0.23 | 3.22–3.27 | 2.80,3.92 |
| ATA (mm) | 11.71 ± 0.39 | 11.67–11.75 | 10.52,12.63 |
| ACW (mm) | 11.72 ± 0.37 | 11.68–11.76 | 10.38,13.02 |
| CLR (µm) | 34.51 ± 159.77 | 17.39–51.63 | -467,492 |
| LT (mm) | 3.67 ± 0.64 | 3.60–3.74 | 2.60,4.70 |
| HSTS (mm) | 11.61 ± 0.38 | 11.57–11.65 | 10.57,12.66 |
| VSTS (mm) | 12.08 ± 0.39 | 12.03–12.12 | 11.04,13.11 |
| CSA (degrees) | 54.24 ± 13.64 | 52.78–55.71 | 10.00,124.25 |
| PPD (mm) | 3.92 ± 0.60 | 3.86–3.99 | 2.56,6.40 |
| AL (mm) | 26.79 ± 1.29 | 26.65–26.93 | 23.48,31.06 |
| ICL size (mm) | |||
| 12.1 | 72 (21.3%) | ||
| 12.6 | 213 (63.0%) | ||
| 13.2 | 39 (11.5%) | ||
| 13.7 | 14 (4.2%) |
WTW white-to-white diameter; ACD anterior chamber depth; ATA angle-to-angle diameter; ACW anterior chamber width; CLR crystalline lens rise; LT lens thickness; HSTS horizontal sulcus-to-sulcus diameter; VSTS vertical sulcus-to-sulcus diameter; CSA ciliary sulcus angle; PPD photopic pupil diameter; AL axial length; ICL implantable collamer lens; SD standard deviation; CI confidence interval
Prediction errors of all formulae
Comparisons of the PE of all seven formulae were statistically significant (P < 0.001, Friedman’s test). In the post hoc comparisons of AE between formulae, all comparisons were statistically significant (all P < 0.001), except for the differences between the WH and KS formulae, the Zhu and ZZ formulae and the Luo and NK formulae, which were not statistically significant (P = 0.218, P = 0.216, and P = 0.06, respectively). Because the AE values of all formulae were non-normally distributed (P < 0.05 using Shapiro-Wilk test), MedAE was used to evaluate the magnitude of error for each formula. The magnitude of error of each formula was evaluated based on SD and ME to determine whether the MedAE was similar between formulae. According to the MedAE comparison, the seven formulae from the smallest to largest error are: KS (101.00), WH (116.65), Luo (123.62), NK (141.50), Hun (152.68), ZZ (196.00), and Zhu (225.98). MedAE was similar in both KS and WH formulae; however, MAE and SD in the KS formula were lower (MAE: 126.55 and SD: 154.48) than in the WH formula (MAE: 131.45 and SD: 157.01). A comparison of the seven formulae (PE, MAE, and MedAE) is shown in Table 2 and Fig. 1.
Table 2.
Prediction performance for each formula in all patients (N = 328)
| Formula | PE ± SD (µm) | MAE (µm) | MedAE (µm) | % of eyes within PE range a | |||
|---|---|---|---|---|---|---|---|
| ± 50 µm (%) | ± 100 µm (%) | ± 200 µm (%) | ± 300 µm (%) | ||||
| KS | 46.38 ± 154.48 | 126.55 | 101.00 | 23.4 | 49.7 | 79.9 | 94.5 |
| WH | -41.65 ± 157.01 | 131.45 | 116.65 | 22.8 | 43.2 | 80.5 | 93.5 |
| Luo | -68.37 ± 157.97 | 142.01 | 123.62 | 20.4 | 42.3 | 75.7 | 91.7 |
| NK | 54.01 ± 185.08 | 156.20 | 141.50 | 19.8 | 36.7 | 68.9 | 88.2 |
| Hun | 174.00 ± 169.99 | 192.01 | 152.68 | 19.2 | 33.4 | 58.9 | 76.6 |
| ZZ | 172.01 ± 234.87 | 237.48 | 196.00 | 13.0 | 23.4 | 50.9 | 70.3 |
| Zhu | 215.46 ± 191.47 | 241.23 | 225.98 | 10.7 | 23.7 | 45.3 | 65.7 |
The PE, MAE, MedAE, SD, and percentage of eyes with PE within ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm for each of the seven formulae. The best MedAE value was found for KS (101.00 µm) and WH (116.65 µm) formulae; the worst result was produced by the ZZ (196.00 µm) and Zhu (225.98 µm) formulae
a = Cochran’s Q test was run to determine whether there were differences in proportion of eyes with a PE within ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm of the formulae included. PE within ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm were statistically significantly different, P < 0.001
PE prediction error; SD standard deviation; MAE mean absolute prediction error; MedAE median absolute prediction error
Fig. 1.
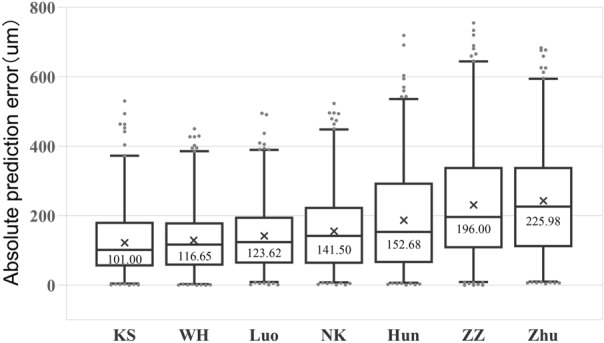
Distribution of the absolute prediction error. Formulae are ranked according to the median absolute prediction error, increasing from left to right. In the post hoc comparisons of absolute prediction error between formulae, all comparisons were statistically significant (P < 0.001), except for the differences between the WH and KS formulae, the Zhu and ZZ formulae, and the Luo and NK formulae, which were not statistically significant (P > 0.05)
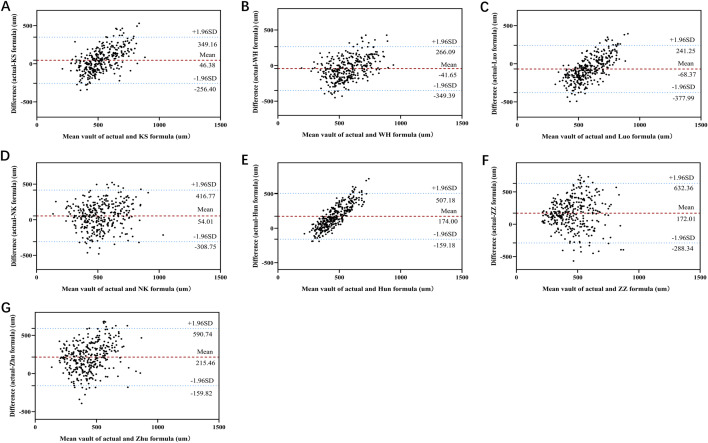
Figure 2 shows the agreement between the actual and predicted results for the seven formulae. The 95% limit of agreement (LoA) between the actual vault and the KS formula for predicted vault (mean ± SD) is the narrowest at – 256.40 to 349.16 µm (46.38 ± 154.48 µm), followed by the WH formula: – 349.39 to 266.09 µm (– 41.65 ± 157.01 µm). The 95% LoA between the actual vault and the ZZ formula for predicted vault (mean ± SD) is the widest at – 288.34 to 632.36 µm (172.01 ± 234.87 µm).
Fig. 2.
Bland-Altman plot of actual vault vs. predicted vault using the seven formulae. A KS formula; B WH formula; C Luo formula; D NK formula; E Hun formula; F ZZ formula; G Zhu formula
Percentages of PE within ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm
The difference in the percentage of PE within ± 50 µm, ± 100 µm, ± 200 µm, and ± 300 µm was statistically significant for all seven formulae (all P < 0.001). More than 90% of the patients with the KS, WH, and Luo formulae had a PE within ± 300 µm. The KS formula yielded the highest percentage of ± 50 µm, ± 100 µm, and ± 300 µm at 23.4%, 49.7%, and 94.5%, respectively. The highest percentage of ± 200 µm was 80.5% for the WH formula. Figure 3 and Table 2 show the specific distributions.
Fig. 3.
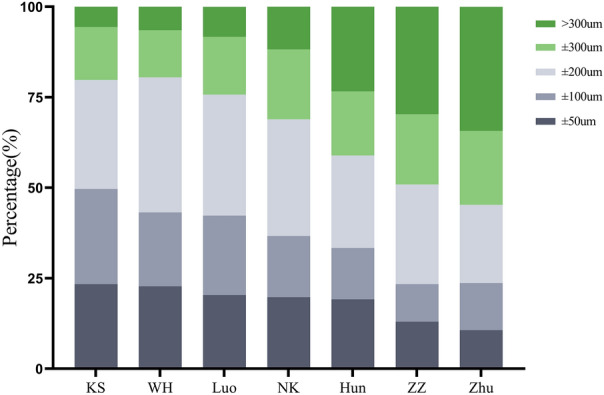
Stacked histogram comparing the percentage of cases with a given prediction error. Formulae are ranked according to the higher percentage for the prediction error within ± 50 µm
Subgroup Analysis According to ICL Size
Figure 4 shows the comparison of formulae by different ICL sizes and the MedAE line graphs of the seven formulae. The majority of the formula errors increase when selecting a large size (13.2, 13.7 µm). The KS formula error was minimized when 12.1 and 12.6 mm were selected with a MedAE of 78.50 and 110.00, respectively; the Luo formula error was minimized when 13.2 mm was selected with a MedAE of 99.89; the WH formula prediction error was minimized when 13.7 mm was selected with a MedAE of 101.67. The seven formulae with different ICL sizes for the PE, SD, MAE, and MedAE are listed in Table 3.
Fig. 4.
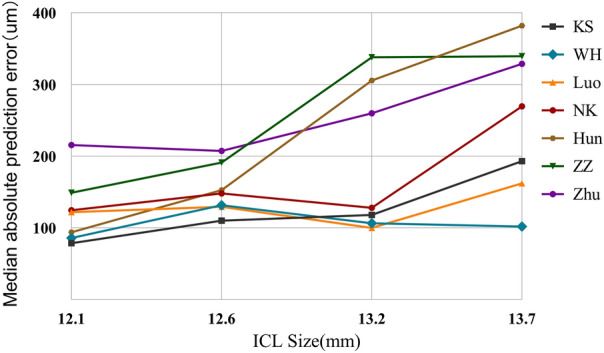
Line graph of median absolute prediction error for the seven vault prediction formulae vs. implantable collamer lens size. ICL implantable collamer lens
Table 3.
Prediction performance based on implantable collamer lens (ICL) sizes
| 12.1 mm (n = 72)a | 12.6 mm (n = 213)a | 13.2 mm (n = 39)a | 13.7 mm (n = 14)a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | SD | MAE | MedAE | PE | SD | MAE | MedAE | PE | SD | MAE | MedAE | PE | SD | MAE | MedAE | |
| Postoperative vault prediction of 7 formulae (n = 338) | ||||||||||||||||
| KS | 35.51 | 108.69 | 89.90 | 78.50 | 33.89 | 157.94 | 129.92 | 110.00 | 76.69 | 161.08 | 140.64 | 118.00 | 207.79 | 193.52 | 224.50 | 193.00 |
| WH | – 33.47 | 119.37 | 100.18 | 85.84 | – 55.23 | 160.85 | 139.58 | 131.50 | – 44.84 | 159.73 | 126.73 | 106.27 | 131.62 | 192.68 | 181.68 | 101.67 |
| Luo | – 103.00 | 109.84 | 127.70 | 121.85 | – 79.50 | 162.87 | 147.24 | 129.28 | 5.89 | 151.93 | 122.03 | 99.89 | – 166.33 | 176.50 | 191.51 | 162.10 |
| NK | 63.82 | 139.51 | 125.63 | 124.50 | 42.60 | 192.41 | 160.93 | 148.00 | 38.26 | 190.65 | 157.95 | 128.00 | 221.14 | 195.37 | 236.71 | 269.50 |
| Hun | 94.49 | 118.27 | 114.45 | 93.68 | 169.81 | 166.62 | 189.87 | 152.56 | 290.03 | 166.13 | 299.68 | 305.59 | 323.45 | 198.89 | 323.45 | 382.05 |
| ZZ | 66.89 | 184.73 | 164.31 | 149.00 | 166.07 | 236.54 | 234.45 | 191.00 | 343.36 | 202.84 | 355.56 | 338.00 | 325.71 | 188.51 | 330.86 | 339.50 |
| Zhu | 226.29 | 137.68 | 227.25 | 215.53 | 205.50 | 208.96 | 241.71 | 207.34 | 203.85 | 163.30 | 223.76 | 259.79 | 343.65 | 190.90 | 354.50 | 328.73 |
The bolded type indicates that the formula predicts that this size gives the best MedAE results. a = comparisons of the absolute prediction error of the seven formulae were statistically significant (P < 0.001, Friedman’s test)
PE prediction error; SD standard deviation; MAE mean absolute prediction error; MedAE median absolute prediction error
Discussion
Vault after ICL implantation is a major clinical concern. Previous studies focused on size selection by creating size-selection formulae [18–20]. However, ICL size is not a continuous numerical variable, and only four ICL sizes are currently available (12.1, 12.6, 13.2, and 13.7 mm), which limits the choice of size. Therefore, direct prediction of the vault after ICL implantation has been proposed to improve the safety of the procedure while indirectly guiding the selection of ICL sizes [10, 16]. Therefore, in recent years, many researchers have developed vault prediction formulae for clinicians to use as references to further quantify postoperative predictions. In this study, we compared the current clinically published vault prediction formulae for the first time, including the KS, WH, Luo, NK, Hun, ZZ, and Zhu formulae, and found that the KS and WH formulae were more accurate than the other formulae.
The results of this study showed statistically significant differences in the PE among the seven formulae. This confirms that the seven formulae exhibit differences in their overall predictability. The seven formulae referenced different metrics for prediction, including the ICL size, ATA, PPD, and AL, and the study showed that there was a difference in the magnitude of the effect of these parameters on the vault [21–23], which may have contributed to the differences in predictions between the formulae. In this study, the KS formula was the most predictive, followed by the WH and Luo formulae. Although in the ranking of the regression model fit of the three formulae, the KS formula had the lowest coefficient of determination of 0.41, and the WH and Luo formulae were 0.67 and 0.66, respectively [10, 12, 16]. This is because the KS formula incorporates fewer predictors than the WH and Luo formulae. This demonstrates that the KS formula is simpler and more accurate for clinical applications. All three formulae predictors included the ICL size. Several factors, such as ICL size, ACD, and CLR, influence the postoperative vault, with ICL size being recognized as the most critical factor [24, 25]. Therefore, the three formulae that incorporated ICL size were the most predictive. In addition, all three formulae included the ATA, and the theoretical STS was more vault-predictive when the haptic was located within the posterior ciliary sulcus after ICL implantation. However, STS can currently only be obtained from UBM measurements, and the interobserver and interdevice repeatability of UBM is poor for contact checking, which is inconvenient [26, 27]. ATA is the distance between the angle recesses, and some studies have shown a higher correlation between ATA and STS than between ATA and WTW [28]. ATA can be objectively measured using AS-OCT, and the repeatability of ATA measurement is high [12]. For ocular transverse size, the ATA may be a better choice. We can reference the ATA for ICL size selection and vault prediction. In this study, the ZZ and Zhu formulae were less predictive than the other formulae. The actual vault is higher than the predicted vault. The lower predictability could be because both formulae included STS in the predicted parameters. Therefore, prediction formulae that include STS should be used with caution between different centers. Meanwhile, both the ZZ and Hun formulae incorporate LT. LT affects vault and is negatively correlated with the vault [29]. The LT size correlates with age and conditioning, with the LT becoming thicker as age progresses [30]. Most patients in this study were young, the LT was not thick, and the measurements were performed under a stable light source with no induced conditioning to cause the crystal to become convex, which may have contributed to the actual vault being higher than the predicted vault.
Achieving error-free vault prediction is impossible and unnecessary; errors within a certain range are acceptable. Therefore, we evaluated the PE range to maintain the error between the actual and predicted vaults within a certain range. In this study, the PE of the KS and WH formulae had the highest range of ± 300 µm. The vault prediction formulae, when used as an indirect guide for sizing, are often based on the sizing inputs corresponding to the 500-µm predicted value for final sizing. Therefore, sizing based on the KS and WH formulae will result in more patients with a postoperative vault within 500 ± 300 µm, with the highest percentage of patients achieving the ideal postoperative vault (250–750 µm). However, future prospective studies are required to validate this finding. A postoperative vault within the range of 250–1000 µm is relatively safe [28, 31]. Therefore, a high vault, within a certain postoperative range, may be safer than a low vault. Based on this viewpoint, the PE in this study showed that all formulae except the WH and Luo formulae predicted a low vault, and the prediction of the postoperative vault based on the WH and Luo formulae would be higher and possibly safer. In addition, we grouped the sizes to determine the magnitude of formula errors. Most formulae showed an increase in prediction error as the size increased, which may be related to the fact that fewer patients with large sizes were included in this study. However, Ando [32] validated the NK and KS formulae and found that prediction error increases with increasing size, which is similar to the results of this study.
This study provides guidelines for clinicians in this regard. First, the results demonstrated the size of each PE formula, and all ICLs in the included patients were placed horizontally, controlling for the effect of orientation on the vault [33]. This provides indirect guidance for the application of the formula. For instance, when choosing the Zhu formula for a 12.6-mm implant with a PE of 205.50 µm, implantation direction can be tilted to reduce the vault and minimize the error of the formula. This improved the accuracy of Zhu’s formula for future use. Second, the formulae used in this study included multiple eye parameters. However, physicians often only consider the WTW and ACD for ICL sizing. Patients with abnormal morphology may have suboptimal postoperative vaults [17, 28]. These patients were referred to the findings of this study. For example, a patient with an abnormal CSA observed on UBM could refer more to the WH formula for vault prediction. Third, we compared the results for different ICL sizes using the formulae. When both formulae recommend an ICL size of 12.1 mm, we can refer more to the results of the KS formula. The KS formula provided the most accurate prediction when a 12.1-mm ICL size was chosen.
This study had several advantages, except for the STS measurements, which were measured preoperatively using devices with high reproducibility [12, 34, 35], such as Sirius, CASIA2, and AL-SCAN, and the formula prediction parameters were obtained reliably. The Hun formula used in this study has not been previously validated [17], and our study was the first to validate it. In addition, some formulae [10, 12, 13, 15] that were previously validated with small sample sizes were revalidated with adequate sample sizes, thereby providing convincing conclusions. Our study had some limitations. The 13.7-mm size included in this study is small, and larger samples should be added in the future. Most patients were Asians, and there were differences in the measurements of ocular parameters between the different races [36]. The results of this experiment should be applied to other races with caution; however, the seven prediction formulae used in this study were established based on Asian populations, which reduced the prediction error due to interracial differences in this study. Finally, we were unable to compare all the currently published vault prediction formulae because some studies did not disclose their formula parameters; therefore, we did not have access to the predicted values of these formulae.
Conclusion
Among the seven prediction formulae, the KS formula was the best for predicting the vault after ICL implantation, followed by the WH formula. Moreover, the KS formula was most accurate when 12.1 mm and 12.6 mm were selected, the Luo formula was most accurate when 13.2 mm was selected, and the WH formula was most accurate when 13.7 mm was selected.
Aknowledgements
Medical Writing, Editorial and Other Assistance
We thank editage (www.editage.cn) for English language editing.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Hao Wu. Methodology: Hao Wu. Formal analysis and investigation: Hao Wu, Ding-juan Zhong, Hua Wang. Writing—original draft preparation: Hao Wu. Writing—review and editing: Hao Wu, Ding-juan Zhong, Hua Wang. Funding acquisition: Dong-qiang Luo, Jiao Chen, Ding-juan Zhong, Hua Wang. Resources: Dong-qiang Luo, Jiao Chen, Ding-juan Zhong, Hua Wang. Supervision: Ding-juan Zhong, Hua Wang.
Funding
This work was supported by the Youth Doctoral Fund of Hunan Provincial People's Hospital under Grant [number BSJJ202214] by Ding-juan Zhong. The journal’s Rapid Service Fee was funded by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Hao Wu, Dong-qiang Luo, Jiao Chen, Hua Wang, Ding-juan Zhong declare that they have no competing interests.
Ethical Approval
The study was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of Hunan Provincial People's Hospital (2022–141). All patients provided informed consent prior to the procedures. The study has also been registered with the Chinese Clinical Trial Registry (https://www.chictr.org.cn/, registration number: ChiCTR2200065501).
Footnotes
Hua Wang and Ding-juan Zhong are considered co-corresponding authors.
Contributor Information
Hua Wang, Email: wanghuaeye@163.com.
Ding-juan Zhong, Email: zdjlxx@163.com.
References
- 1.Packer KT, Vlasov A, Greenburg DL, Coggin A, Weightman JW, Beltran T, Berry-Cabán CS, Carroll RB. U. S. military implantable collamer lens surgical outcomes: 11-year retrospective review. J Cataract Refract Surg. 2022;48(6):649–656. doi: 10.1097/j.jcrs.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 2.Montés-Micó R, Ruiz-Mesa R, Rodríguez-Prats JL, Tañá-Rivero P. Posterior-chamber phakic implantable collamer lenses with a central port: a review. Acta Ophthalmol. 2021;99(3):e288–e301. doi: 10.1111/aos.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Plaza E, López-de la Rosa A, López-Miguel A, Holgueras A, Maldonado MJ. EVO/EVO+ Visian Implantable Collamer Lenses for the correction of myopia and myopia with astigmatism. Expert Rev Med Devices 2023; 20 (2), 75–83. 10.1080/17434440.2023.2174429. [DOI] [PubMed]
- 4.Fu M, Li M, Xian Y, Yu Z, Zhang H, Choi J, Niu L, Wang X, Zhou X. Two-Year Visual Outcomes of Evolution Implantable Collamer Lens and Small Incision Lenticule Extraction for the Correction of Low Myopia. Front Med (Lausanne) 2022;9:780000. doi: 10.3389/fmed.2022.780000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi H, Ryu IH, Lee IS, Kim JK, Yoo TK. Comparison of implantation of EVO-ICL and laser vision correction in terms of corneal endothelial cells: a 3-year observational paired-eye study. J Cataract Refract Surg. 2023 doi: 10.1097/j.jcrs.0000000000001246. [DOI] [PubMed] [Google Scholar]
- 6.Gimbel HV, LeClair BM, Jabo B, Marzouk H. Incidence of implantable Collamer lens-induced cataract. Can J Ophthalmol. 2018;53(5):518–522. doi: 10.1016/j.jcjo.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Srirampur A, Mansoori T, Balijepalli P, Gadde AK. Management of anisocoria and high vault in an eye with implantable collamer lens. Indian J Ophthalmol. 2020;68(12):3070–3072. doi: 10.4103/ijo.IJO_3030_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida T, Kojima T, Kataoka T, Isogai N, Yoshida Y, Nakamura T. Prediction of the trabecular iris angle after posterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2022;48(5):604–610. doi: 10.1097/j.jcrs.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 9.Wei R, Li M, Aruma A, Knorz MC, Yang D, Yu Y, Wang X, Choi J, Yao P, Zhou X. Factors leading to realignment or exchange after implantable collamer lens implantation in 10 258 eyes. J Cataract Refract Surg. 2022;48(10):1190–1196. doi: 10.1097/j.jcrs.0000000000000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Zhong D-J, Luo D-Q, Zhang L-Y, Liu J, Wang H. Improvement in the ideal range of vault after implantable collamer lens implantation: a new vault prediction formula. Front Med (Lausanne) 2023;10:1132102. doi: 10.3389/fmed.2023.1132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Q-J, Xing X-Y, Zhu M-H, Ma L, Yuan Y, Song E. Validation of the vault prediction model based on the sulcus-to-sulcus diameter and lens thickness: a 925-eye prospective study. BMC Ophthalmol. 2022;22(1):463. doi: 10.1186/s12886-022-02698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi A, Shimizu K, Kato S, Kamiya K. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg. 2019;45(8):1099–1104. doi: 10.1016/j.jcrs.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Optimization of implantable collamer lens sizing based on swept-source anterior segment optical coherence tomography. J Cataract Refract Surg. 2020;46(5):742–748. doi: 10.1097/j.jcrs.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Vila-Arteaga J, Beltran J, Baviera J. Dynamic Assessment of Light-Induced Vaulting Changes of Implantable Collamer Lens With Central Port by Swept-Source OCT: Pilot Study. Transl Vis Sci Technol. 2018;7(3):4. doi: 10.1167/tvst.7.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Shao J, Zheng L, Zhao X, Chen S. Implantable collamer lens sizing based on measurement of the sulcus-to-sulcus distance in ultrasound biomicroscopy video clips and ZZ ICL formula. BMC Ophthalmol. 2022;22(1):363. doi: 10.1186/s12886-022-02583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Y, Li Y, Luo Y. Prediction of Implantable Collamer Lens Vault Based on Preoperative Biometric Factors and Lens Parameters. J Refract Surg. 2023;39(5):332–339. doi: 10.3928/1081597X-20230207-03. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Kang DSY, Choi JY, Ha BJ, Kim EK, Seo KY, Kim T-I. Analysis of pre-operative factors affecting range of optimal vaulting after implantation of 12.6-mm V4c implantable collamer lens in myopic eyes. BMC Ophthalmol. 2018;18(1):163. doi: 10.1186/s12886-018-0835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima, T.; Yokoyama, S.; Ito, M.; Horai, R.; Hara, S.; Nakamura, T.; Ichikawa, K. Optimization of an implantable collamer lens sizing method using high-frequency ultrasound biomicroscopy. Am J Ophthalmol 2012, 153 (4). 10.1016/j.ajo.2011.06.031. [DOI] [PubMed]
- 19.Moshirfar M, Placide J, da Silva NHV, Durnford KM, Ronquillo YC, McCabe SE, Hoopes PC. Assessing the Efficacy of Four Diagnostic Devices and Four Nomograms in Posterior Chamber Phakic Intraocular Lens Size Selection. J Refract Surg. 2022;38(2):106–111. doi: 10.3928/1081597X-20211109-01. [DOI] [PubMed] [Google Scholar]
- 20.Kang EM, Ryu IH, Lee G, Kim JK, Lee IS, Jeon GH, Song H, Kamiya K, Yoo TK. Development of a Web-Based Ensemble Machine Learning Application to Select the Optimal Size of Posterior Chamber Phakic Intraocular Lens. Transl Vis Sci Technol. 2021;10(6):5. doi: 10.1167/tvst.10.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Y, Wang L, Jian W, Shang J, Wang X, Ju L, Li M, Zhao J, Chen X, Ge Z, Wang X, Zhou X. Big-data and artificial-intelligence-assisted vault prediction and EVO-ICL size selection for myopia correction. Br J Ophthalmol. 2023;107(2):201–206. doi: 10.1136/bjophthalmol-2021-319618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerpa Manito S, Sánchez Trancón A, Torrado Sierra O, Baptista AM, Serra PM. Biometric and ICL-related risk factors associated to sub-optimal vaults in eyes implanted with implantable collamer lenses. Eye Vis (Lond) 2021;8(1):26. doi: 10.1186/s40662-021-00250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T, Kim SJ, Lee BY, Cho HJ, Sa BG, Ryu IH, Kim JK, Lee IS, Han E, Kim H, Yoo TK. Development of an implantable collamer lens sizing model: a retrospective study using ANTERION swept-source optical coherence tomography and a literature review. BMC Ophthalmol. 2023;23(1):59. doi: 10.1186/s12886-023-02814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ang RET, Reyes EKF, Ayuyao FAJ, Umali MIN, Cruz EM. Comparison of white-to-white measurements using four devices and their determination of ICL sizing. Eye Vis (Lond) 2022;9(1):36. doi: 10.1186/s40662-022-00308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Ye Y, Yao H, Liu C, He A, Hou X, Zhao K, Cui Z, Li Y, Qiu J, Chen P, Yang Y, Zhuang J, Yu K. Predicting post-operative vault and optimal implantable collamer lens size using machine learning based on various ophthalmic device combinations. Biomed Eng Online. 2023;22(1):59. doi: 10.1186/s12938-023-01123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guber I, Bergin C, Perritaz S, Majo F. Correcting Interdevice Bias of Horizontal White-to-White and Sulcus-to-Sulcus Measures Used for Implantable Collamer Lens Sizing. Am J Ophthalmol. 2016 doi: 10.1016/j.ajo.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama S, Kojima T, Horai R, Ito M, Nakamura T, Ichikawa K. Repeatability of the ciliary sulcus-to-sulcus diameter measurement using wide-scanning-field ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(7):1251–1256. doi: 10.1016/j.jcrs.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty PJ, Rivera RP, Schneider D, Lane SS, Brown D, Vukich J. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13–18. doi: 10.1016/j.jcrs.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Meng L, Zhao X, Chen Y, Luo Y. Clinical Prediction of Inadequate Vault in Eyes With Thick Lens After Implantable Collamer Lens Implantation Using Iris Morphology. Front Med (Lausanne) 2022;9:906433. doi: 10.3389/fmed.2022.906433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Y, Cao Y, Fan S, Xu M, Yang Z, Zhou L, Shi L, Ou L, Li Y, Qing W, Zou Z, Mao F, Wang N, Duh EJ, Yi W, Liu X. Nonhuman Primate Eyes Display Variable Growth and Aging Rates in Alignment With Human Eyes. Invest Ophthalmol Vis Sci. 2023;64(11):23. doi: 10.1167/iovs.64.11.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Tan W, Lei X, Pan C, Jin L, Zeng Q, Wang Z. Clinical Prediction of Excessive Vault After Implantable Collamer Lens Implantation Using Ciliary Body Morphology. J Refract Surg. 2020;36(6):380–387. doi: 10.3928/1081597X-20200513-02. [DOI] [PubMed] [Google Scholar]
- 32.Ando W, Kamiya K, Hayakawa H, Takahashi M, Shoji N. Comparison of Phakic Intraocular Lens Vault Using Conventional Nomogram and Prediction Formulas. J Clin Med. 2020;9(12):4090. doi: 10.3390/jcm9124090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei R, Cheng M, Niu L, Wang L, Luo X, Li M, Zhou X, Wang X, Zhou X, Yao P. Outcomes of the EVO ICL Using a Customized Non-horizontal or Horizontal Implanting Orientation Based on UBM Measurement: A Pilot Study. Ophthalmol Ther. 2022;11(3):1187–1198. doi: 10.1007/s40123-022-00498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar M, Shetty R, Jayadev C, Rao HL, Dutta D. Repeatability and agreement of five imaging systems for measuring anterior segment parameters in healthy eyes. Indian J Ophthalmol. 2017;65(4):288–294. doi: 10.4103/ijo.IJO_729_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X, Chen H, Savini G, Zheng Q, Song B, Tu R, Huang J, Wang Q. Precision of a new ocular biometer in children and comparison with IOLMaster. Sci Rep. 2018;8(1):1304. doi: 10.1038/s41598-018-19605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chansangpetch S, Tran B, Perez CI, Siguan-Bell C, Lau K, Nguyen A-H, Thai A, He M, Wang D, Nguyen N, Lin SC. Comparison of Anterior Segment Optical Coherence Tomography Parameters Among Vietnamese, Chinese, and Whites. Am J Ophthalmol. 2018;195:72–82. doi: 10.1016/j.ajo.2018.07.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.