Abstract
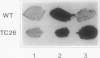
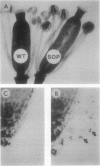
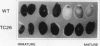
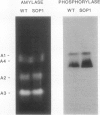
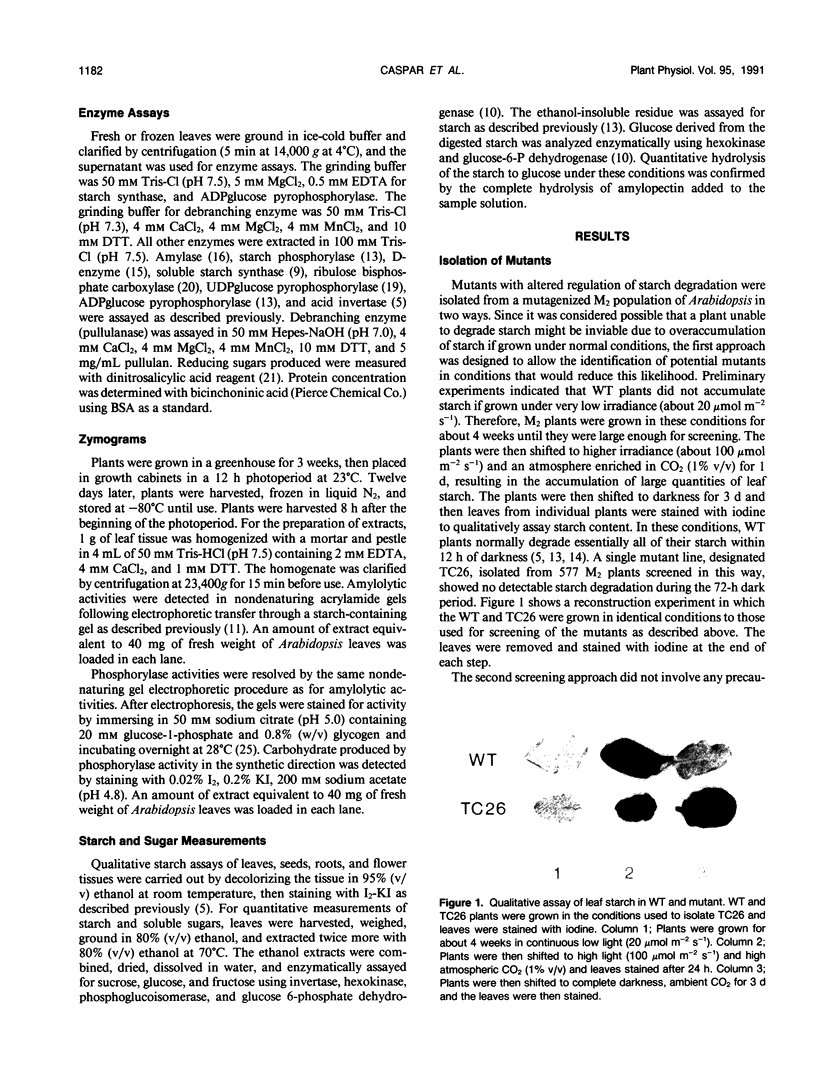
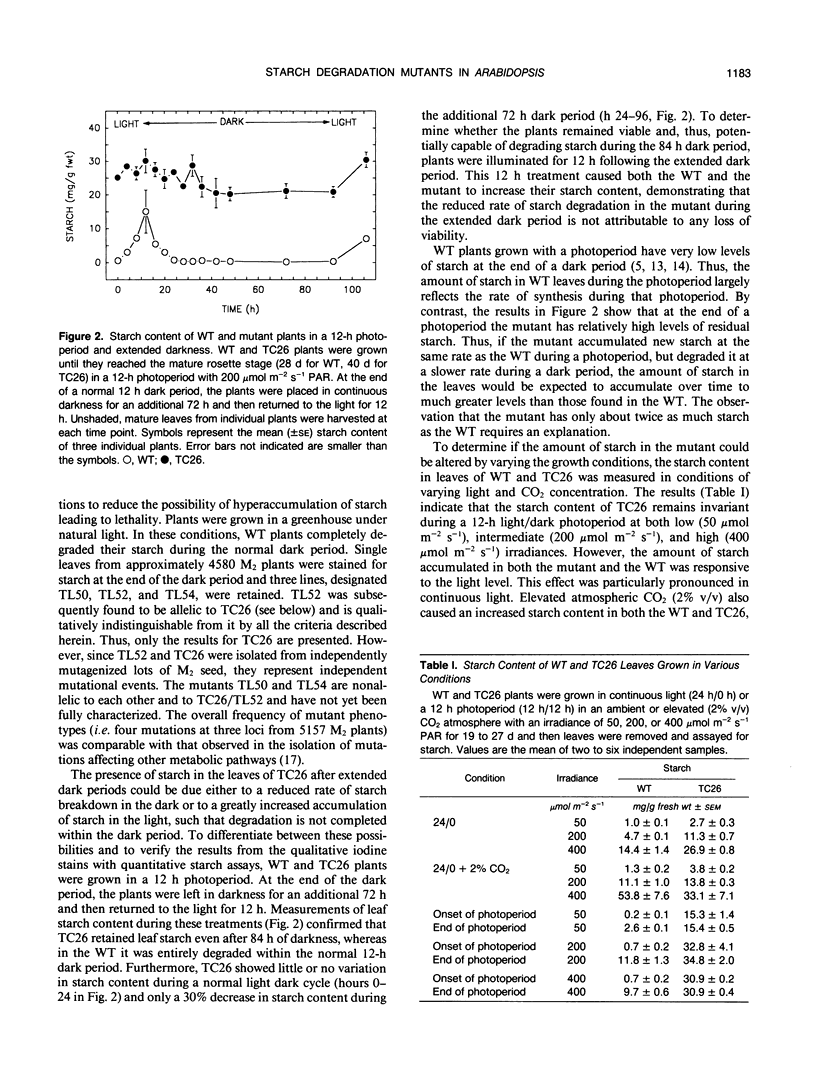
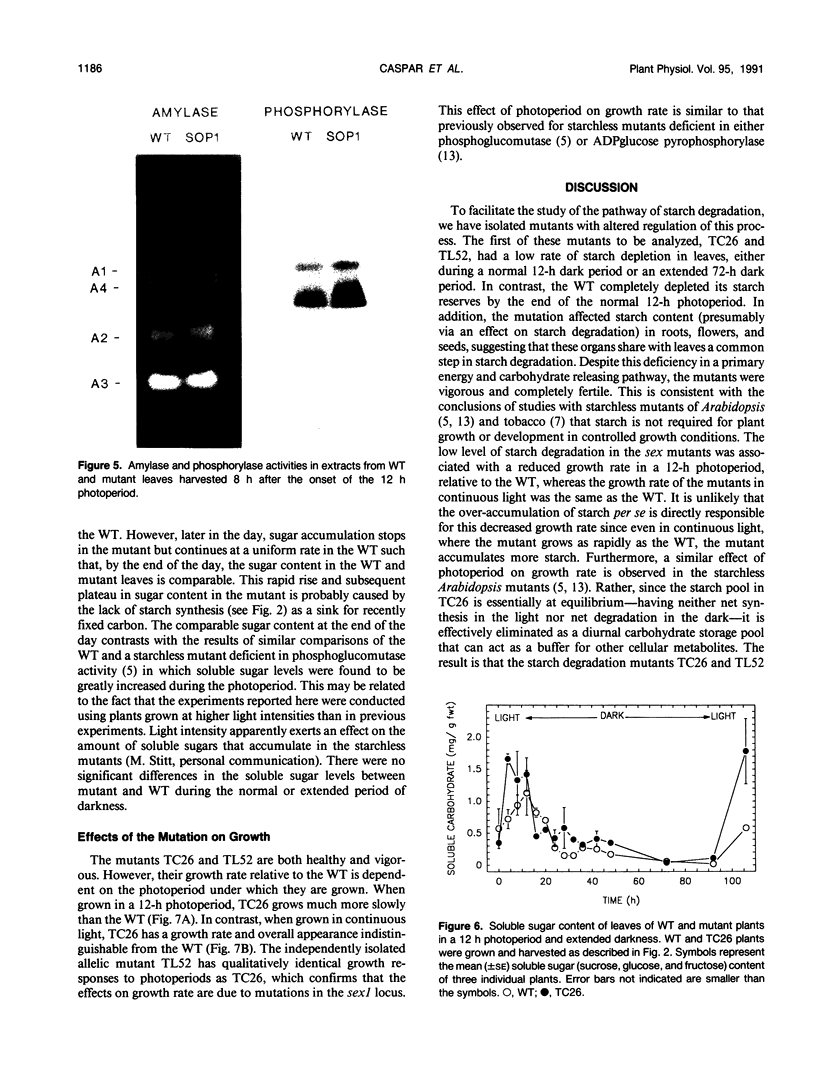
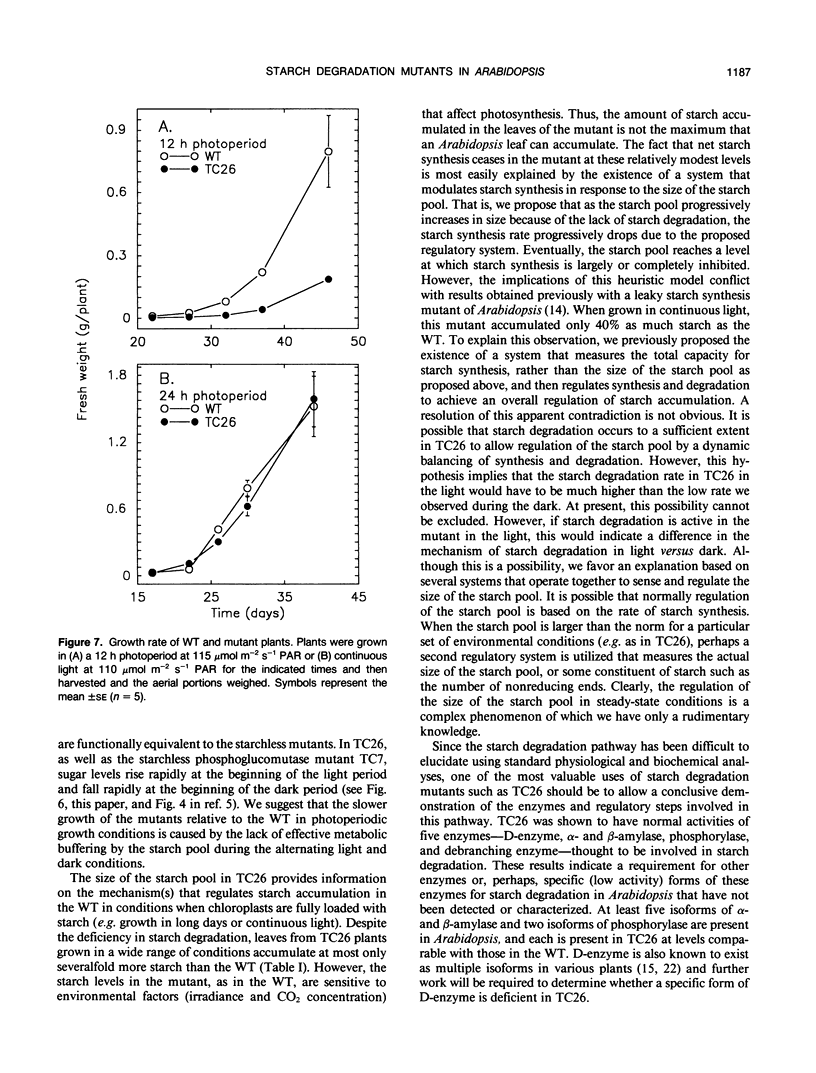
Mutants of Arabidopsis thaliana (L.) Heynh. with altered regulation of starch degradation were identified by screening for plants that retained high levels of leaf starch after a period of extended darkness. The mutant phenotype was also expressed in seeds, flowers, and roots, indicating that the same pathway of starch degradation is used in these tissues. In many respects, the physiological consequences of the mutations were equivalent to the effects observed in previously characterized mutants of Arabidopsis that are unable to synthesize starch. One mutant line, which was characterized in detail, had normal levels of activity of the starch degradative enzymes α-amylase, β-amylase, phosphorylase, D-enzyme, and debranching enzyme. Thus, it was not possible to establish a biochemical basis for the phenotype, which was due to a recessive mutation at a locus designated sex1 at position 12.2 on chromosome 1. This raises the possibility that hitherto unidentified factors, altered by the mutation, play a key role in regulating or catalyzing starch degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY J. M., WHELAN W. J. Physical properties of starch. I. Relationship between iodine stain and chain length. J Biol Chem. 1961 Apr;236:969–973. [PubMed] [Google Scholar]
- Caspar T., Lin T. P., Monroe J., Bernhard W., Spilatro S., Preiss J., Somerville C. Altered regulation of beta-amylase activity in mutants of Arabidopsis with lesions in starch metabolism. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5830–5833. doi: 10.1073/pnas.86.15.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T., Pickard B. G. Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R., McHale N. A. A Starchless Mutant of Nicotiana sylvestris Containing a Modified Plastid Phosphoglucomutase. Plant Physiol. 1988 Nov;88(3):838–844. doi: 10.1104/pp.88.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker J. S., Ozbun J. L., Ozaki H., Greenberg E., Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974 Feb;160(2):530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Electrophoretic transfer as a technique for the detection and identification of plant amylolytic enzymes in polyacrylamide gels. Plant Physiol. 1984 May;75(1):278–280. doi: 10.1104/pp.75.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C. R., Preiss J. A Starch Deficient Mutant of Arabidopsis thaliana with Low ADPglucose Pyrophosphorylase Activity Lacks One of the Two Subunits of the Enzyme. Plant Physiol. 1988 Dec;88(4):1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Preiss J. Characterization of d-Enzyme (4-alpha-Glucanotransferase) in Arabidopsis Leaf. Plant Physiol. 1988 Jan;86(1):260–265. doi: 10.1104/pp.86.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Spilatro S. R., Preiss J. Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol. 1988 Jan;86(1):251–259. doi: 10.1104/pp.86.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Greenberg E., Lammel C., Preiss J. Starch Synthetase, Phosphorylase, ADPglucose Pyrophosphorylase, and UDPglucose Pyrophosphorylase in Developing Maize Kernels. Plant Physiol. 1973 Jan;51(1):1–5. doi: 10.1104/pp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., McCurry S. D., Mulligan R. M., Tolbert N. E. Activation and assay of ribulose-1,5-bisphosphate carboxylase/oxygenase. Methods Enzymol. 1982;89(Pt 500):47–55. doi: 10.1016/s0076-6879(82)89011-3. [DOI] [PubMed] [Google Scholar]
- Suiter K. A., Wendel J. F., Case J. S. LINKAGE-1: a PASCAL computer program for the detection and analysis of genetic linkage. J Hered. 1983 May-Jun;74(3):203–204. doi: 10.1093/oxfordjournals.jhered.a109766. [DOI] [PubMed] [Google Scholar]
- Tandecarz J. S., Sivak M. N., Cardini C. E. A primer independent form of potato tuber phosphorylase. Biochem Biophys Res Commun. 1978 May 15;82(1):157–164. doi: 10.1016/0006-291x(78)90590-9. [DOI] [PubMed] [Google Scholar]