Abstract
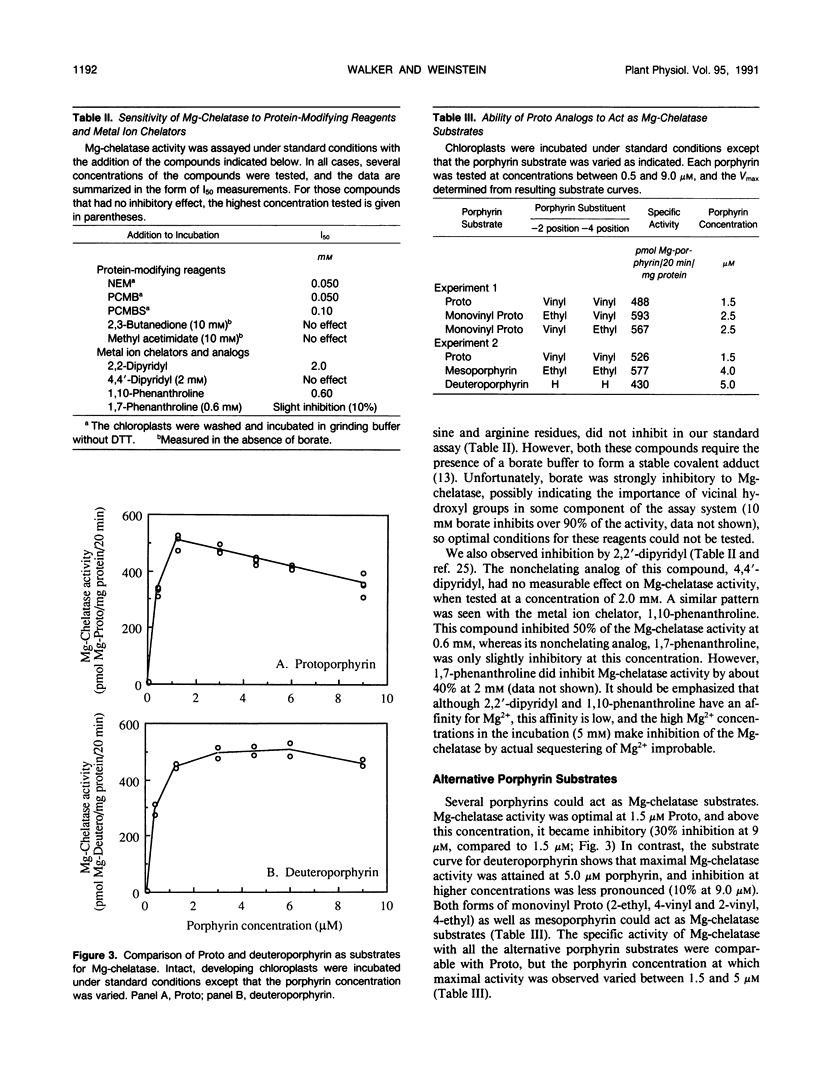
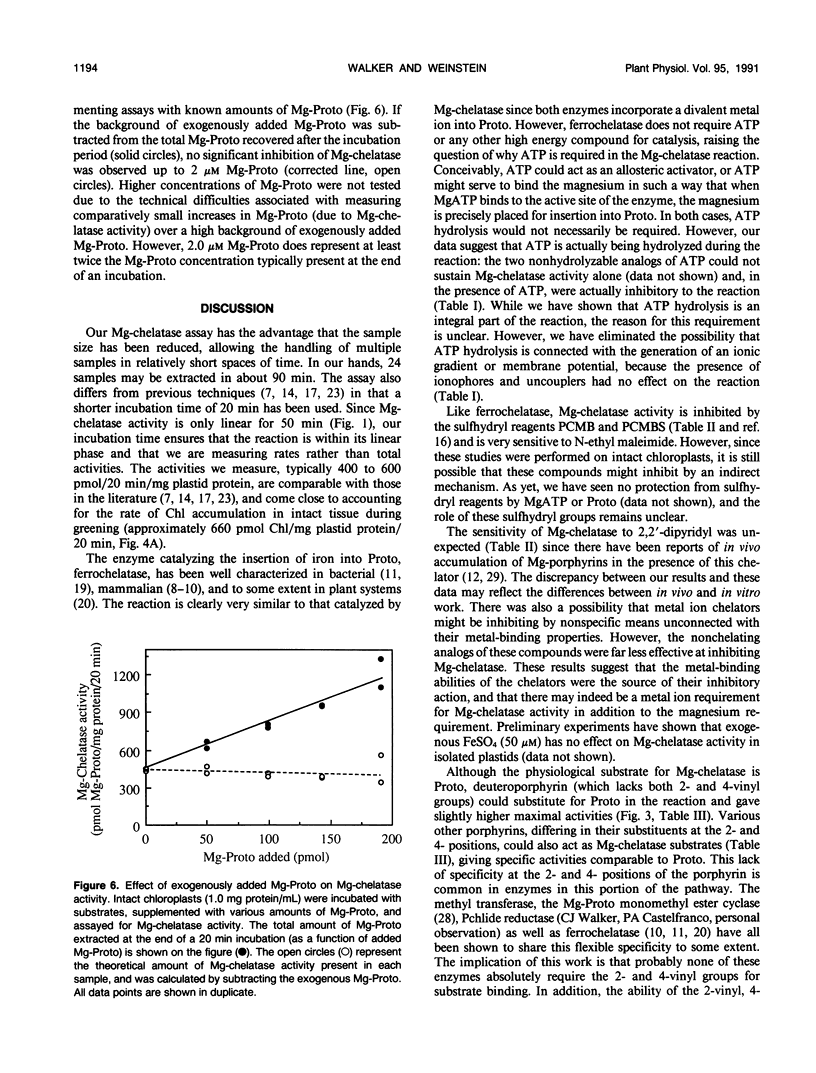
Mg-chelatase catalyzes the first step unique to the chlorophyll branch of tetrapyrrole biosynthesis, namely the insertion of Mg into protoporphyrin IX (Proto). Mg-chelatase was assayed in intact chloroplasts from semi-green cucumber (Cucumis sativus, cv Sumter) cotyledons. In the presence of Proto and MgATP, enzyme activity was linear for 50 minutes. Plastid intactness was directly related to (and necessary for) Mg-chelatase activity. Uncouplers and ionophores did not inhibit Mg-Chelatase in the presence of ATP. The nonhydrolyzable ATP analogs, β,γ-methylene ATP and adenylylimidodiphosphate, could not sustain Mg-chelatase activity alone and were inhibitory in the presence of ATP (I50 10 and 3 millimolar, respectively). Mg-chelatase was also inhibited by N-ethylmaleimide (I50, 50 micromolar) and the metal ion chelators 2,2′-dipyridyl and 1, 10 phenanthroline (but not to the same degree by their nonchelating analogs). In addition to Proto, the following porphyrins acted as Mg-chelatase substrates, giving comparable specific activities: deuteroporphyrin, mesoporphyrin, 2-ethyl, 4-vinyl Proto and 2-vinyl, 4-ethyl Proto. Mg-chelatase activity and freely exchangeable heme levels increased steadily with greening, reaching a maximum and leveling off after 15 hours in the light. Exogenous protochlorophyllide, chlorophyllide, heme, and Mg-Proto had no measurable effect on Mg-chelatase activity. The potent ferrochelatase inhibitors, N-methylmesoporphyrin and N-methylprotoporphyrin, inhibited Mg-chelatase at micromolar concentrations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Chen N. C. N-Methyl Mesoporphyrin IX Inhibits Phycocyanin, but Not Chlorophyll Synthesis in Cyanidium caldarium. Plant Physiol. 1983 Feb;71(2):263–268. doi: 10.1104/pp.71.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Holroyd J. A., Vernon D. I., Troxler R. F., Smith K. M. The effect of N-methylprotoporphyrin IX on the synthesis of photosynthetic pigments in Cyanidium caldarium. Further evidence for the role of haem in the biosynthesis of plant billins. Biochem J. 1982 Nov 15;208(2):487–491. doi: 10.1042/bj2080487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Dailey H. A. Effect of sulfhydryl group modification on the activity of bovine ferrochelatase. J Biol Chem. 1984 Mar 10;259(5):2711–2715. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E., Harbin B. M. Ferrochelatase from Rhodopseudomonas sphaeroides: substrate specificity and role of sulfhydryl and arginyl residues. J Bacteriol. 1986 Jan;165(1):1–5. doi: 10.1128/jb.165.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A. Spectroscopic examination of the active site of bovine ferrochelatase. Biochemistry. 1985 Mar 12;24(6):1287–1291. doi: 10.1021/bi00327a003. [DOI] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Hanamoto C. M., Castelfranco P. A. Separation of Mg-Protoporphyrin IX and Mg-Protoporphyrin IX Monomethyl Ester Synthesized de novo by Developing Cucumber Etioplasts. Plant Physiol. 1982 Feb;69(2):421–423. doi: 10.1104/pp.69.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wong Y. S., Castelfranco P. A. Localization of Mg-Chelatase and Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase Activities within Isolated, Developing Cucumber Chloroplasts. Plant Physiol. 1984 Jul;75(3):662–664. doi: 10.1104/pp.75.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wright L. A., Castelfranco P. A. Properties of Magnesium Chelatase in Greening Etioplasts: METAL ION SPECIFICITY AND EFFECT OF SUBSTRATE CONCENTRATIONS. Plant Physiol. 1981 Feb;67(2):246–249. doi: 10.1104/pp.67.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fufsler T. P., Castelfranco P. A., Wong Y. S. Formation of Mg-Containing Chlorophyll Precursors from Protoporphyrin IX, delta-Aminolevulinic Acid, and Glutamate in Isolated, Photosynthetically Competent, Developing Chloroplasts. Plant Physiol. 1984 Apr;74(4):928–933. doi: 10.1104/pp.74.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. D., Honeybourne C. L., Smith K. M., Tabba H. D., Jones O. T. The use of N-methylprotoporphyrin dimethyl ester to inhibit ferrochelatase in Rhodopseudomonas sphaeroides and its effect in promoting biosynthesis of magnesium tetrapyrroles. Biochem J. 1982 Nov 15;208(2):479–486. doi: 10.1042/bj2080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. Ferrochelatase of spinach chloroplasts. Biochem J. 1968 Mar;107(1):113–119. doi: 10.1042/bj1070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Wu S. M., Kuhadja M., Daniell H., Perkins E. J. Chlorophyll a biosynthetic routes and chlorophyll a chemical heterogeneity in plants. Mol Cell Biochem. 1983;57(2):97–125. doi: 10.1007/BF00849189. [DOI] [PubMed] [Google Scholar]
- Thomas J., Weinstein J. D. Measurement of heme efflux and heme content in isolated developing chloroplasts. Plant Physiol. 1990 Nov;94(3):1414–1423. doi: 10.1104/pp.94.3.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy B. C., Rebeiz C. A. Chloroplast biogenesis. Demonstration of the monovinyl and divinyl monocarboxylic routes of chlorophyll biosynthesis in higher plants. J Biol Chem. 1986 Oct 15;261(29):13556–13564. [PubMed] [Google Scholar]
- Walker C. J., Mansfield K. E., Rezzano I. N., Hanamoto C. M., Smith K. M., Castelfranco P. A. The magnesium-protoporphyrin IX (oxidative) cyclase system. Studies on the mechanism and specificity of the reaction sequence. Biochem J. 1988 Oct 15;255(2):685–692. [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Mansfield K. E., Smith K. M., Castelfranco P. A. Incorporation of atmospheric oxygen into the carbonyl functionality of the protochlorophyllide isocyclic ring. Biochem J. 1989 Jan 15;257(2):599–602. doi: 10.1042/bj2570599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]


