Highlights
-
•
The investigation focuses on the effects of three edible oils, monoglyceride concentration (MG), and production temperature on oleofoam properties.
-
•
The storage modulus and loss modulus of oleofoams, as well as their firmness and melting point, increase as the production temperature decreases.
-
•
The stability, overrun, and foamability of oleofoams improve when produced at low temperatures and with high MG concentrations.
-
•
Oleofoams prepared using sunflower oil with high (MG) concentration at low temperature production exhibit excellent stability.
Keywords: Oleofoam, Rheological properties, Stability, Fatty acid, Monoglyceride
Abstract
This study explores the impact of oil type, surfactant concentration, and production temperature on oleofoam properties. Oleofoams were prepared using different concentrations (5, 8, and 10 % w/w) of monoglyceride (MG) in olive, soybean, and sunflower oils at temperatures of 25 °C and 5 °C. The results indicate that higher surfactant concentrations and lower production temperatures enhance the stability, foamability, melting behavior, and hardness of the oleofoams, while minimizing oil drainage. Microscopic analysis reveals that lower production temperatures result in smaller bubble sizes in all oil blends which reduces oil loss and increases the hardness of the oleofoam. Also, oleofoams derived from different oils exhibit solid-like behavior. Among the oils studied, the oleofoam prepared with sunflower oil, at a concentration of 10 % MG and a production temperature of 5 °C, demonstrates superior properties. These findings provide valuable insights into optimizing oleofoam properties by controlling the oil type, surfactant concentration, and production temperature.
Introduction
Oleofoam is a soft material composed of a three-dimensional network of oil droplets and wax crystals, with air bubbles dispersed throughout the system, which is prepared by combining vegetable oils with a foaming agent, such as a surfactant or an emulsifier, followed by whipping or agitating the mixture to create a foam-like texture (Da Silva, 2022) . Oleofoams have several unique properties that make them useful in lots of applications, including low cost, zero calories, being free of synthetic surfactants, and the lightweight nature of the air bubbles, in addition to hypoallergenic properties (Fameau et al., 2015). Oleofoams also cause a significant decrease in microbial spoilage, thereby reducing the need for preservatives (Metilli et al., 2022) and extending the product shelf-life without substantial degradation or deterioration (Qiu et al., 2022). In food applications, oleofoams are explored as a way to create new types of foams and aerated foods such as cake, confectionary and sauce (Mohanan et al., 2023; Saremnejad et al., 2020; Chisholm et al., 2019, Gunes et al., 2018). Oleofoams are promising novel materials with many potential applications, and ongoing research has explored their properties and possible uses. Generally, oleofoam is a vital component of many food products and can be used to develop a wide range of textures and flavors. Versatility and adaptability make Oleofoam an exciting area of research and development in the food industry.
According to the literature, forming and controlling air bubbles in large quantities is difficult owing to their tendency to move and combine with other substances. Despite these challenges, significant efforts have been made to overcome them (Murray, 2019). However, many factors influence the structure of the resulting oleofoam, some of which are related to the cooling rate (Andriotis et al., 2022; Liu & Binks et al., 2021), shear rate (Mishra et al., 2019), and crystal properties (Heymans et al., 2018, Truong et al., 2019), the length and type of the hydrophobic chain (Callau et al., 2020; Zhao et al., 2020), the concentration and the type of the foaming agent (Du et al., 2021, Grossi et al., 2023; Qiu et al., 2022), solubility boundary (Pugh, 2016), The whipping ability of oleogels and the whipping time (Gu et al., 2023) are crucial for forming oleofoams. These factors affect the formation and the ability of the oleogel to retain air, resulting in a stable foam.
Several studies have examined the influence of structural agents and their combinations on the physical, microstructural, stability, and rheological properties of oleofoam (Fameau and Saint-Jalmes, 2020, Heymans et al., 2017). However, limited research has been conducted on the impact of oil type on the properties of oleofoam, despite the significant role of oil in structure formation. Liu et al. (2021) investigated the stability and microstructure of oleofoams produced from refined peanut oil and extra virgin olive oil under specific temperature conditions. Their findings revealed that triglyceride crystals with a high melting point contained a higher proportion of saturated fatty acids compared to the original oil. Subsequently, Liu and Binks (2022) explored the foaming behavior of mixtures comprising 10 % Span 80 and various oils, including rapeseed oil, sesame oil, extra virgin olive oil, refined peanut oil, corn oil, high oleic sunflower oil, and soybean oil. They observed distinct foaming behaviors among different oils, but the reasons for these variations were not elucidated. Additionally, Goli et al. (2023) investigated the effects of coconut oil, rapeseed oil, sunflower oil, flaxseed oil, and a mixture of glycerol monostearate and beeswax (GMS-BW; 1:1) as an oleogelator on oleofoam properties. Their results indicated that flaxseed oil oleofoam outperformed other oils, exhibiting higher melting and crystallization enthalpy.
However, it is still not well understood the effect of gelator concentration, the nature of the oil, and production temperature on oleofoam properties. The hypothesis of this study is that manipulating the oil type, surfactant concentration, and production temperature will have a significant impact on the stability and functional characteristics of oleofoam. Specifically, we predict that specific combinations of oil type, surfactant concentration, and production temperature can lead to improvements in the stability, foamability, melting behavior, and hardness of the oleofoam. By carefully controlling these factors, we anticipate achieving a more favorable microstructure and rheological properties in the oleofoam, ultimately enhancing its overall quality.
Materials and methods
Materials
The surfactant Distilled monoglyceride, a mixture of about 95 % MG, 1 % free glycerol, and 1.5 % free fatty acids with a melting point of 65 °C, was purchased from Palsgaard, Denmark. Sunflower oil (relative density 0.918 and viscosity 58 cP at 20 °C), olive oil (relative density 0.909 and viscosity 62 cP at 20 °C), and soybean oil (relative density 0.915 and viscosity 54 cP at 20 °C) purchased from a local market in Iran.
Oleofoam preparation
The oleofoam samples were prepared based on a previously published method with some modifications (Saremnejad et al., 2020). Specific amounts of the oil were heated and mixed with the MG (5, 8, and 10 w/w) at 80 °C. Then, the mixtures were cooled in a refrigerator at 5 °C for 24 h, afterward, the samples were allowed to equilibrate to room temperature (25 °C), except the ones prepared in a cold-water bath at 5 °C. The capability of foam formation can be regulated by adjusting the surfactant concentration at a constant temperature or producing foams with a constant surfactant concentration at different temperatures. Therefore, in this study, the effects of the oil type (olive, soybean, and sunflower), temperature (25 and 5 °C), and MG level (5, 8, and 10 % w/w) were investigated on the oleofoam properties. The foams were prepared by whipping the oil and the surfactant at the highest speed of a 5-speed mixer (Gosonic, model GHM-818, 250 W, China). An external thermometer monitored individual samples' temperature during the aeration process. Whipping was done intermittently for 60 min, performed as 5 min of whipping followed by 5 min of rest (total whipping time of 30 min). The resulting oleofoams were used for further analyses conducted in triplicate.
Oleofoam characterization
Overrun and foamability
Overrun was measured by determining the amount of air incorporated in the oleofoam. At 5-min intervals of whipping, three 10-ml plastic cups were used to compare the foam volume with the unwhipped mixtures. The overrun was calculated using Eq. (1) (Binks & Marinopoulos, 2017), and the measure of foamability in oleofoams was determined by Eq. (2) (Saremnejad et al., 2020)
| (1) |
| (2) |
Oleofoam stability
10 ml of the oleofoam was transferred to a 50 ml centrifugal tube to evaluate its long-term stability at 25 °C throughout three months,. The stability was computed using Eq. (3) (sheng et al., 2018).
| (3) |
Optical microscopy and bubble size distribution
A sample was removed from the center of the foam and sealed by being gently pressed between two cover slides. Light microscopy (Olympus BX41, Japan) was used to observe the sample at ambient temperature. The micrographs were captured using a digital camera (Canon EOS 1000D). The dimensions of about 500 bubbles from at least three images were quantified using ImageJ 1.50f for Windows.
Differential scanning calorimetry (DSC)
A DSC device (PerkinElmer, USA) was used to investigate the thermal properties of the oleofoams. Approximately 5 mg of the sample was weighed in a 5-ml aluminum pan and sealed with a press machine. Calibration was performed using an empty aluminum pan as the reference. The DSC measurements of the oleofoams were conducted using a heating cycle between 0 and 100 °C at a constant rate of 5 °C/min in absolute value.
Texture analysis
The texture of the oleofoam samples using a TA.XT plus texture analyzer (stable micro-systems, England) equipped with an acrylic cylindrical probe with a diameter of 25 mm and a height of 35 mm was assessed. After refrigerating the samples for 24 h, the plastic cups (with a volume of 35.0 ml and a diameter of 3.5 cm) were tested at 25 °C. The puncture tests were conducted at the probe speed of 1 mm/s, penetrating the sample to a depth of 20 mm. The trigger force was set at 0.01 N. The hardness was defined as the maximum force (N) recorded by the probe (Heymans et al., 2013).
Rheological properties
The rheological properties of the oleofoams were measured at 25 °C using the Physica MCR 301 rheometer (Anton-Paar, GmbH, Graz, Austria) with a serrated parallel-plate geometry, 40 mm in diameter (gap 1000 µm). To determine the viscoelastic properties of the oleofoams, dynamic rheological properties were calculated, which can be divided into two separate parts as follows: 1- Strain sweep test performed at a constant frequency of 1 Hz and in the strain range of 0.1–10 %. This test was conducted on all the samples to determine the linear viscoelastic region. 2- Frequency sweep test in which the frequency varied between 0.1 and 100 Hz at a constant strain of 0.01 %. G', G“, loss tangent (tan δ), and complex viscosity (η*) were quantified.
Statistical analysis
The collected data were analyzed statistically and expressed as mean ± standard deviation (SD). The analysis of variance (ANOVA) was performed using the statistical software Minitab v17.0 (Minitab, Inc., State College, PA) to determine the significant differences in the mean values; Duncan's test was utilized at a significance level of P < 0.05. All figures were drawn using origin pro 2022 (v.9.9.0.225).
Results and discussion
Overrun and foamability
As shown in Fig. 1 (A, B), the foamability and overrun of all the samples significantly increased with a rise in the MG level from 5 to 10 %, which could be attributed to the adsorption of the surfactant at the oil-air interface. The formation of firmer network structures and increased thickness of the crystal adsorption layer at the interface can be attributed to a higher number of tiny crystals present in the system, as reported by Jakubczyk and Niranjan (2006) and (Matsuo (2022), These changes may enhance the foaming ability of the system by improving the retention of air bubbles within the continuous phase. MG reaches the solubility phase boundary at high concentrations in oil, and the insoluble MG increases the foam stability. This finding was confirmed previously reported by (Saremnejad et al., 2020; Binks et al., 2011).
Fig. 1.
Overrun of oleofoam samples produced at 25 °C and 5 °C (A) and foamability of oleofoam samples produced at 25 °C and 5 °C (B).
The foamability and overrun of the samples were significantly influenced by the temperature increase from 5 to 25 °C. It was observed that the foamability of all samples reached its maximum at 5 °C and decreased at 25 °C. The presence of an adequate amount of solid fat content in the system was found to be crucial for successful foaming. This is supported by previous research conducted by Himawan et al. (2006), which suggests that a higher degree of supercooling can enhance the nucleation and growth kinetics of triglycerides. These findings underscore the importance of crystal formation in achieving optimal foaming ability. Additionally, Liu and Binks, (2021) investigated the production of oleofoam from olive and peanut oils and observed that the highest foamability was obtained at the lowest temperature. However, it slightly lowered as the temperature was elevated. Mishra et al. (2020) have reported that the rise in an overrun of whole samples at lower temperatures (5 °C) can be explained by the formation of α crystals, which transform to β crystals during the aeration process.
However, the effect of the oil type on the foam volume was less pronounced than that of the MG concentration and production temperature as seen in Fig. 1 (A, B). The results demonstrated that the olive oleofoams had the highest foam volume; due to the fact that olive oil has a higher percentage of saturated fatty acid crystals (18.89 %) compared to soybean (16.56 %) oils and sunflower (13.45 %), which contain low-melting point triglycerides. This higher saturation, in combination with (MG), enhances its ability to stabilize air bubbles by co-crystallizing with MAG during blending. As a result, olive oil exhibits superior foaming properties compared to other oils. This result agrees with Liu and Banks (2021 and 2022), who also reported that sesame oil showed higher foamability than other oils due to high saturated fatty acid content (Liu and Banks, 2022). It could be suggested that high MG levels and low temperatures have a synergetic effect on the foam volume.
The effect of whipping time on the foamability of the samples is depicted in (Fig. 2). During beating for 60 min (5 min of whipping followed by 5 min of rest; i.e., total whipping time of 30 min), the foam volume of the entire samples considerably increased in the first 5 min and approximately remained constant after that. The initial increase in foamability observed within the first 5 min of whipping can be attributed to the high adsorption of MG crystals at the O-A interface, allowing for the incorporation of more air. However, the foam volume remains constant due to the limited availability of emulsifiers. Samples with low MG concentration, such as SFO 5 % and SO 5 %, exhibit a soft foam structure prone to collapse, resulting in decreased foam volume during whipping. This collapse is due to the combined effects of increasing temperature and mechanical stress from the mixer. Similar results have been reported for the whipping of sunflower oleofoams prepared with different concentrations of mono and diglyceride (Saremnejad et al., 2020) and high-oleic acid sunflower oleofoams prepared with myristic acid at different concentrations (Liu and Binks, 2021).
Fig. 2.
Relationship between whipping time and foam volume of 5, 8, and 10 wt% MG-oil mixtures up to 1 h at different temperatures at 25 °C and 5 °C, (A) sunflower oil, (B) Olive oil and (C) soybean oil.
Foam stability
The stabilities of the olive, soybean, and sunflower oleofoams after three months of storage are denoted in (Fig. 3 A, B). Oil drainage and air expulsion are the reasons behind foam instability, both observed in this research. The results indicated that all the factors significantly impacted the foam stability, as increased MG content and decreased production temperature raised the foam stability. The oil type had a noticeable effect on the response. The oleofoam of the sunflower oil had higher stability than the other oils. In the olive oil samples, only oil drainage was the reason for instability, and this phenomenon is caused by an increase in coalescence due to a low quantity of crystals in the continuous phase, resulting in a decrease in viscosity. However, it was resolved with a rise in the MG level due to a sufficient number of crystals in the bulk phase or a reduction in the production temperature, which increases the solid fat content. In the soybean oleofoams, instability was caused by the collapse of the air bubbles and oil drainage at 25 °C; however, only the air bubbles collapse was observed at 5 °C. This difference can originate from the difference in the fatty acid composition of the oils because research has proved that oleofoam stability increases as the content of saturated fatty acids is elevated (Liu et al., 2022). The results also indicated that the highest stability (100 %) of the sunflower oleofoam containing 5, 8 and 10 % of the MG was obtained at 5 °C. Among all the sunflower oleofoams, a slight collapse of the air bubbles was only observed in the ones prepared at 25 °C with the lowest level of the MG, which was not observed in the ones prepared at 5 °C. Oil drainage was observed in all the olive oleofoams. The lowest stability (46.5 %) belonged to the soybean oleofoams containing 5 % of the MG and prepared at 25 °C, in which both the instability phenomena (bubble collapse and oil drainage) were observed. Bubble collapse occurred in the whole soybean samples, except the one prepared with 10 % MG at 5 °C, which proved that sufficient crystals in the oil/air (A/O) surface prevented bubble air collapse. Qiu et al. (2021) assessed the production of soybean oleofoam with medium-long chain diacylglycerol (MLCD) and β-sitosterol (St). They observed the air bubbles collapse during storage, conforming to the results of the present study. They also found that the foam stability rose as the MLCD concentration was increased to 20 % (w/w), which diminished the oil phase separation. This was ascribed to the larger amount of MLCD crystals, which prevented the coalescence and collapse of the air bubbles. At the same time, using high concentrations of MLCD did not result in oleofoams with sufficient stability, as the oil drainage began immediately after production and continued for 96 h. This was since the formation of an elastic oleogel was a precursor to the inhibition of the oil drainage and the reduction in the rate of the air bubbles coalescence (Heymans et al., 2017, Hunter et al., 2008, Qiu et al., 2021).
Fig. 3.
Stability of oleofoam samples at different MG levels (5, 8 and 10 wt%) and temperatures (5 °C and 25C°) (A) and the picture of oleofoams after storage (B).
The MG concentration also had a significant effect on the foam stability. The higher the MG percentage, the more stable the foam, as the surfactant molecules cover the oil-air interface and restrain the foam collapse (Mishima et al., 2016; Saremnejad et al., 2020). Additionally, by increasing the concentration of surfactant, the average size of crystals decreases, leading to a corresponding increase in the stability of oleofoams. MG adsorbs at the A/O interface at low concentrations, but its presence is insufficient to prevent bubble dissolution within the system. Similarly, the foam produced at 25 °C using sunflower oil and 2 wt% MDG- was slightly stable, whereas it was stable during long-term storage when the surfactant level was 10 % (Saremnejad et al., 2020). Fameau & Saint-Jalmes (2017) also confirmed the high stability of the oleofoam at myristic acid concentrations above 8 %. Temperature also affected the foam stability significantly.
Production temperature was also a compelling factor in the stability of the oleofoams, as the samples produced at 5 °C were more stable than those prepared at 25 °C because lower temperature increases the number of crystals in the oil phase, giving rise to the foam stability (Arnaudova et al., 2022); Additionally, low temperatures enhance the formation of small bubbles, the size and the homogeneity of the initial bubble size distribution are essential for the stability of oleofoam. Small bubbles can be perfectly surfaced with a dense layer to protect them from coalescence and Oswald ripening (Callau et al., 2020). This finding is consistent with Callau et al.'s study, which showed that fast cooling can decrease the size of bubbles and increase their stability against oil loss. Also, Binks et al. (2016) discovered that the formation of stable foams was restricted to lower temperatures (22 °C and 30 °C) when utilizing crystal dispersions. Conversely, no foam formation was achievable at elevated temperatures (35 °C and 40 °C) when employing molecular solutions. Another reason could be the nucleation rate of the α crystals under rapid cooling, then during whipping, the heating may cause the transformation of the crystalline state, and the β nucleation starts following the melting of α (Mishima et al., 2016). As a result, applying sunflower oil at high concentrations and low temperatures could improve the stability of the foam.
Optical microscopy and bubble size distribution
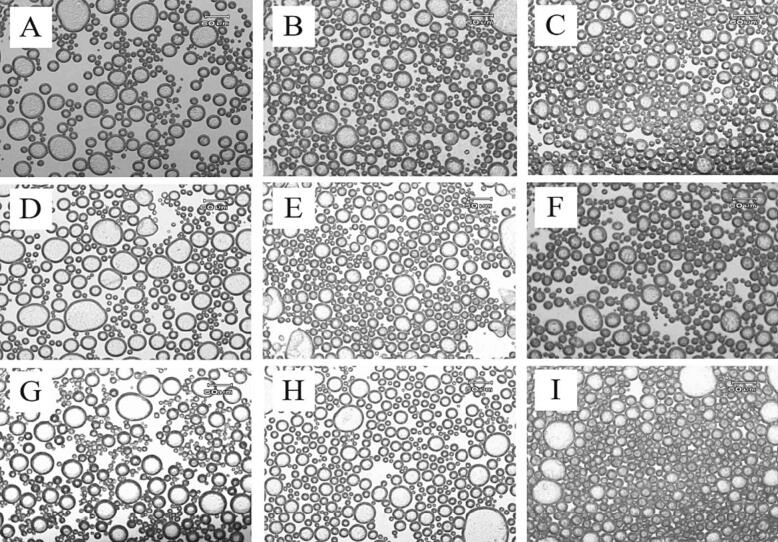
In an emulsifier-rich system, bubble size distribution can be associated with the size and shape of crystals and determine the bubbles' stability and microscopic images are significant as they provide a detailed view of the structure and properties of oleofoam. Previous studies have shown that the process of foam production highly influences this characteristic (Heymans et al., 2017). In (Fig. 4a, b), it can be observed that the stabilized oleofoams exhibited interfacial crystallization; all bubbles are surrounded with dark layers indicated by the close packing of crystal layers around the air bubbles, which is evidence of the presence of crystals adsorbed at the A/O surface that hinders shape relaxation. the size of the bubbles varied as a function of MG concentration; at high concentrations of MG (Fig. 5). The bubbles shrank in all prepared samples as the MG concentration was elevated from 5 % to 10 %. Smaller bubbles predominated at the highest percentage of the MG (10 %), and the largest ones were observed at 5 % MG. These results agreed with those previously declared by (Du et al., 2020, ؛ Saremnejad et al., 2020؛ Grossi ، et al., 2023). The A/O interface is covered with a few crystals at low MG concentrations, which cannot stabilize the bubbles properly. On the other hand, at high levels of MG, the sample is filled with more tightly arranged bubbles. The complete bubble structure can be formed when enough crystals are adsorbed at the interface. In other words, they can be regarded as Pickering stabilization-based oleofoams in which crystals act as specific solid particles (Du et al., 2020). The higher the surfactant concentration, the smaller the bubbles and the more stable the foam (Saremnejad et al., 2020) since smaller bubble is less deformable than lager air bubbles (according to the Laplace pressure theory), that is why it decreases of drainage (Du et al., 2021).
Fig. 4a.
Micrographs of oleofoams contained sunflower oil with 5 % MG (A), sunflower oil with 8 % MG (D), sunflower oil with 10 % MG (G), Olive oil with 5 % MG (B), Olive oil with 8 % MG (E), Olive oil with 10 % MG (H) and soybean oil with 5 % MG (C), soybean oil with 8 % MG (F), soybean oil with 10 % MG (I) oleofoams prepared at 25 °C (scale bar = 60 µm).
Fig. 4b.
Micrographs of oleofoams contained sunflower oil with 5 % MG (A), sunflower oil with 8 % MG (D), sunflower oil with 10 % MG (G), Olive oil with 5 % MG (B), Olive oil with 8 % MG (E), Olive oil with 10 % MG (H) and soybean oil with 5 % MG (C), soybean oil with 8 % MG (F), soybean oil with 10 % MG (I) oleofoams prepared at 5 °C (scale bar = 60 µm).
Fig. 5.
The relative frequency distribution of bubble sizes at various MG levels (5, 8 and 10 wt%) and temperatures (5 °C and 25C°) determined in soybean oil (A), Olive oil (b) and sunflower oil (C).
As shown in (Fig. 4a, b), the air bubbles in the oleofoams produced at 5 °C were more spherical and the structure was more condensed than those made at 25 °C due to a sufficient and rigid crystal layer around the bubbles and higher solid fat content at low temperatures, which hindered shape relaxation. The production temperature influenced the bubble size distribution, as the small bubbles formed in the olive and sunflower oleofoams at 5 °C accounted for more than 80 %. In contrast, their percentage was in the range of 60–70 % at 25 °C. This can be attributed to the rise in the oils' viscosity at low temperatures, leading to the formation of small air bubbles (Wildmoser et al., 2004).
Furthermore, the results showed that the sunflower and olive oils did not affect the bubble size distribution considerably because they had similar behaviors at both production temperatures and all the MG levels. On the other hand, the number of small bubbles was smaller in the soybean oleofoams and equal to about 50 % at 5 °C and 60 % at 25 °C, which can be ascribed to the chemical composition of soybean oil.
Melting resistance
The peak melting temperature (Tm) of an oleofoam indicates its melting resistance and ability to retain its structure at various temperatures. It can be observed in (Fig. 6) that Tm significantly increased as the MG concentration was raised, which was more pronounced in the sunflower oleofoams. As the MG level was increased from 5 to 10 %, the Tm of the sunflower samples prepared at 5 °C rose from 61 to 66 °C and from 62.8 to 65.6 °C in the case of those produced at 25 °C significantly. This confirmed that the rise in the MG concentration led to forming of a stronger crystal network with more heat resistance. The oleofoams collapsed when the temperature was increased because the MG crystals were melted at the oil-air interface, and the effect of the air stabilization was lost. The dependence of oleogels' melting resistance on the concentration of oleo gelators has already been reported. Previous studies on the thermal analysis of oleogels prepared with MAG (Pérez-Monterroza et al.,2016) and oleofoams manufactured from soybean oil at different concentrations of MG (Du et al., 2020) have shed light on this relationship.
Fig. 6.
Peak melting temperatures of oleofoam samples (Tm) for oleofoams with different MG concentrations, production temperatures and vegetable oils.
The oil type significantly influenced the melting points of the samples (P < 0.05). The Tm of the olive oleofoams was the lowest of all, which ranged from 59.1 to 63.6 °C for those prepared at 25 °C and from 57.8 to 61.3 °C for those produced at 5 °C. The highest Tm was 63.6 °C, belonging to the sample containing 10 % MG and prepared at 5 °C. The literature has shown that fatty acids and esters' melting and crystallization behavior tightly depends on structural properties such as chain length, the position and conformation of double and triple bonds and functional groups (Knothe and Dunn, 2009). In general, unsaturated fatty acids have lower melting points than the corresponding saturated ones with the same chain length (Kostik et al., 2013). The lower Tm of the olive oleofoams could be because olive oil has more content of monounsaturated fatty acids (66.88) % than the soybean and sunflower ones. Additionally, olive oil contains more saturated fatty acids than sunflower and soybean oil, further contributing to its lower melting point. Similarly, Fasina et al., 2008 reported that the amount of the monounsaturated or polyunsaturated is highly correlated with the onset melting temperature, peak melting temperature, and melting enthalpy for the 12 vegetable oil samples.
The results also demonstrated that the production temperature significantly affected the Tm (P < 0.05). The processing time and temperature strongly impact the final properties of the oil-crystal mixture, particularly in the case of MGs (Andriotis et al., 2022). All the oleofoam samples prepared at 5 °C had higher melting points than those designed at 25 °C, probably owing to forming a firmer network at 5 °C, leading to the rise in the melting point. The low production temperature (5 °C) resulted in the transformation of sub-α crystals to the more stable β ones, which melt at higher temperatures (Chen and Terentjev, 2009). Also, it was reported that fast cooling promotes the growth of smaller crystals in high numbers in the A-o interface (Callau et al.,2020). (Andriotis et al., (2022) reported similar results for oleogels produced with medium-chain triglyceride (MCT), 20 % glycerol monostearate (GMS), and 5 % glycerol monoolein (GMO) and stored at 4 °C for 24 h. Similarly, (Wei et al., 2021) examined the effects of cooling temperature and crystal morphology on the oleogel-structured emulsion to enhance the oxidative stability of perilla oil and found that the emulsion containing stearic acid prepared at 4 °C had a higher melting point (56.04 °C) than that prepared at 20 °C (52.56 °C).
Texture analysis
The texture of the sample was analyzed after 24 h of refrigerated storage. The results showed that the production temperature did not significantly affect the foams' hardness (Fig. 7). At the same time, the oil type and the MG concentration significantly affected this response. The variations in texture characteristics may have resulted from the different types of fatty acids found in oils used as the dispersing medium and the high level of crystal formation and interaction of crystals in the continuous phase (Heymans et al., 2013). The highest hardness was associated with the sunflower oleofoams, but no significant differences existed between the olive and soybean samples. Therefore, it sounds that the large amount of air in the olive oil oleofoams did not cause a rise in viscosity and hardness, which is consistent with that reported by (Heymans et al., 2018), who evaluated the hardness of the oleofoam of sunflower oil and MG, as they realized that there was a strong negative correlation between overrun and hardness. Moreover, the hardness of the samples was raised as the MG level was increased, which can be owing to the formation of more tightly arranged bubbles, more concentrated bubble size distribution(a high quantity of small air bubbles), and stronger surface tension in the oleofoams at higher MG concentrations. Du et al., 2021 produced oleofoams with different percentages of MG and declared that a rise in the MG level remarkably elevated the hardness and surface tension of the system containing oil and air, which was more resistant to external stress.
Rheological properties
Dynamic rheological tests (strain sweep and frequency sweep) were conducted to investigate the mechanical properties of the oleofoam samples. It is depicted in (Fig. 8a) that all the samples had a solid-like behavior with G' > G“ below the crossover strain except sample soybean oil with 5 % MG at 25C where LVR was observed at low strain, G' > G,” indicating the formation of an elastic structure. In other words, the samples with larger G' values were more stable, leading to a greater ability to withstand external stress. The soybean oil sample with 5 % MG at 25C has a weak gel behavior, illustrating its lower stability. Additionally, it was observed that G' and G“ were elevated as the MG concentration rose, revealing the effectiveness of the MG level in the rheological properties of the oleofoams, as the higher MG contents could enhance the rigidity of the network to stabilize more air bubbles at the oil-air interface and accelerate the formation of the oleofoam network structure, thus improving the rheological properties of the foam system (Zhang et al., 2023). A regular decrease in the crossover point of storage modulus (G') and loss modulus (G”) was observed with increasing monoglyceride (MG) concentrations, indicating that oleofoams with higher concentrations of MG exhibit greater resistance to external stress, this result in agreement with (Du et al., 2020). Low-temperature production increased the G' and G“ values but did not affect the elastic structure significantly. Observations indicate that oleofoam produced using sunflower oil displays the highest storage and loss modulus within the linear viscoelastic region. This phenomenon may be attributed to the presence of a rigidly-structured adsorbed MG layer surrounding the dispersed bubbles, which is likely a result of the fat's crystalline properties of the oil.
Loss tangent (tanδ) is defined as the ratio between G' and G“. It is a measure for evaluating the viscoelastic behavior of an oleofoam (Mandala et al., 2004). The tanδ < 1 of the oleofoams revealed their elastic behavior and capability of forming weak gels. As the strain percentage was increased, G' and G” declined, and the crossover point appeared in the curve, signifying the foam-liquid transition and the breakdown of the interactions present in the solid-like structure of the oleofoams (Lei et al., 2020).
The results of the frequency sweep test performed at a constant strain and 25 °C are illustrated in (Fig. 8b). For all the samples, G' was bigger than G“ within the applied frequency range and exhibited solid-like behavior. The values of both storage modulus (G') and loss modulus (G”) were found to increase with an increase in monoglyceride (MG) concentration, which is consistent with the higher levels of crystalline MG present in the oleofoams. At low temperatures (5 °C), the oleofoams exhibited increased G' and G“ values, which can be attributed to suitable structuring surfactant crystals and higher levels of solid fat content. This, in turn, promotes the efficient adhesion of small crystalline particles to the air-oil (A-O) interface and increases the hardness of the oleofoam. Lei et al. (2019) revealed that cooling increased the hardness of the fully hydrogenated palm oil – Diacylglycerol (FHPO-DAG) oleofoam. Between studied oils (olive, soybean, and sunflower), sunflower oil had the higher G' values it is probably due to its TAG composition; it was indicated that control of oil temperature could induce crystallization of triglyceride molecules with high melting points, resulting in the formation of a concentrated layer of adsorbed crystals at the interfaces of bubbles. While triglyceride molecules with low melting points remain liquid and fortify the gel network in the continuous oil phase (Fameau et al., 2021). At low frequencies (<0.1 Hz), a crossover point was observed for the olive and sunflower oleofoams produced at 25 °C and the soybean prepared at 5 °C. At this point, the rheological behavior is transitioned from elastic to viscous, and it might be due to the internal structure of the solid lipid placket crystals having the ability to release stress and promote relaxation, as reported by Fameau et al., 2015, Heymans et al., 2018 and Shrestha et al. (2010) have observed that the foam structure may undergo rearrangement during testing. Therefore, it can be mentioned that all the oleofoam samples were able to form a weak gel, which was intensified with the increase in the MG concentration. The G' and G” obtained from the frequency sweep and strain sweep tests were in complete agreement. The tanδ < 1 and the dependence of the moduli on the frequency showed the ability of the oleofoams to form a weak gel. It was also realized that the G' and G“ of the sunflower oleofoams containing 10 % MG produced at 5 °C were higher than those of the corresponding soybean and olive samples. This indicated the stronger network of the sunflower oleofoams compared with the others. These results were in line with those of the texture analysis.
Conclusions
The results of this study showed that the concentration of the surfactant, oil type, and production temperature had significant impacts on all the characteristics of the oleofoams. The samples prepared at the highest MG concentration and low production temperature had smaller air bubbles and higher stability than the others, and all the oleofoams had gel-like and viscoelastic properties. It was also found that the type of oil significantly affected the quality indices of the oleofoams. The extent of these changes and their effects on the oleofoam properties depended on the fatty acid composition of the oils. It was realized that sunflower oil, the highest MG concentration and the low production temperature, had desirable properties for use as a fat substitute in food products.
CRediT authorship contribution statement
Fayza Hussein Alhasan: Conceptualization, Methodology, Writing – original draft, Investigation, Software. Mostafa Mazaheri Tehrani: Supervision, Writing – review & editing, Project administration. Mehdi Varidi: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Ferdowsi University of Mashhad (FUM) grant number [3/54227].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101033.
Contributor Information
Mostafa Mazaheri Tehrani, Email: mmtehrani@um.ac.ir.
Mehdi Varidi, Email: m.varidi@um.ac.ir.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Andriotis E.G., Monou P.K., Komis G., Bouropoulos N., Ritzoulis C., Delis G.…Fatouros D.G. Effect of glyceryl monoolein addition on the foaming properties and stability of whipped oleogels. Gels. 2022;8(11):705. doi: 10.3390/gels8110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaudova T., Mitrinova Z., Denkov N., Growney D., Brenda R., Tcholakova S. Foamability and foam stability of oily mixtures. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2022;653 doi: 10.1016/j.colsurfa.2022.129987. [DOI] [Google Scholar]
- Binks B.P., Marinopoulos I. Ultra-stable self-foaming oils. Food Research International. 2017;95:28–37. doi: 10.1016/j.foodres.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Callau M., Sow-Kébé K., Jenkins N., Fameau A.L. Effect of the ratio between fatty alcohol and fatty acid on foaming properties of whipped oleogels. Food Chemistry. 2020;333 doi: 10.1016/j.foodchem.2020.127403. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Terentjev E.M. Aging and metastability of monoglycerides in hydrophobic solutions. Langmuir. 2009;25(12):6717–6724. doi: 10.1021/la9002065. [DOI] [PubMed] [Google Scholar]
- Chisholm J., Rigby J.R., Bayliss M., Berg D.A., Dahle H., Gladders M., Sharon K. Constraining the metallicities, ages, star formation histories, and ionizing continua of extragalactic massive star populations∗. The Astrophysical Journal. 2019;882(2):182. doi: 10.3847/1538-4357/ab3104. [DOI] [Google Scholar]
- Da Silva F.L. In: EEG - fMRI. Mulert C., Lemieux L., editors. Springer; Cham: 2022. EEG: origin and measurement. [DOI] [Google Scholar]
- Du L., Jiang Q., Li S., Zhou Q., Tan Y., Meng Z. Microstructure evolution and partial coalescence in the whipping process of oleofoams stabilized by monoglycerides. Food Hydrocolloids. 2021;112 doi: 10.1016/j.foodhyd.2020.106245. [DOI] [Google Scholar]
- Fameau A.L., Carl A., Saint-Jalmes A., Von Klitzing R. Responsive aqueous foams. ChemPhysChem. 2015;16(1):66–75. doi: 10.1002/cphc.201402580. [DOI] [PubMed] [Google Scholar]
- Fameau A.L., Saint-Jalmes A. Non-aqueous foams: Current understanding on the formation and stability mechanisms. Advances in Colloid and Interface Science. 2017;247:454–464. doi: 10.1016/j.cis.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Fameau A.L., Saint-Jalmes A. Recent advances in understanding and use of oleofoams. Frontiers in Sustainable Food Systems. 2020;4:110. doi: 10.3389/fsufs.2020.00110. [DOI] [Google Scholar]
- Fasina O.O., Craig-Schmidt M., Colley Z., Hallman H. Predicting melting characteristics of vegetable oils from fatty acid composition. LWT-Food Science and Technology. 2008;41(8):1501–1505. doi: 10.1016/j.lwt.2007.09.012. [DOI] [Google Scholar]
- Goli S.A.H., Rezvani M., Abdollahi M. Beeswax and monoglycerol-based oil foam: Effect of oil type and oleogelator concentration on physicochemical, rheological properties and storage stability. Food Structure. 2023;37 [Google Scholar]
- Grossi M., Fang B., Rao J., Chen B. Oleofoams stabilized by monoacylglycerides: Impact of chain length and concentration. Food Research International. 2023;169 doi: 10.1016/j.foodres.2023.112914. [DOI] [PubMed] [Google Scholar]
- Gu X., Du L., Meng Z. Thermal-reversible lacquer wax-based oleofoams in dual stabilization with high ambient stability. Food Research International. 2023;167 doi: 10.1016/j.foodres.2023.112650. [DOI] [PubMed] [Google Scholar]
- Gunes D.Z., Engmann J., Gehin-Delval C., Schmitt C., Leser M.E. Foam films and foams. CRC Press; 2018. Foams in food; pp. 379–400. [Google Scholar]
- Heymans R., Tavernier I., Danthine S., Rimaux T., Van der Meeren P., Dewettinck K. Correction: Food-grade monoglyceride oil foams: The effect of tempering on foamability, foam stability and rheological properties. Food & Function. 2018;9(7):4036. doi: 10.1039/c8fo90023j. [DOI] [PubMed] [Google Scholar]
- Heymans R., Tavernier I., Dewettinck K., Van der Meeren P. Crystal stabilization of edible oil foams. Trends in Food Science & Technology. 2017;69:13–24. doi: 10.1039/C8FO00536B. [DOI] [Google Scholar]
- Himawan C., Starov V.M., Stapley A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Advances in Colloid and Interface Science. 2006;122(1–3):3–33. doi: 10.1016/j.cis.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Hunter T.N., Pugh R.J., Franks G.V., Jameson G.J. The role of particles in stabilising foams and emulsions. Advances in Colloid and Interface Science. 2008;137(2):57–81. doi: 10.1016/j.cis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Jakubczyk E., Niranjan K. Transient development of whipped cream properties. Journal of Food Engineering. 2006;77(1):79–83. doi: 10.1016/j.jfoodeng.2005.06.046. [DOI] [Google Scholar]
- Knothe G., Dunn R.O. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. Journal of the American Oil Chemists' Society. 2009;86(9):843–856. doi: 10.1007/s11746-009-1423-2. [DOI] [Google Scholar]
- Kostik V., Memeti S., Bauer B. Fatty acid composition of edible oils and fats. Journal of Hygienic Engineering and Design. 2013;4:112–116. ISSN 978–608-4565-03-1. [Google Scholar]
- Lei M., Zhang N., Lee W.J., Tan C.P., Lai O.M., Wang Y., Qiu C. Non-aqueous foams formed by whipping diacylglycerol stabilized oleogel. Food Chemistry. 2020;312 doi: 10.1016/j.foodchem.2019.126047. [DOI] [PubMed] [Google Scholar]
- Liu Y., Binks B.P. A novel strategy to fabricate stable oil foams with sucrose ester surfactant. Journal of Colloid and Interface Science. 2021;594:204–216. doi: 10.1016/j.jcis.2021.03.021. [DOI] [PubMed] [Google Scholar]
- Liu Y., Binks B.P. Fabrication of stable oleofoams with sorbitan ester surfactants. Langmuir. 2022;38(48):14779–14788. doi: 10.1021/acs.langmuir.2c02413. https://pubs.acs.org/doi/full/10.1021/acs.langmuir.2c02413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala I.G., Savvas T.P., Kostaropoulos A.E. Xanthan and locust bean gum influence on the rheology and structure of a white model-sauce. Journal of Food Engineering. 2004;64(3):335–342. doi: 10.1016/j.jfoodeng.2003.10.018. [DOI] [Google Scholar]
- Matsuo, K. (2022). Fabrication and Characterization of Oleofoams Composed of Tribehenoyl-glycerol: Toward a Stable and Higher Air-content Colloidal System. https://www.authorea.com/doi/full/10.22541/au.165841922.24895621. [DOI] [PubMed]
- Metilli L., Storm M., Marathe S., Lazidis A., Marty-Terrade S., Simone E. Application of X-ray microcomputed tomography for the static and dynamic characterization of the microstructure of oleofoams. Langmuir. 2022;38(4):1638–1650. doi: 10.1021/acs.langmuir.1c03318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima S., Suzuki A., Sato K., Ueno S. Formation and microstructures of whipped oils composed of vegetable oils and high-melting fat crystals. Journal of the American Oil Chemists' Society. 2016;93:1453–1466. doi: 10.1007/s11746-016-2888-4. [DOI] [Google Scholar]
- Mishra K., Bergfreund J., Bertsch P., Fischer P., Windhab E.J. Crystallization-induced network formation of tri-and monopalmitin at the middle-chain triglyceride oil/air interface. Langmuir. 2020;36(26):7566–7572. doi: 10.1021/acs.langmuir.0c01195. [DOI] [PubMed] [Google Scholar]
- Mishra K., Dufour D., Windhab E.J. Yield stress dependent foaming of edible crystal-melt suspensions. Crystal Growth & Design. 2019;20(2):1292–1301. doi: 10.1021/acs.cgd.9b01558. [DOI] [Google Scholar]
- Mohanan S., Guan X., Liang M., Karakoti A., Vinu A. Stimuli-responsive silica silanol conjugates: Strategic nanoarchitectonics in targeted drug delivery. Small. 2023;2301113 doi: 10.1002/smll.202301113. [DOI] [PubMed] [Google Scholar]
- Murray B.S. Pickering emulsions for food and drinks. Current Opinion in Food Science. 2019;27:57–63. doi: 10.1016/j.cofs.2019.05.004. [DOI] [Google Scholar]
- Pérez-Monterroza E.J., Ciro-Velásquez H.J., Tobón J.C.A. Study of the crystallization and polymorphic structures formed in oleogels from avocado oil. Revista Facultad Nacional de Agronomía Medellín. 2016;69(2):7945–7954. https://doi. org/10.15446/rfna.v69n2.59139 [Google Scholar]
- Pugh R.J. Cambridge University Press; 2016. Bubble and foam chemistry. [Google Scholar]
- Qiu C., Lei M., Lee W.J., Zhang N., Wang Y. Fabrication and characterization of stable oleofoam based on medium-long chain diacylglycerol and β-sitosterol. Food Chemistry. 2021;350 doi: 10.1016/j.foodchem.2021.129275. [DOI] [PubMed] [Google Scholar]
- Saremnejad F., Mohebbi M., Koocheki A. Practical application of nonaqueous foam in the preparation of a novel aerated reduced-fat sauce. Food and Bioproducts Processing. 2020;119:216–225. doi: 10.1016/j.fbp.2019.11.004. [DOI] [Google Scholar]
- Sheng L., Wang Y., Chen J., Zou J., Wang Q., Ma M. Influence of high-intensity ultrasound on foaming and structural properties of egg white. Food Research International. 2018;108:604–610. doi: 10.1016/j.foodres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Shrestha R.G., Shrestha L.K., Solans C., Gonzalez C., Aramaki K. Nonaqueous foam with outstanding stability in diglycerol monomyristate/olive oil system. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2010;353(2–3):157–165. doi: 10.1016/j.colsurfa.2009.11.007. [DOI] [Google Scholar]
- Truong T., Prakash S., Bhandari B. Effects of crystallisation of native phytosterols and monoacylglycerols on foaming properties of whipped oleogels. Food Chemistry. 2019;285:86–93. doi: 10.1016/j.foodchem.2019.01.134. [DOI] [PubMed] [Google Scholar]
- Wei F., Miao J., Tan H., Feng R., Zheng Q., Cao Y., Lan Y. Oleogel-structured emulsion for enhanced oxidative stability of perilla oil: Influence of crystal morphology and cooling temperature. LWT. 2021;139 doi: 10.1016/j.lwt.2020.110560. [DOI] [Google Scholar]
- Wildmoser H., Scheiwiller J., Windhab E.J. Impact of disperse microstructure on rheology and quality aspects of ice cream. LWT-Food Science and Technology. 2004;37(8):881–891. doi: 10.1016/j.lwt.2004.04.006. [DOI] [Google Scholar]
- Zhang Y., Xu J., Tang C., Li Y. Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration. Foods. 2023;12(2):345. doi: 10.3390/foods12020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.