Abstract
BACKGROUND:
Whether functional status is associated with survival to pediatric lung transplant is unknown. We hypothesized that completely dependent functional status at waitlist registration, defined using Lansky Play Performance Scale (LPPS), would be associated with worse outcomes.
METHODS:
Retrospective cohort study of pediatric lung transplant registrants utilizing United Network for Organ Sharing’s Standard Transplant Analysis and Research files (2005–2020). Primary exposure was completely dependent functional status, defined as LPPS score of 10–40. Primary outcome was waitlist removal for death/deterioration with cause-specific Cox (CSHR) regression. Subdistribution hazard regression (SHR, Fine and Gray) was used for the secondary outcome of waitlist removal due to transplant/improvement with a competing risk of death/deterioration. Confounders included: sex, age, race, diagnosis, ventilator dependence, extracorporeal membrane oxygenation, year, and listing center volume.
RESULTS:
A total of 964 patients were included (63.5% ≥ 12 years, 50.2% cystic fibrosis [CF]). Median waitlist days were 95; 20.1% were removed for death/deterioration and 68.2% for transplant/improvement. Completely dependent functional status was associated with removal due to death/deterioration (adjusted CSHR 5.30 [95% CI 2.86–9.80]). This association was modified by age (interaction p = 0.0102), with a larger effect for age ≥12 years, and particularly strong for CF. In the Fine and Gray model, completely dependent functional status did not affect the risk of removal due to transplant/improvement with a competing risk of death/deterioration (adjusted SHR 1.08 [95% CI 0.77–1.49]).
CONCLUSIONS:
Pediatric lung transplant registrants with the worst functional status had worse pretransplant outcomes, especially for adolescents and CF patients. Functional status at waitlist registration may be a modifiable risk factor to improve survival to lung transplant.
Keywords: lung transplantation, pediatrics, functional status, survival analysis, rehabilitation
Waitlist removal due to death or clinical deterioration occurs for 20% to 25% of adolescents and children listed for lung transplantation.1,2 Factors associated with higher pretransplant mortality include adolescent age (≥12 years old), male sex, higher lung allocation score (LAS) at listing, shorter height, and requiring extracorporeal membrane oxygenation (ECMO).1,2
Despite limited evidence, participation in active rehabilitation is encouraged by pediatric lung transplant centers to maintain or improve functional status while on the waitlist in an effort to maximize survival to transplant and potentially to improve post-transplant outcomes.3 Critically ill pediatric lung transplant candidates are also increasing,2 in part because of strategies such as noninvasive or invasive mechanical ventilation use or ECMO to help maximize pretransplant functional capacity.4,5 It is unknown if worse functional status of pediatric lung transplant candidates is associated with survival to transplant.
The Lansky Play Performance Scale (LPPS) has been reported in the United Network for Organ Sharing (UNOS) registry since 2005 and is a functional status measure assigned by each transplant center for each patient. The LPPS was originally developed to measure the global functional status in children and adolescents with cancer,6 but it has also been used to assess functional status in pediatric solid organ transplant populations.7,8
We sought to determine whether functional status at the time of waitlisting was associated with pretransplant outcomes in pediatric candidates for lung transplantation. We hypothesized that completely dependent functional status, defined as LPPS score between 10 and 40, at the time of waitlist registration would be associated with a higher waitlist mortality or removal from the waitlist due to clinical deterioration prior to transplant.
Materials and methods
Study design and population
We performed a multicenter retrospective cohort study of pediatric (ages 1–17 years) lung transplant registrants between January 1, 2005, and December 31, 2020, utilizing the UNOS’s Standard Transplant Analysis and Research files. Only the first listing for a patient within the study period was used and patients must have had an LPPS score at the time of lung transplant registration. Exclusion criteria included if the reason for waitlist removal was missing (n = 5), refused transplant (n = 7), unable to contact (n = 6), or received a living donor transplant (n = 2) (Figure 1). There were minimal missing data with 3.2% of patients excluded for missing LPPS (Figure 1) and there were no missing data for any of the covariates used in the multivariable models in the final cohort.
Figure 1.
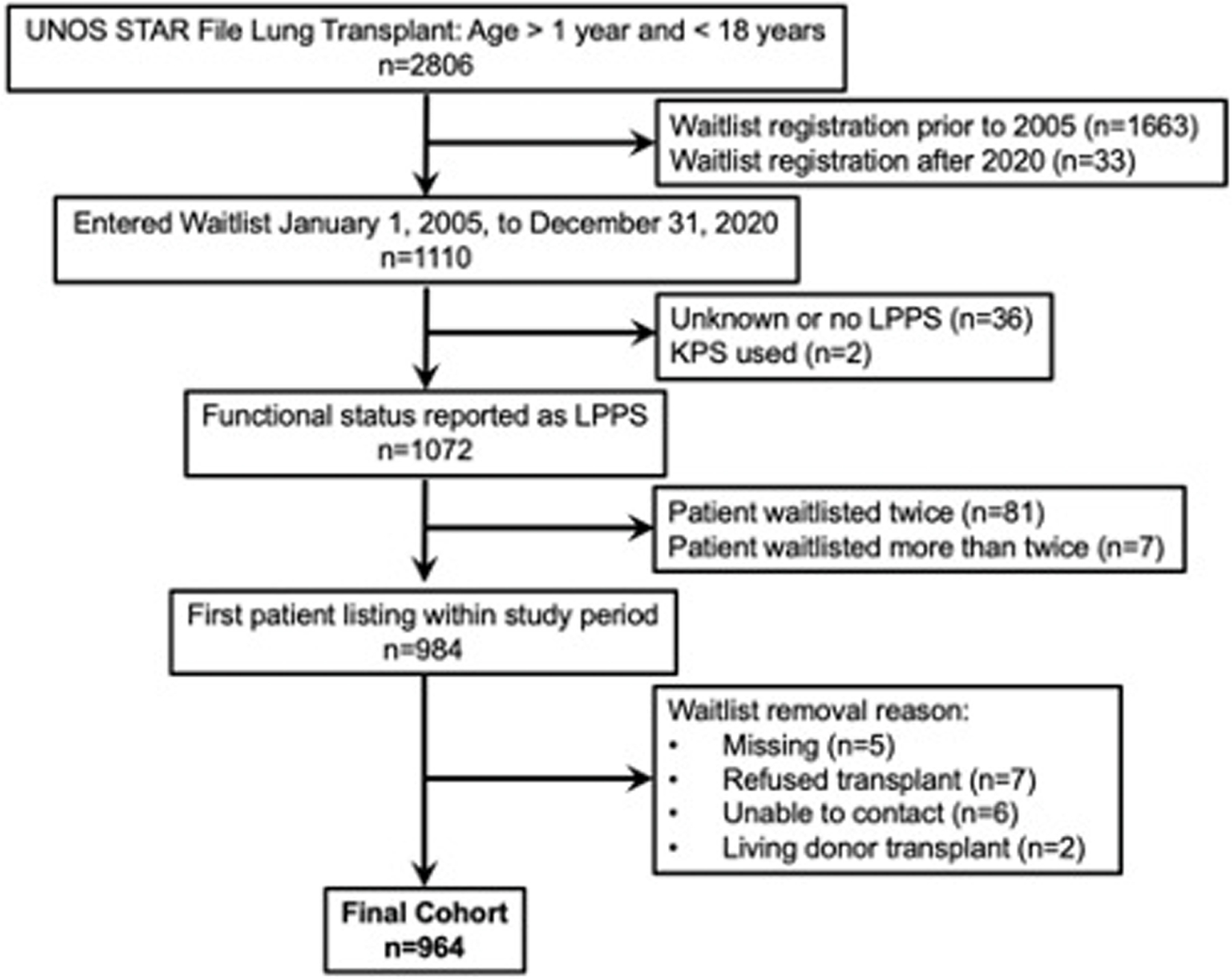
Study flow diagram. LPPS, Lansky Play Performance Scale; STAR, Standard Transplant Analysis and Research; UNOS, United Network for Organ Sharing.
The study was deemed exempt by the Institutional Review Board at the Children’s Hospital of Philadelphia and was in compliance with the International Society for Heart and Lung Transplantation Ethics statement.
Primary Exposure Definition
A 3-category consolidated LPPS score at the time of waitlist registration was used: completely dependent (LPPS 10–40), moderately dependent (LPPS 50–70), and minimally dependent/independent (LPPS 80–100). This LPPS grouping has been used to analyze functional status in pediatric liver transplant patients7 and mirrors grouping of the Karnofsky Performance Status scale used in adult lung transplant registrants and recipients.9 The primary exposure was completely dependent functional status (LPPS 10–40).
Outcome Definitions
The primary outcome was waitlist removal due to death or clinical deterioration. The secondary outcome was waitlist removal due to deceased donor transplant or clinical improvement. Patients who remained on the waitlist were censored at the end of the follow-up time.
Confounder Definitions
Confounders of the association between LPPS category and outcomes were determined based on literature review and the construction of a directed acyclic graph (Figure S1). Confounders examined included sex, age category (< 12 years and ≥12 years), UNOS race category (White, Black, Multiracial, Hispanic, Asian, other), primary diagnosis (cystic fibrosis [CF], pulmonary hypertension [PH], obliterative bronchiolitis, pulmonary fibrosis, and other), ventilator dependence at registration, ECMO at registration, waitlist year, and listing center volume (0–24 cases, 25–49 cases, and > 50 cases over the entire study period).
Statistical Analyses
Statistical analyses were conducted with STATA/IC 16.1 SE (StataCorp, College Station, TX). Data were summarized as median and interquartile range (IQR) or number and percentages. Continuous data were compared with Wilcoxon’s rank sum test and/or Cuzick’s nonparametric test of trend. Categorical data were compared by Pearson’s chi-square. Dunn’s test with Bonferroni correction was used as a test for nonparametric multiple comparisons.
To have the most comprehensive understanding of the competing risks structure of the outcomes, both cause-specific hazards method10 and subdistribution hazard regression with the method of Fine and Gray11 clustered by listing center were used. Two cause-specific Cox hazards models were constructed (using stcox, cause-specific hazard ratio [CSHR]) to separately test for the association of functional status category with the outcomes of (1) waitlist removal for death or clinical deterioration and (2) waitlist removal for transplant or clinical improvement. Age category (< 12 or ≥12 years) and sex were specifically assessed for effect modification. Subgroup analyses were performed for the primary diagnoses of CF and PH and for those who were ventilator dependent or on ECMO at the time of waitlist registration. Proportional hazards assumptions were tested with log–log plots of survival, plot of empirical survival vs predicted survival, the score test using Schoenfeld residuals, and with assessment for influential observations. The overall fit of the Cox models was assessed with Cox–Snell residuals. A subdistribution hazard model was constructed (using stcrreg, subdistribution hazard ratio [SHR]) for a primary outcome of waitlist removal for transplant or clinical improvement and a competing risk of waitlist removal for death or clinical deterioration.
For all survival analyses, time zero was the date of waitlist entry. Follow-up time was measured from the date of waitlist entry to the outcome of interest for Cox regression models, to the outcome of interest or competing outcome for competing risk regression, or censored at last follow-up if a patient remained waitlisted. The same confounders were used in the cause-specific hazards and competing risk regression models. Two sensitivity analyses were performed: (1) exclusion of CF patients from the cohort and (2) performance of mixed effects Cox regression models with listing center as a random effect (using mestreg). For the CF and PH subgroup analyses, the same confounders were used except for “primary diagnosis” which was excluded from the models. A cumulative incidence function curve and a cumulative subhazard function curve were constructed for the primary outcome of waitlist removal for transplant or clinical improvement and a competing risk of waitlist removal for death or clinical deterioration (using stcurve). p-values < 0.05 were considered significant.
Results
A total of 964 patients were included in the analysis: median age was 13 years (IQR 9–16); 63.5% were ≥12 to 17 years of age; 58.5% were female; and 69.0% were White (Table 1). The most common primary diagnoses for transplant listing were cystic fibrosis (50.2%) and pulmonary hypertension (13.5%); most patients (64.4%) were waitlisted at high-volume centers (Table 1). At the time of waitlist registration, patient functional status categorization was 25.6% completely dependent, 52.5% moderately dependent, and 21.9% minimally dependent/independent (Table 1).
Table 1.
Cohort Demographics and Characteristics With Comparison by Functional Status (N = 964 Unless Otherwise Noted)
| Characteristic | Total N = 964 |
Completely dependent (LPPS 10–40) n = 247 |
Moderately dependent (LPPS 50–70) n = 506 |
Minimally dependent/independent (LPPS 80–100) n = 211 |
p a | p b |
|---|---|---|---|---|---|---|
| Age (years) | 13.0 (9.0–16.0) | 12.0 (6.0–15.0) | 14.0 (10.0–16.0) | 13.0 (8.0–15.0) | < 0.001 | 0.210 |
| Sex | 0.20 | - | ||||
| Female | 564 (58.5%) | 155 (62.8%) | 294 (58.1%) | 115 (54.5%) | ||
| Male | 400 (41.5%) | 92 (37.2%) | 212 (41.9%) | 96 (45.5%) | ||
| UNOS race category | 0.40 | - | ||||
| White | 665 (69.0%) | 163 (66.0%) | 350 (69.2%) | 152 (72.0%) | ||
| Black | 71 (7.4%) | 18 (7.3%) | 34 (6.7%) | 19 (9.0%) | ||
| Multiracial | 12 (1.2%) | 2 (0.8%) | 8 (1.6%) | 2 (0.9%) | ||
| Hispanic | 179 (18.6%) | 50 (20.2%) | 95 (18.8%) | 34 (16.1%) | ||
| Asian | 27 (2.8%) | 9 (3.6%) | 16 (3.2%) | 2 (0.9%) | ||
| Other | 10 (1.0%) | 5 (2.0%) | 3 (0.6%) | 2 (0.9%) | ||
| Weight (kg) | 36.2 (23.7–45.7) | 34.4 (16.1–45.5) | 37.1 (26.1–46.3) | 35.1 (22.4–44.9) | 0.004 | 0.290 |
| Height (cm) | 146.0 (124.4–156.6) | 141.0 (103.5–155.0) | 147.3 (129.0–157.0) | 143.7 (122.6–157.0) | < 0.001 | 0.092 |
| BMI (kg/m2) | 17.1 (15.2–19.3) | 17.3 (15.3–19.6) | 17.2 (15.3–19.2) | 17.0 (15.0–18.9) | 0.40 | 0.202 |
| Initial Calc LAS (n = 942) | 34.6 (32.0–38.6) | 37.0 (30.7–55.5) | 34.8 (32.8–38.1) | 33.4 (31.9–34.9) | < 0.001 | < 0.001 |
| Offer/removal/current Calc LAS (n = 964) | 35.7 (31.7–43.5) | 40.0 (30.9–74.7) | 36.0 (32.8–41.3) | 33.8 (28.6–36.8) | < 0.001 | < 0.001 |
| Primary diagnosis | < 0.001 | - | ||||
| Cystic fibrosis | 484 (50.2%) | 81 (32.8%) | 273 (54.0%) | 130 (61.6%) | ||
| Pulmonary fypertension | 130 (13.5%) | 39 (15.8%) | 64 (12.6%) | 27 (12.8%) | ||
| Obliterative bronchiolitis | 56 (5.8%) | 15 (6.1%) | 31 (6.1%) | 10 (4.7%) | ||
| Pulm fibrosis – other | 52 (5.4%) | 21 (8.5%) | 23 (4.5%) | 8 (3.8%) | ||
| All others | 242 (25.1%) | 91 (36.8%) | 115 (22.7%) | 36 (17.1%) | ||
| Reason for WL removal | < 0.001 | - | ||||
| Deceased donor Tx | 605 (62.8%) | 136 (55.1%) | 344 (68.0%) | 125 (59.2%) | ||
| Transfer | 16 (1.7%) | 2 (0.8%) | 6 (1.2%) | 8 (3.8%) | ||
| Died | 147 (15.2%) | 50 (20.2%) | 63 (12.5%) | 34 (16.1%) | ||
| Other | 89 (9.2%) | 26 (10.5%) | 45 (8.9%) | 18 (8.5%) | ||
| Improved | 49 (5.1%) | 11 (4.5%) | 22 (4.3%) | 16 (7.6%) | ||
| Deteriorated | 47 (4.9%) | 20 (8.1%) | 21 (4.2%) | 6 (2.8%) | ||
| Transfer (multiple) | 8 (0.8%) | 0 (0.0%) | 4 (0.8%) | 4 (1.9%) | ||
| Died during Tx | 3 (0.3%) | 2 (0.8%) | 1 (0.2%) | 0 (0.0%) | ||
| WL removal for death or deterioration | 194 (20.1%) | 70 (28.3%) | 84 (16.6%) | 40 (19.0%) | < 0.001 | - |
| WL removal for transplant or improvement | 657 (68.2%) | 149 (60.3%) | 367 (72.5%) | 141 (66.8%) | 0.003 | - |
| Listing center volume | < 0.001 | - | ||||
| Low (0–24) | 247 (25.6%) | 73 (29.6%) | 134 (26.5%) | 40 (19.0%) | ||
| Medium (25–49) | 96 (10.0%) | 17 (6.9%) | 68 (13.4%) | 11 (5.2%) | ||
| High (> 50) | 621 (64.4%) | 157 (63.6%) | 304 (60.1%) | 160 (75.8%) | ||
| Total Days on WL | 95 (30–304) | 37 (10–82) | 120 (40–290) | 257 (59–667) | < 0.001 | < 0.001 |
| Ventilator Dep at WL | 106 (11.0%) | 93 (37.7%) | 8 (1.6%) | 5 (2.4%) | < 0.001 | - |
| ECMO at WL | 63 (6.5%) | 60 (24.3%) | 2 (0.4%) | 1 (0.5%) | < 0.001 | - |
Key: Calc, calculated; cm, centimeters; Dep, dependence; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; kg, kilograms; LAS, Lung Allocation Score; LPPS, Lansky Play Performance Scale; m, meters; Pulm, pulmonary; STAR, Standard Transplant Analysis and Research; Tx, transplant; UNOS, United Network for Organ Sharing; WL, waitlist.
Data are presented as median (IQR) for continuous measures and n (%) for categorical measures.
Kruskal–Wallis or Pearson’s chi-square test.
Cuzick’s nonparametric test of trend for continuous variables.
The median time on the waitlist was 95 days (IQR 30–304); 20.1% of patients were removed from the waitlist due to death or clinical deterioration and this was highest in the completely dependent functional status category; 68.2% were removed from the waitlist due to transplant or clinical improvement and this was least in the completely dependent functional status category (Table 1). Over the study time period, the proportion of those waitlisted as completely dependent increased from 17.6% to 28.9% and those as minimally dependent decreased from 23.8% to 18.3% (Dunn’s test p = 0.03, Figure 2). The proportion of patients waitlisted by high volume centers was similar throughout the study time period (Dunn’s test p = 1.0, Figure S2).
Figure 2.
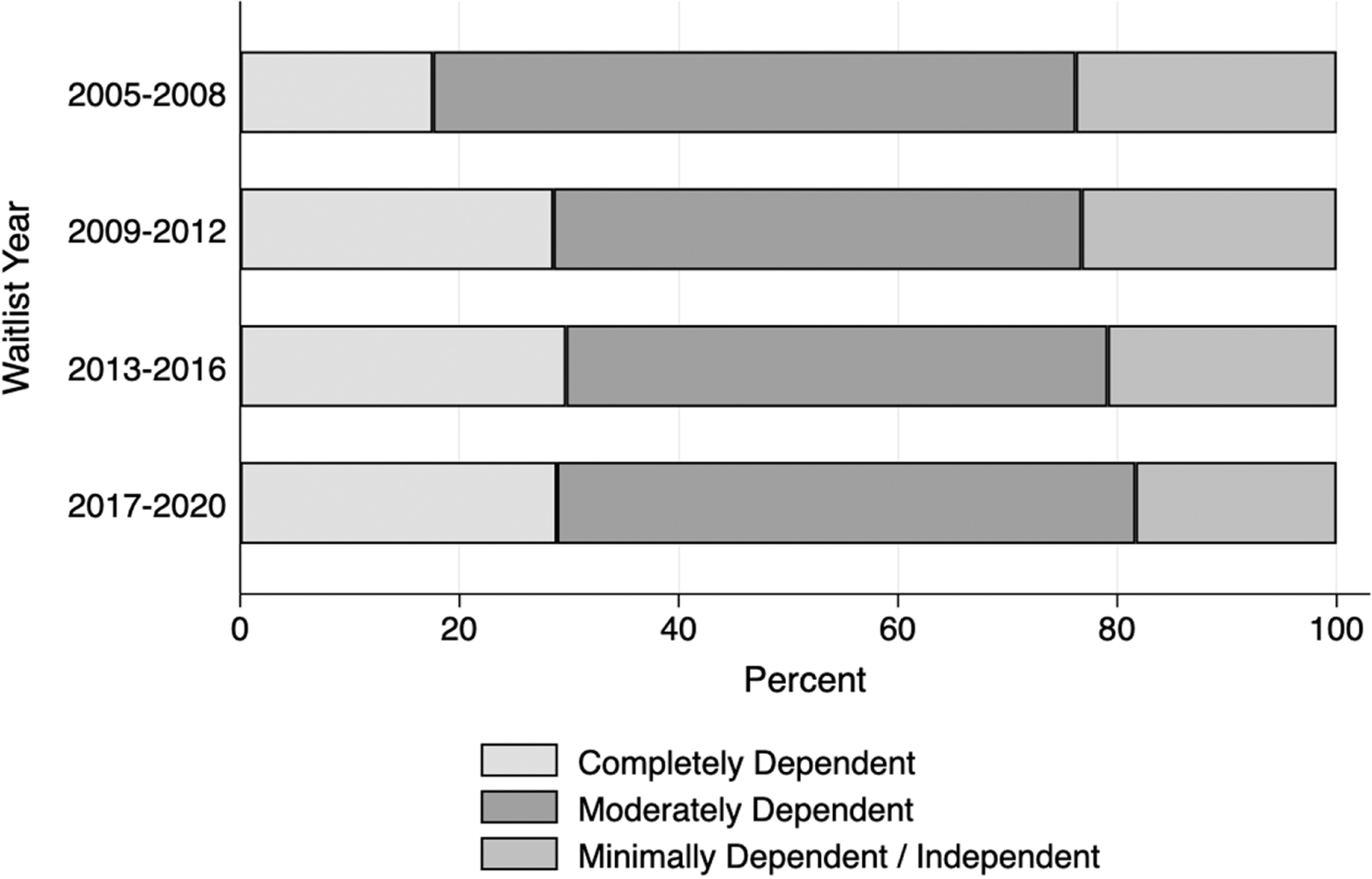
Functional status by waitlist epoch.
In multivariable cause-specific hazard analysis, controlling for sex, age category, ethnicity, ventilator dependence, ECMO, waitlist year, primary diagnosis, and listing center volume, completely dependent functional status was associated with (1) an increased hazard of waitlist removal due to death or clinical deterioration (adjusted CSHR 5.30 [95% CI 2.86–9.80], p < 0.001) and (2) an increased hazard of waitlist removal due to lung transplant or clinical improvement (adjusted CSHR 2.30 [95% CI 1.53–3.46], p < 0.001) compared to minimally dependent/independent patients (Table 2). Age modified the relationship between the association of completely dependent functional status with waitlist removal for death or clinical deterioration (interaction p = 0.0102), as age ≥12 had a higher hazard (stratified CSHR 9.26 [95% CI 3.95–21.71], p < 0.001) compared to those < 12 (stratified CSHR 3.81 [95% CI 2.31–6.30], p < 0.001). In contrast, sex did not modify this association (interaction p = 0.1982). Age category (interaction p = 0.1688) or sex (interaction p = 0.0930) did not modify the association between functional status and waitlist removal for lung transplant or clinical improvement. The full unadjusted and adjusted cause-specific hazard models are shown in Tables S1 and S2, respectively.
Table 2.
Comparison of Multivariable Models Clustered by Listing Center for Cause-specific Hazard Ratios (CSHR) for the Primary Outcome of Waitlist Removal for Death or Clinical Deterioration (CSHR Adjusted Model #1), CSHR for the Secondary Outcome of Waitlist Removal for Lung Transplant or Clinical Improvement (CSHR Adjusted Model #2), and for Multivariable Model for Subdistribution Hazard Ratios (SHR, Fine and Gray method) for the Outcome of Waitlist Removal for Lung Transplant or Clinical Improvement With the Competing Risk of Death or Clinical Deterioration
| CSHR (95% CI)a |
SHR (95% CI)a |
|||||
|---|---|---|---|---|---|---|
| LPPS category | Adjusted model #1: Waitlist removal for death or clinical deterioration | p | Adjusted model #2: Waitlist removal for transplant or clinical improvement | p | Waitlist removal for transplant or clinical improvement with the competing risk of death or clinical deterioration | p |
| Minimally dependent/independent | Ref | - | Ref | - | Ref | - |
| Moderately dependent | 1.37 (1.02–1.83) | 0.036 | 1.61 (1.07–2.41) | 0.022 | 1.36 (1.02–1.82) | 0.037 |
| Completely dependent | 5.3 (2.86–9.80) | < 0.001 | 2.3 (1.53–3.46) | < 0.001 | 1.08 (0.77–1.49) | 0.665 |
Key: ECMO, extracorporeal membrane oxygenation; Ref, reference; UNOS, United Network for Organ Sharing.
Adjusted for sex, age category, UNOS race category, ventilator dependence, ECMO, waitlist year, primary diagnosis, and listing center volume.
In multivariable subdistribution hazard regression, moderately dependent functional status increased the risk of waitlist removal due to lung transplant or clinical improvement (adjusted SHR 1.36 [95% CI 1.02–1.82], p < 0.037) when compared to minimally dependent/independent patients (Table 2, Figure S3). The full unadjusted and adjusted subdistribution hazard models are shown in Table S3. Completely dependent functional status had minimal effect on the cumulative incidence of waitlist removal for transplant or clinical improvement compared to minimally dependent/independent functional status and had less of an effect compared to moderately dependent functional status (Figure 3). A sensitivity analysis with CF patients removed from the cohort showed similar results for the primary analysis for the CSHR models, albeit with smaller effect sizes, and the SHR model became nonsignificant (Table S4). A sensitivity analysis using mixed effects Cox regression models with listing center as a random effect showed similar effect sizes to the primary analysis (Table S5).
Figure 3.
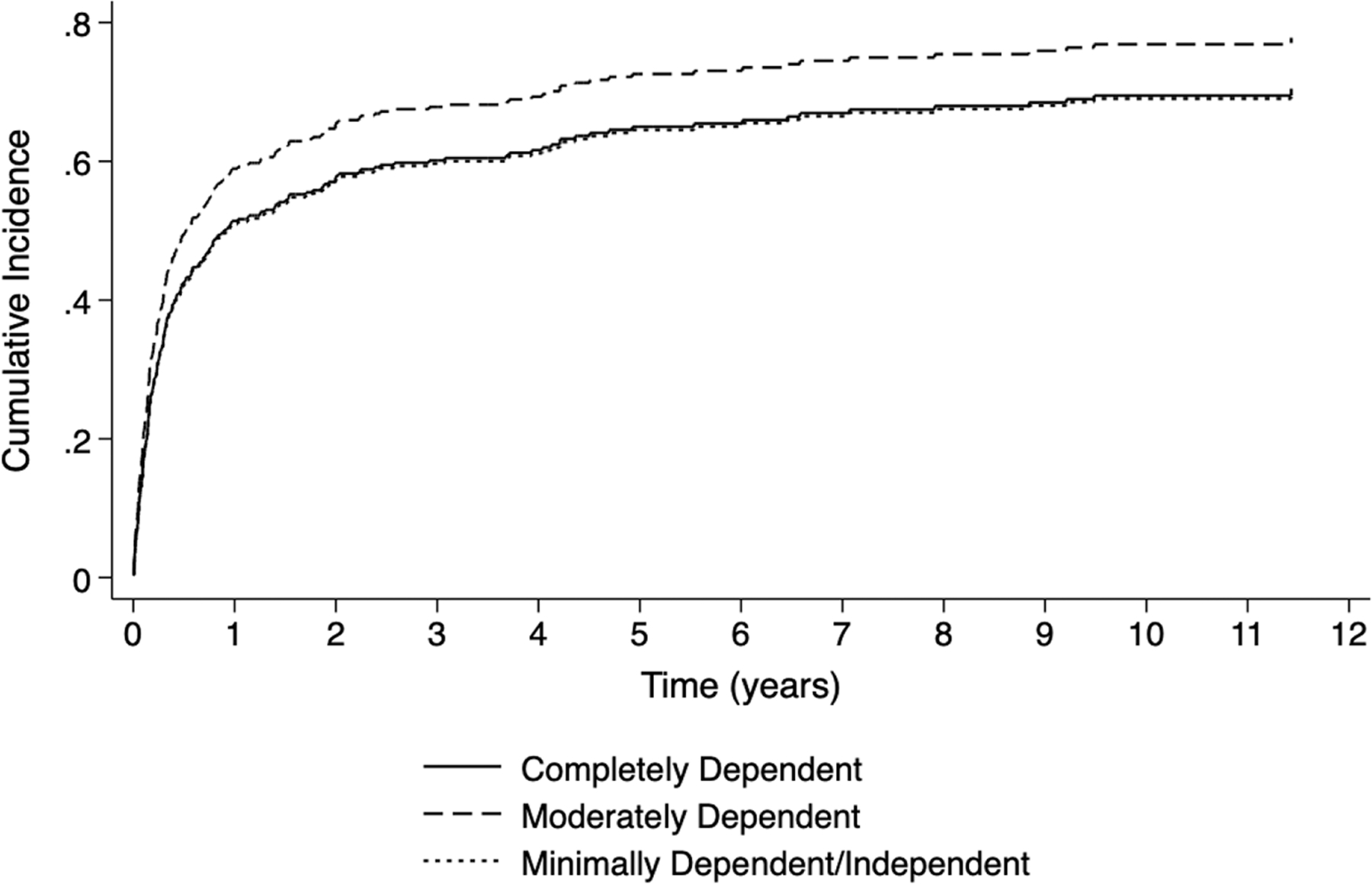
Cumulative incidence function curves by pediatric lung transplant registrant functional status for the outcome of waitlist removal for transplant or clinical improvement (log rank test p < 0.001).
Subgroup analyses of patients who were ventilator dependent or on ECMO at the time of registration and patients with primary diagnoses of CF or PH are presented in the Supplemental Material, Figure S4, Table S6, and Table S7.
Discussion
Completely dependent functional status of pediatric lung transplant registrants was highly associated with waitlist removal due to death or clinical deterioration when controlling for sex, age category, UNOS race category, ventilator dependence, ECMO, waitlist year, primary diagnosis, and transplant listing center volume in this retrospective cohort study involving the UNOS registry. This association was stronger for adolescents compared to younger children and particularly strong for patients with CF. Moderately dependent functional status had the highest risk for waitlist removal for lung transplant or clinical improvement in the presence of the competing risk of death or clinical deterioration in the total cohort and was higher in patients with CF. Functional status at waitlist registration may be an important and potentially modifiable risk factor to improve survival to lung transplant for children and adolescents.
As technology-dependent and critically ill pediatric lung transplant candidates are increasing in number,2 maximizing functional capacity both inside and outside of the hospital environment may be important. Our results suggest that this may be especially true for adolescent patients and for patients with CF. Further, the vast majority of patients who are ventilator dependent or on ECMO at waitlist registration are at a relatively low functional status. As waitlist removal due to death or clinical deterioration occurs for 20% to 25% of pediatric lung transplant patients,1,2 earlier interventions or targeted prewaitlist interventions for high-risk patients that increase or maintain functional capacity and/or listing prior to a significant functional decline may improve their quality of life and pretransplant outcomes. In adults, severely limited functional capacity without rehabilitation potential is a contraindication for lung transplantation.12 While rehabilitation potential can be difficult to estimate for many pediatric patients, the trajectory of functional status prior to and at waitlist registration is potentially another important clinical consideration that may need to be weighted differently for different patients. For example, the safety and goals of active rehabilitation for an adolescent with CF may be different than an adolescent with PH or a younger child with interstitial lung disease. As LPPS in the UNOS registry was only reported at the time of waitlist registration and at the time of transplantation (i.e., not reported for those removed from the waitlist due to death or clinical deterioration), we were unfortunately unable to investigate the effect of the trajectory of LPPS-derived functional status on pretransplant outcomes. Future studies should focus on the effect of both the trajectory of and interventions to increase functional capacity during the prewaitlist and waitlist periods on pre-transplant outcomes.
The 2 methods of survival analyses provide complementary and congruent evidence for active rehabilitation for pediatric patients who are listed for lung transplantation. The cause-specific hazard analysis showed that completely dependent functional status was associated with death or clinical deterioration at a hazard over 4-fold that of moderately dependent and over 5-fold that of minimally dependent or independent functional statuses, respectively (Table 2). The complementary analysis involving competing risk regression using the method of Fine and Gray allowed us to determine the incidence and risk of functional status on transplant or clinical improvement when taking the competing risk of death or clinical deterioration into account, and showed that moderately dependent functional status had the highest risk of waitlist removal for transplant or clinical improvement (Table 2). Taken together, the cause-specific hazard and competing risk regression analyses show that functional status category matters for good (waitlist removal due to transplant or clinical improvement) and bad (waitlist removal for death or clinical deterioration) outcomes.
Given that the landscape of pediatric lung transplantation is changing with PH and interstitial lung disease being rising indications and CF patients decreasing,13 we performed subgroup analyses on patients who were waitlisted due to the primary diagnoses of CF and PH. Patients with CF contributed largely to the overall effects observed of functional status on pretransplant outcomes but, as demonstrated in the sensitivity analysis with CF patients removed from the cohort, this was not the complete story. One of the challenges of pediatric lung transplantation is the heterogeneity of diagnoses, ages, comorbidities, and (luckily) the relatively rare need for lung transplantation. Future research should focus particularly on these growing populations of non-CF diagnoses.
We used LPPS as the measure of functional status for this analysis. Although only validated in children with cancer,6 LPPS has provided insights into the association of functional status of children and adolescents pre and postliver transplant7 and preheart transplant.8 In the UNOS registry, LPPS was reported by each listing center at the time of waitlisting. However, there was no standardization across sites and, therefore, LPPS scores may have been prone to observer bias. To partially mitigate this potential bias and potential site variability, our analysis focused on 3 categories of functional status as has been used in a prior pediatric liver transplant study7 and similar to the Karnofsky Performance Statu scale categories used in a study of adult lung transplant candidates and recipients.9 While LPPS is not incorporated into the pediatric LAS, the LAS does include a qualitative measure of functional status (no assistance, some assistance, or total assistance needed with activities of daily living) and has several elements that are associated with functional status, such as 6-minute walk distance. Because the LAS is influenced by both functional status and by the outcome measures of this study, we considered LAS a collider (i.e., on the causal pathway downstream from LPPS) and, therefore, LAS at any point during the waitlist period was not controlled for in our competing risk analyses.
Given these limitations to the current methods of functional status assessment and given effect modification by age on the association between functional status and pre-transplant outcomes, it would be valuable for pediatric centers to utilize validated and age-specific functional status measures. For example, the adult lung transplant community uses qualitative assessments, quantitative measurements, and plasma biomarkers to determine frailty phenotypes that provide clinically important information for both pre- and post-transplant outcomes.14–17 As has recently been published for patients with CF,18 pediatric and diagnosis-specific frailty in the lung transplant population may be better defined using pediatric-centered and validated assessments such as the PedsQL19 or the Functional Status Score20 in combination with other quantitative, plasma, and imaging biomarkers. We do not wish to imply that older children who are completely dependent should be less eligible for lung transplantation but rather hope to spark more research into the understanding of pediatric frailty and ways it can be addressed to potentially improve outcomes.
Limitations
As this was a retrospective cohort study of the UNOS dataset, we were only able to report associations and the dataset was restricted to centers in the United States. As mentioned previously, LPPS was not standardized across institutions and susceptible to observer bias. Further, younger age could either bias toward a higher or lower LPPS because of developmental considerations during functional status assessment. This would bias any association with outcome toward the null and could partly explain why we found a greater impact of poor functional status in adolescents. We did not investigate post-transplant outcomes as it was unclear how functional status at the time of waitlist registration would directly influence post-transplant outcomes. For those who survived to transplant in our dataset and who had LPPS scores at the time of transplant (n = 569), the median time to death (n = 79) was 618 days (IQR 326–908) and median time to retransplant (n = 58) was 1453 days (IQR 807–2035). Given the timing and relative low numbers of patients, we believe the potential interpretation of analyses in regard to the association of LPPS at the time of transplant with these post-transplant outcomes of interest to be very limited. We were unable to investigate if pretransplant location was an important confounder as our dataset did not contain pretransplant location as a variable. Finally, we were unable to investigate functional status trajectory during the waitlist period due to the reporting frequency of LPPS in the UNOS registry.
Conclusions
Children and adolescents waitlisted for lung transplantation with the worst functional status had worse pretransplant outcomes and had the greatest risk of waitlist removal due to death or clinical deterioration when controlling for sex, age category, race, ventilator dependence, ECMO, waitlist year, primary diagnosis, and transplant listing center volume. This association was stronger for adolescents compared to younger children and particularly strong for patients with CF. Functional status at the time of waitlist may be an important and potentially modifiable risk factor to improve survival to lung transplant for pediatric patients.
Supplementary Material
Acknowledgments
Disclosure statement
Grant funding was paid to the affiliated institutions in support of ongoing work of Adam S. Himebauch (NIH 5K23-HL153759), Nadir Yehya (NIH 5R01-HL14805403), Douglas E. Schaubel (NIH NIDDK R01-DK070869), Robert A. Berg (NIH 5R01-HL147616–03, NIH 1RL1HD107777–01), Steven M. Kawut (NIH K24-HL103844), and Jason D. Christie (NIH U01HL145435). Jason D. Christie is on the Board of Directors of the International Society for Heart and Lung Transplantation. Maureen B. Josephson has no disclosures.
List of nonstandard abbreviations:
- CSHR
cause-specific hazard ratio
- DAG
directed acyclic graph
- ECMO
extracorporeal membrane oxygenation
- IQR
interquartile range
- ISHLT
International Society for Heart and Lung Transplantation
- LAS
lung allocation score
- LPPS
Lansky Play Performance Scale
- SHR
subdistribution hazard ratio
- STAR
Standard Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.healun.2023.07.003.
References
- 1.Keeshan BC, Rossano JW, Beck N, et al. Lung transplant waitlist mortality: height as a predictor of poor outcomes. Pedia Transplant 2015;19:294–300. [DOI] [PubMed] [Google Scholar]
- 2.Lancaster TS, Miller JR, Epstein DJ, et al. Improved waitlist and transplant outcomes for pediatric lung transplantation after implementation of the lung allocation score. J Heart Lung Transplant 2017;36:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberger D, Gould Delaney A, Forbes P, Manley D, Visner GA. Pediatric lung transplant: correlation of pretransplant condition with post-transplant outcomes. Pediatr Transplant 2021;25:e13889. [DOI] [PubMed] [Google Scholar]
- 4.Toprak D, Midyat L, Freiberger D, Boyer D, Fynn-Thompson F, Visner G. Outcomes of mechanical support in a pediatric lung transplant center. Pedia Pulmonol 2017;52:360–6. [DOI] [PubMed] [Google Scholar]
- 5.Turner DA, Cheifetz IM, Rehder KJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Crit Care Med 2011;39:2593–8. [DOI] [PubMed] [Google Scholar]
- 6.Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer 1987;60:1651–6. [DOI] [PubMed] [Google Scholar]
- 7.Perito ER, Bucuvalas J, Lai JC. Functional status at listing predicts waitlist and post-transplant mortality in pediatric liver transplant candidates. Am J Transplant 2019;19:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulic A, Maeda K, Zhang Y, et al. Functional status of United States children supported with a left ventricular assist device at heart transplantation. J Heart Lung Transplant 2017;36:890–6. [DOI] [PubMed] [Google Scholar]
- 9.Grimm JC, Valero III V, Kilic A, et al. Preoperative performance status impacts perioperative morbidity and mortality after lung transplantation. Ann Thorac Surg 2015;99:482–9. [DOI] [PubMed] [Google Scholar]
- 10.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data 2nd ed., New York: John Wiley,; 2002. [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 12.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014 – an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1–15. [DOI] [PubMed] [Google Scholar]
- 13.Avdimiretz N, Benden C. The changing landscape of pediatric lung transplantation. Clin Transplant 2022;36:e14634. [DOI] [PubMed] [Google Scholar]
- 14.Singer JP, Diamond JM, Gries CJ, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med 2015;192:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin MR, Singer JP, Huang D, et al. Refining low physical activity measurement improves frailty assessment in advanced lung disease and survivors of critical illness. Ann Am Thorac Soc 2017;14:1270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: a prospective cohort study. Am J Transplant 2018;18:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venado A, McCulloch C, Greenland JR, et al. Frailty trajectories in adult lung transplantation: a cohort study. J Heart Lung Transplant 2019;38:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koutsokera A, Sykes J, Theou O, et al. Frailty predicts outcomes in cystic fibrosis patients listed for lung transplantation. J Heart Lung Transplant 2022;41:1617–27. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Seid M, Kurtin PS. PedsQL 4 0: Reliab validity Pediatr Qual Life Inventory version 4 0 generic core scales health patient population. Med Care 2001;39:800–12. [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Holubkov R, Glass P, et al. Functional status scale: new pediatric outcome measure. Pediatrics 2009;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


