Abstract
Surveillance of endemic pathogens is essential for disease control, providing an evidence base for policy and advice. Bovine Herpes Virus Type 1 (BoHV-1), the causative agent of Infectious Bovine Rhinotracheitis (IBR), has been found to have high seroprevalence within the Irish cattle population. The aim of the present study was to establish seroprevalence levels for culled cattle in Ireland aged < 30 months and to establish whether BVD exposure and other factors was associated with BoHV-1 exposure. We employed random effects logit models coupled with repeated bootstrap sampling to provide robust estimates. The final dataset contained results for 5273 animals tested over two study years, 2018 and 2020. The animal-level seroprevalence of BoHV-1 was 21.43% (1130/5273; 95%CI: 20.32–22.53%). Univariable analysis suggested that BoHV-1 seropositivity risk was associated with BVDV serodiagnosis status, age, sex, year sampled, herd type, herd-size, and metrics of movement into the herd. Final random-effects multivariable models suggested increased risk associated with increasing herd size of the last herd, movements made by animals during the previous year, and the year the animal was sampled. Despite BVDV status and sex being retained in the final model, repeated bootstrap sampling of the regression model to estimate biased-corrected 95%CI suggested that these associations were not robust. The overall apparent prevalence of BoHV-1 exposure for culled cattle in Ireland declined in 2020 relative to 2018 (from 23.32 to 17.61%). Herd-size and the movement of animals were found to be important factors associated with animal-level risk, but there was less statistical support for sex-based or BVDV status associations.
Subject terms: Risk factors, Ecological epidemiology
Introduction
Bovine Herpes Virus 1 (BoHV-1) infection occurs worldwide (reviewed by1, and is an economically significant pathogen due to its impact on production losses2,3. BoHV-1 cause life-long infection4 that reactivates under stress and may result in viral excretion5. Infection with BoHV1 reduces milk yield impacting farm profitability2,6.
BoHV-1 is endemic in Ireland11–13. Infection with BoHV1 was first described in Ireland in the seventies. Historically, prevalence was low (9% seroprevalence) during the 1980s14. Higher prevalence has been described more recently11,15,16; with Sayers et al.7 reporting 80% of bulk milk samples test positive for BoHV1.
Research has suggested a potential association between BoHV-1 infection and Bovine Viral Diarrhea Virus (BVDV), both in vivo7,8; and in vitro9,10. High levels of cooccurrence of BoHV-1 and BVDV have been reported in cattle herds in Ireland7,12,17. However, the impact of co-infection and the changes in BVDV prevalence over the course of the national BVD Free programme remains unexplored in Ireland.
The Terrestrial manual of the OIE18 outlines the requirements for a country to qualify for disease free status; while the Commission delegated Regulation (EU) 2020/68919 prohibits vaccination and set sets out the requirement to achieve 99.8% of bovine establishments, representing at least 99.9% of all cattle are free from BoHV-1. Eradication programmes have been successful elsewhere in Europe1, and the establishment of a control programme is being considered in Ireland. It is well known animal diseases can reduce efficiency and productive outputs, and therefore requiring increased inputs to overcome disease relative impacts driving up carbon emissions20. Control of IBR has the potential to increase farm efficiencies and help mitigate some carbon outputs21. As a consequence, the Climate Action Plan 202122 set out the objective of launching an IBR control programme in Ireland.
The primary aim of this study was to establish seroprevalence levels of BoHV-1 infection among younger cattle slaughtered in Ireland. Secondly, the study sought to establish whether BVD exposure, and other factors, were associated with BoHV-1 infection, as part of a broader aim of informing policy and control options.
Methods
Sampling and laboratory methods
Information on the survey methods have been published elsewhere23, but briefly, a random sample of Irish cattle aged under 30 months was undertaken, using sera collected for routine serological surveillance purposes. For the present study, IBR testing was undertaken during the years 2018 and 2020. No sampling of live animals was undertaken. Blood sampling was undertaken at slaughter i.e. at post-mortem by veterinary professionals and support staff. It should be noted that the animals were not slaughtered for the purposes of this study. Sampled animals, given their age, were reflective of a population managed for BVD risk as all animals were born since the national BVD eradication scheme was initiated (in 2013). A sample size estimation for seroprevalence was undertaken prior to sampling, indicating that a minimum sample size of 1013 per survey (assuming a design prevalence of 10%; SE: 95%; SP: 99%; confidence: 95%; precision: 0.02) was required24. Note, all serum was sampled as part of routine national disease surveillance; all samples were collected at post-mortem after routine slaughter at abattoir, and therefore sampling was not subject to animal welfare guidelines.
All testing (both for BoHV-1 and BVDV) occurred within a single laboratory (Cork Regional Veterinary Laboratory), and therefore there was no inter-lab variation. Samples were tested for BVD using the IDEXX BVDV/MD/BVD p80 Protein Antibody Kits, according to the manufacturers’ instructions. According to the manufacturers, the BVD assay has a diagnostic sensitivity (dSE) and specificity (dSP) of 98% and 97%, respectively. Samples were tested for BoHV-1 also using IDEXX gE ELISA test kits, according to the manufacturers’ instructions. The dSE and dSP for this assay has been estimated at 99% and 99.7%, respectively25.
Statistical methods
The outcome variable of interest throughout was the IBR serology test status, which was modelled as a binary outcome. All inconclusive results were considered test negatives throughout. The apparent and estimated true seroprevalence was reported. True seroprevalence was based on the Rogan-Gladen estimator, assuming dSE and dSP reported above, and confidence interval estimated via the Blaker methodology26–28.
Generalised linear mixed models were used to model the data, with a random effect included for herd due to the non-independence of multiple observations (i.e. animals sampled) from the same herd. As animals could be clustered via their birth herd or the [last] herd they resided when sampled, two comparative null models without fixed affects were compared. Models with a lower Akaike’s Information Criterion (AIC) and Schwarz Bayesian Information Criterion (BIC) was used as an indicator of the preferred random effect structure.
Descriptive exploration of the independent variables and their relationship with the outcome variable was undertaken. Independent variables included age, breed, sex, movement metrics and herd type (dairy; beef finishing; suckler (beef production where calves are left to suckle from their mothers); other (including mixed enterprises)). Descriptive information on these variables are presented in Table 1.
Table 1.
Descriptive statistics and univariable associations between selected animal- and herd-level factors and IBR seropositivity risk.
| BVD | IBR- | IBR+ | Total | OR | P | Upper 95%CI | Lower 95%CI |
|---|---|---|---|---|---|---|---|
| Neg | 3975 | 1052 | 5027 | Ref | |||
| % | 79.07 | 20.93 | 100 | ||||
| Pos | 168 | 78 | 246 | 1.928 | 0.014 | 1.140 | 3.259 |
| % | 68.29 | 31.71 | 100 | ||||
| Year | |||||||
| 2018 | 1802 | 619 | 2421 | Ref | |||
| % | 74.43 | 25.57 | 100 | ||||
| 2020 | 2341 | 511 | 2852 | 0.521 | < 0.001 | 0.394 | 0.688 |
| % | 82.08 | 17.92 | 100 | ||||
| Age (q) | |||||||
| Q1 | 1009 | 294 | 1303 | Ref | |||
| % | 77 | 22.56 | 100 | ||||
| Q2 | 975 | 345 | 1320 | 1.537 | 0.009 | 1.116 | 2.118 |
| % | 73.86 | 26.14 | 100 | ||||
| Q3 | 1090 | 240 | 1330 | 0.811 | 0.226 | 0.579 | 1.138 |
| % | 81.95 | 18.05 | 100 | ||||
| Q4 | 1069 | 251 | 1320 | 0.905 | 0.575 | 0.638 | 1.284 |
| % | 80.98 | 19.02 | 100 | ||||
| Breed | |||||||
| AA | 720 | 214 | 934 | Ref | |||
| % | 77.09 | 22.91 | 100 | ||||
| CH | 752 | 192 | 944 | 0.717 | 0.117 | 0.473 | 1.087 |
| % | 79.66 | 20.34 | 100 | ||||
| FR | 830 | 217 | 1,047 | 0.756 | 0.183 | 0.500 | 1.142 |
| % | 79.27 | 20.73 | 100 | ||||
| HE | 608 | 184 | 792 | 0.851 | 0.462 | 0.554 | 1.308 |
| % | 76.77 | 23.23 | 100 | ||||
| LM | 785 | 208 | 993 | 0.590 | 0.011 | 0.393 | 0.886 |
| % | 79.05 | 20.95 | 100 | ||||
| Other | 448 | 115 | 563 | 0.663 | 0.083 | 0.416 | 1.056 |
| % | 79.57 | 20.43 | 100 | ||||
| Sex | |||||||
| F | 1742 | 454 | 2196 | Ref | |||
| % | 79.33 | 20.67 | 100 | ||||
| M | 2401 | 676 | 3077 | 1.386 | 0.017 | 1.059 | 1.815 |
| % | 78.03 | 21.97 | 100 | ||||
| Herd change | |||||||
| No change | 1213 | 247 | 1460 | ||||
| % | 83.08 | 16.92 | 100 | ||||
| Change | 2930 | 883 | 3813 | 1.924 | < 0.001 | 1.413 | 2.619 |
| % | 76.84 | 23.16 | 100 | ||||
| Movements | |||||||
| 0 | 1189 | 242 | 1431 | Ref | |||
| % | 83.09 | 16.91 | 100 | ||||
| 1 | 1619 | 437 | 2056 | 1.577 | 0.007 | 1.133 | 2.196 |
| % | 78.75 | 21.25 | 100 | ||||
| 2 | 984 | 317 | 1301 | 2.095 | < 0.001 | 1.462 | 3.001 |
| % | 75.63 | 24.37 | 100 | ||||
| ≥ 3 | 351 | 134 | 485 | 2.950 | < 0.001 | 1.875 | 4.642 |
| % | 72.37 | 27.63 | 100 | ||||
| Type of last herd | |||||||
| BEEF | 1438 | 473 | 1911 | 1.731 | 0.001 | 1.247 | 2.403 |
| % | 75.25 | 24.75 | 100 | ||||
| DAIRY | 769 | 203 | 972 | 1.166 | 0.460 | 0.776 | 1.752 |
| % | 79.12 | 20.88 | 100 | ||||
| OTHER | 376 | 83 | 459 | 1.001 | 0.996 | 0.594 | 1.688 |
| % | 81.92 | 18.08 | 100 | ||||
| SUCKLER | 1560 | 371 | 1931 | Ref | |||
| % | 80.79 | 19.21 | 100 | ||||
| Type of birth herd | |||||||
| BEEF | 228 | 58 | 286 | 0.832 | 0.497 | 0.490 | 1.414 |
| % | 79.72 | 20.28 | 100 | ||||
| DAIRY | 2248 | 660 | 2908 | 1.423 | 0.011 | 1.083 | 1.870 |
| % | 77.3 | 22.7 | 100 | ||||
| OTHER | 184 | 46 | 230 | 0.902 | 0.731 | 0.501 | 1.624 |
| % | 80 | 20 | 100 | ||||
| SUCKLER | 1483 | 366 | 1849 | Ref | |||
| % | 80.21 | 19.79 | 100 | ||||
| Birth herd size (median: 130; mean: 170.7) | |||||||
| Q1 (< 71) | 1042 | 258 | 1300 | Ref | |||
| % | 80.15 | 19.85 | 100 | ||||
| Q2 (71–138) | 1041 | 279 | 1320 | 1.199 | 0.280 | 0.863 | 1.666 |
| % | 78.86 | 21.14 | 100 | ||||
| Q3 (139–243) | 1044 | 289 | 1333 | 1.385 | 0.058 | 0.989 | 1.940 |
| % | 78.32 | 21.68 | 100 | ||||
| Q4 (244–1920) | 1016 | 304 | 1320 | 1.474 | 0.031 | 1.036 | 2.099 |
| % | 76.97 | 23.03 | 100 | ||||
| Last herd size (Median: 96; mean: 148.1) | |||||||
| Q1 (< 53) | 1120 | 173 | 1293 | Ref | |||
| % | 86.62 | 13.38 | 100 | ||||
| Q2 (53–109) | 1079 | 248 | 1327 | 1.875 | 0.002 | 1.265 | 2.780 |
| % | 81.31 | 18.69 | 100 | ||||
| Q3 (110–224) | 1035 | 291 | 1326 | 2.321 | < 0.001 | 1.560 | 3.455 |
| % | 78.05 | 21.95 | 100 | ||||
| Q4 (> 225) | 909 | 418 | 1327 | 5.963 | < 0.001 | 3.885 | 9.154 |
| % | 68.5 | 31.5 | 100 | ||||
AA, Aberdeen angus; CH, Charolais; FR, Friesian; HE, Hereford; LM, Limousin.
Univariable mixed models, controlling for herd clustering, was fitted to each independent variable respectively and reported. Throughout, linear predictors were categorised based on quartiles. The BVD serodiagnosis result was dichotomised by assuming inconclusive test results were negative, following Barrett et al.23. Breed categories were simplified by classing “pure” breeds with their reported “cross breeds”, for example, both Aberdeen angus (AA) and Aberdeen Angus crosses (AAX) were considered being within the same category. Due to their being several rarer breeds that were poorly represented in the dataset, we also classified any breed types with < 700 observations as “other”, yielding a categorical predictor with 6 classes. Movements of animals between enterprises during the last year was simplified, with animals having 3 or more moves being grouped into a single category.
Multivariable models were fitted controlling for the non-independence of observations from the same herd with the inclusion of a random effect. Year was controlled for as a fixed effect. All other potential explanatory variables were added as fixed effects. Backward elimination was used to build parsimonious candidate models using a cut-point of P < 0.05, but preferred models were informed by information criteria. Both Akaike’s and Bayesian information criteria were used to compare competing models. Bayesian information criteria penalise models with greater numbers of parameters, relative to Akaike’s information criteria. Models were considered equivalent if the difference in AIC was < 2, and models with a difference range of 2–7 having some support29. Following Raftery30, differences greater than 10 in the BIC value between models was considered very strong evidence against the more complex model. To improve inference and avoid overfitting, bias corrected bootstrapping estimates with 1000 iterations was also calculated31,32 for the final model. This approach resamples the distribution, with replacement, and iteratively fits models while collecting the estimated standard errors and associated 95%CI. A bias statistic calculates how the bootstrap estimates deviates from the fitted model, with inference based on the confidence intervals derived from the bootstrapped dataset, providing a better indication of the generalisability (by avoiding overfitting) of the model. 1000 iterations were chosen as we were interested in estimates of the 95%CI, as well as the standard error estimates33,34. All analyses were undertaken with Stata 16.1MP35.
Results
There were 5542 IBR test results in the dataset from 3687 last herds, of which 213 were missing contemporaneously sampled BVD test results. Furthermore, there was 56 animals which were over 30-months of age (mean: 48.4 months; IQR: 33–42 months; Max: 223 months). These data were not included in any further modelling (therefore, n = 5273 for all multivariable models), but their descriptive statistics are presented in the Supplementary material. There was no significant difference in the proportion IBR positive in the 213 records with missing data (OR: 1.20; P = 0.252; 95%CI: 0.88–1.65), however the seroprevalence amongst the 56 older animals was significantly higher (35.7%) than the proportion positive amongst the study cohort (21.43%; OR: 2.04; P = 0.011; 95%CI: 1.17–3.53).
Univariable analysis
The seroprevalence of IBR was 21.43% (1130/5273; 95%CI: 20.32–22.53), with an additional 61 inconclusive results. Estimated true seroprevalence was 21.41% (95%CI: 20.31–22.55%).
The seroprevalence of BVD from the random sample was 4.67% (246/5273; 95%CI: 4.15–5.30), with an additional 68 suspect (inconclusive) results.
There was a higher proportion of animals BVD seropositive when seropositive for IBR (n = 78; 6.96% positive), in comparison with animals with seronegative IBR results (n = 168; 4.11%; Pearson χ2 (1) = 15.80; Pr < 0.001). Univariate mixed effect logit models found significant positive associations between IBR test results and BVD status, with random effects for either the last herd (OR: 1.92; 95%CI: 1.13–3.24) or birth herd (OR: 2.27; P = 0.001; 95%CI: 1.40–3.68). Models accounted for clustering effects within the last-herd cattle were sampled, relative to herds within which they were born, appeared to fit the data better (ΔAIC and ΔBIC > 300). The marginal predicted IBR seroprevalence being 25.44% (95%CI: 18.19–20.17) in animals coinfected with BVD, and 19.40% (95%CI: 18.19–20.62) otherwise.
The relationship between the probability of IBR seropositivity and selected animal- and herd-level factors are presented in Table 1 below. All univariable models were fitted with a random effect for the last-herd the animal was sampled in. The seroprevalence was significantly higher in 2020 (23.32%; 95%CI: 21.23–25.41%; marginal predictions from a random effect model) than in 2018 (17.61%; 95%CI: 16.21–19.00%). At the animal level, IBR risk appeared to peak for animals in second age-quartile at 24.4–26.4 months old. While Aberdeen angus (and their crosses) appeared to have the higher IBR positivity rates, the actual variation across all breed categories was not significant (Wald χ2(DF: 5) = 7.40; Prob > χ2 = 0.193). Males appeared to be a slightly increased risk, relative to female animals, though the size effect was small. Animals who were sampled at a different herd that where they were born were significantly more likely to test positive for IBR, and furthermore, it appears that there was a linear increase in risk with the more movements made in the last year (see ORs in Table 1). Animals sampled in beef and dairy herd-types appear to be of greater risk than suckler or “other” herd types, though there was less variation in IBR positivity risk depending on the birth herd types. Herd size was a significant positively associated risk factor for IBR positivity, for both birth herd and the herd the animal was sampled in. However, there appeared to be a greater effect size for the last herd the animal was sampled within, relative to the birth herd size effect.
Multivariable model
Several competing models had similar AIC values, with the largest difference being ΔAIC = 8.17 (Table 2 Supplementary material). Given the difference in the number of parameters between models, the BIC was more discriminatory (max ΔBIC = 120.98), ranking the most parsimonious (DF: 11) model highest. There was strong evidence to suggest that there was significant clustering at the [last] herd level (LR test of ρ = 0: χbar2(01) = 464.08; Prob > = χbar2 < 0.001). The final model exhibited an AUC of 0.65 (95%CI 0.63–0.67). Bootstrapping indicated there were some bias in the final model (BIC ranked) predictions, based on the biased-corrected confidence intervals. The final model with observed odds-ratios with bootstrapped standard errors and bias-corrected 95%CI is presented in Table 2.
Table 2.
Multivariable model of the risk of BoHV1 animal-level test positivity in relation to animal- and herd-level factors.
| Obs. OR | Obs. P-value | Bootstrap | Bootstrap | Normal-based bootstrap | Bias corrected bootstrap | |||
|---|---|---|---|---|---|---|---|---|
| SE | P-value | Lower 95%CI | Upper 95%CI | Lower 95%CI | Upper 95%CI | |||
| Last herd size (quartiles) | ||||||||
| 1 | Ref. | |||||||
| 2 | 1.722 | 0.001 | 0.336 | 0.005 | 1.175 | 2.525 | 1.265 | 1.907 |
| 3 | 2.239 | < 0.001 | 0.458 | < 0.001 | 1.500 | 3.343 | 1.794 | 2.280 |
| 4 | 4.719 | < 0.001 | 1.029 | < 0.001 | 3.078 | 7.234 | – | – |
| No. moves | ||||||||
| 0 | Ref. | |||||||
| 1 | 2.005 | < 0.001 | 0.383 | < 0.001 | 1.380 | 2.915 | 1.267 | 2.365 |
| 2 | 2.632 | < 0.001 | 0.572 | < 0.001 | 1.718 | 4.030 | 1.642 | 3.164 |
| ≥ 3 | 3.494 | < 0.001 | 0.884 | < 0.001 | 2.128 | 5.736 | 2.143 | 4.463 |
| Sex | ||||||||
| Female | Ref. | |||||||
| Male | 1.231 | 0.032 | 0.179 | 0.154 | 0.925 | 1.637 | 0.975 | 1.287 |
| Year | ||||||||
| 2018 | Ref. | |||||||
| 2020 | 0.634 | < 0.001 | 0.099 | 0.003 | 0.467 | 0.861 | 0.518 | 0.939 |
| BVDV status | ||||||||
| Neg. | Ref. | |||||||
| Pos. | 1.602 | 0.048 | 0.487 | 0.121 | 0.883 | 2.906 | 0.881 | 2.875 |
| Constant | 0.038 | < 0.001 | 0.011 | < 0.001 | 0.021 | 0.067 | ||
| Herd level var. | 1.754 | 0.098 | 1.572 | 1.958 | ||||
| ICC (rho) | 0.483 | 0.028 | 0.429 | 0.538 | ||||
BoHV1 positivity was associated with increasing herd size, with animals sampled from herds within the largest quartile having 4.719 times the odds of being seropositive relative to animals coming from herds with sizes within the first quartile (Fig. 1). The probability of an animal being BoHV1 positive was also significantly associated with the number of movements that the animal had during the previous year prior to sampling, with animals with ≥ 3 movements being 3.494 (bias corrected 95%CI: 2.143–4.463) times the odds of test positivity relative to animals that did not make any movements (Fig. 1). Year was retained in the final model, with lower animal-level risk of test positivity in 2020 relative to 2018 (OR: 0.634; bias corrected 95%CI: 0.518–0.939). Point estimates of the odds ratios for BVDV status and animal sex, suggested higher risk of BoHV1 positivity when animals were males (OR: 1.231) and BVDV positive (OR: 1.602), respectively. However, bias corrected 95%CI of the odds ratios straddled 1 for both being male (bias corrected 95%CI: 0.975–1.287) and BVDV status (bias corrected 95%CI: 0.881–2.875), suggesting insufficient statistical support that these associations could be considered robust (Table 2).
Figure 1.
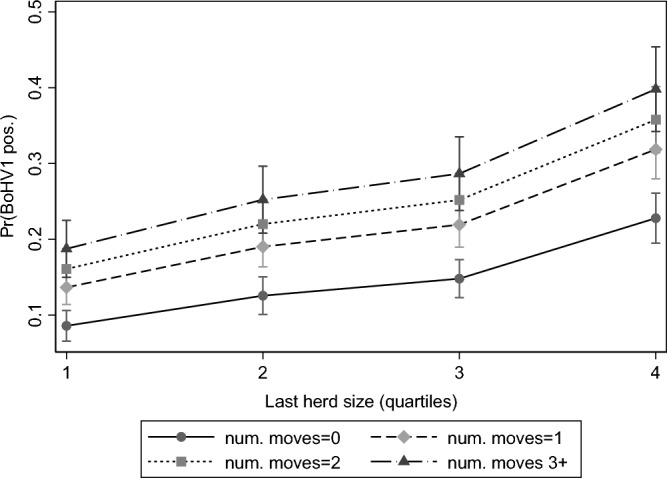
The marginal predicted difference in probability of an animal testing positive to IBR depending on herd size and animal movements, controlling to clustering effects of animals residing in the same herd at test.
Discussion
Over the two years of the study, we found an average BoHV-1 seroprevalence of 21.43%. However, there was a significant reduction in the seroprevalence from 23.32% in 2018 to 17.61% in 2020. The current study differs from previous studies on BoHV-1 in Ireland in that it focuses on cattle less than 30 months of age in commercial cattle herds (see Table 3 for an overview of previous studies). In a study of 161 suckler cow herds, Barrett et al. (2018) found an overall mean within-herd prevalence of 39.8% and a herd-level prevalence of 90%8. In a study of young bulls being submitted to AI (artificial insemination), O’Grady et al.15 found an animal seroprevalence of 28% and a herd level prevalence of 73%. A previous study examining the seroprevalence of BoHV-1 among cattle tested for the national brucellosis eradication recorded a herd-level seroprevalence of 74.9%11. While we do not have a herd level prevalence from this study, the adult animal level prevalence tended to be lower than those previously reported in Ireland (cf: Ring et al.36). The result from the present study may, in part, be related to the age of the cattle surveyed in this study, as other studies have focused on older age cohorts. Indeed, Martinez-Ibeas et al.12 found that increasing age was a significant risk factor for BoHv-1 seropositivity. An exception that proves the rule, is the study by Sayers et al.7. Sayers et al.7 sampled 2171 weanling with a mean age of only 291 days and found a very low exposure with 5.4% of these calves being seropositive for BoHV1.
Table 3.
Review of previous studies relating to apparent prevalence of BoHV1 exposure in cattle in Ireland.
| Study | Year | Herd, within-herd, or animal level | Serum or bulk milk | n | Sample year(s) | Apparent Prevalence |
|---|---|---|---|---|---|---|
| 15 | 2008 | Herd | Serum | 41 beef herds [bulls] | 2007 | 73% (30/41) |
| 15 | 2008 | Within-herd | Serum | 30 [infected] beef herds [bulls] | 2007 | 28% (SD: 20); Median herd size: 55 |
| 11 | 2011 | Herd | Serum | 1175 dairy and beef herds | 2009 | 75% (95% CI 70–80%) |
| 11 | 2011 | Herd | Bulk milk | 111 dairy herds | 2009 | 71% (79/111) |
| 7 | 2015 | Herd | Bulk milk | 305 dairy herds | 2009 | 80% (244/305) |
| 7 | 2015 | Animal | Serum | 2171 weanlings | 2009 | 5% (117/2171) |
| 12 | 2015 | Animal | Serum | 529 bulls | 2009 | 17% (87/529) |
| 36 | 2018 | Animal | Serum | 6534 female cattle (24 herds) | 2010–2013 | 26% |
| 36 | 2018 | Animal | Serum | 10,669 cows (67 herds) | 2015 | 23% |
| 8 | 2018 | Herd | Serum | 161 beef herds | 2014–2015 | 90% |
| 8 | 2018 | Within-herd | Serum | 6049 cows | 2014–2015 | 40% |
The size of the herd the animal resided in immediately prior to slaughter, the number of herds the animal resided in, the year the animal was sampled in, the sex of the animal and the animal’s BVD serostatus were all significant in the final logistic regression model. Herd size is a well-recognised risk factor associated with the occurrence of several diseases in Ireland and elsewhere including bovine tuberculosis37, BVD23 and BoHV-15,11. Increasing herd size increases the probability of an individual animal becoming exposed to pathogens when other infected cattle are present in that herd. This may be especially important in the epidemiology of BoHV-1, where infection may be re-activated in latently infected cattle when they become stressed38. Cattle slaughtered out of herds with more than 225 cattle, were almost 5 times as likely to seroconvert to BHV-1 as cattle slaughtered out of herds with less than 53 cattle. This finding would suggest that there could be value in focusing an BoHV-1 control programme in larger herds, and a strategy that could be tested using simulation tools (for example, Brock et al.39).
The number of herds the animal resided in over its lifetime was found to be the next most important risk factor, which has been reported previously40. The risk of serconversion among cattle that resided in two herds was twice that of those that resided in only one herd from birth. This increased to 2.6 times and 3.5 times to cattle that resided in three and four or more herds respectively. Similar to the herd size risk factor, movement between herds represents an opportunity for increased exposure to infected cattle, but also could represent a stressor41 that could impact on susceptibility or reactivation. Another aspect of movement not addressed in this study was the movement of cattle was facilitated through cattle marts, where several hundred cattle may be assembled from several herds on the same day. Marts can be important connecting nodes amongst cattle movement networks42, which can facilitate infectious pathogen spread43. This potential exposure at markets could provide an added opportunity for the transmission and spread, across connecting trading ‘nodes’ of the network, of BoHV-1.
We found male cattle were 1.2 times more likely to seroconvert to BoHV-1 than their female counterparts, however, bootstrapping of the final multivariable model suggested that this association was not robust. There is no obvious biological reason for a sex bias, but the pattern has been reported elsewhere in the literature (e.g.5,44). However, such sex biases in terms of risk may relate to the overall management of the males versus females. We did not make a distinction between bulls and bullocks (steers) and we would suggest that bulls may be more intensively managed than either bullocks or heifers and this may have contributed to any differential risk, but we do not have the data to investigate this further. Bulls may also be exposed to more at-risk contacts, either by their behaviour at the individual-level or by their inter-herd movements44.
We found that there were an increased odds of cattle slaughtered in 2018 being test positive relative to animals slaughtered in 2020, controlling for covariables within the model. In addition, we found that cattle seropositive for BVD were 1.6 times more likely to seroconvert to BoHV-1 than cattle seronegative for BVD, controlling for year. While these were both significant independent variables in the final model, they may be associated. There has been a BVD eradication programme in Ireland since 2013, where the herd level prevalence of BVD has decreased from 11% in 2013 to 0.59% in 202045. The variance explained by the “year” variable is possibly reflecting some of the effects of BVD control, the biosecurity efforts of farmers in Ireland in recent years46, and the unobserved dynamics not captured by this analysis.
A previous Irish study of 6000 beef suckler cows documented an association between the presence of BVDV antibody positive animals and seroconversion to BoHV-18, which is possibly due to the immunosuppressive effects of BVD. This would suggest that the eradication of BVD may assist in reducing the seroprevalence of BoHV-1. A study by Sayers et al.7 found that 72% of herds sampled for BVD and BoHV-1 using bulk milk samples were concurrently infected in Ireland in 2009. In fact, during that study, only 10 herds (from 305 total herds) were bulk milk seronegative to both pathogens. Even though there was evidence of coinfection associations in previous studies with BVDV in Ireland (e.g.7,8), in the present study, this association was not very statistically robust. Despite this, we know that biosecurity and other infectious disease control interventions from one programme, can have indirect benefits to other pathogens without formal control programmes.
Limitations
The data generated for this study was part of overall surveillance activities and not solely for the surveillance of BoHV-1. The serosurvey was not carried out in 2019, so data from the middle year of the study period is not available. The study was observational and retrospective so therefore any associations cannot be necessarily considered causative. However, most of these associations were previously documented and are generally biologically plausible. We are confident that vaccination would not have interfered with the outcome, as the test kit used in the study for BoHV-1 Ab testing is based in gE detection, a non-structural protein deleted in all commercially available vaccines in Ireland, thus differentiating natural infection from vaccinal antibodies.
Conclusions and implications
This study has demonstrated the BoHV 1 status of cattle at slaughter is associated with the size of the herd the cattle are slaughtered out of, the number of herds the animal resided within and, to a lesser degree, the cattle’s BVD serostatus. This emphasises the need to focus on cattle in larger herds and cattle which move between multiple herd in any effort to control BoHV-1, a finding that could be useful for several other countries currently working towards eradication47. This study also emphasises the potential benefits the eradication of BVD will bring to the control of BoHV-1.
Supplementary Information
Author contributions
D.B.: conceptualization; project administration; writing—review and editing. E.L.: project administration; writing—review and editing. J.M.L.: investigation; methodology; writing—review and editing. K.O.K.: investigation; methodology. A.W.B.: conceptualization; formal analysis; project administration; visualisation; supervision; writing—original draft; writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
No explicit funding was provided explicitly for this research.
Data availability
The datasets analysed during the current study are available from the data controller DAFM on reasonable request and subject to anonymisation of any personal data compliant with GDPR. For data requests please contact the first author or ohssu@agriculture.gov.ie.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-50433-5.
References
- 1.Ackermann M, Engels M. Pro and contra IBR-eradication. Vet. Microbiol. 2006;113:293–302. doi: 10.1016/j.vetmic.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Sayers RG. Associations between exposure to bovine herpesvirus 1 (BoHV-1) and milk production, reproductive performance, and mortality in Irish dairy herds. J. Dairy Sci. 2017;100:1340–1352. doi: 10.3168/jds.2016-11113. [DOI] [PubMed] [Google Scholar]
- 3.Hanrahan, K. et al. Analysis of the economics of BoHV-1 infection in Ireland. A report produced by Teagasc and published by Animal Health Ireland. https://animalhealthireland.ie/assets/uploads/2021/04/AHI-IBR-Economic-Analysis-Report-2020.pdf (2020).
- 4.Ackermann M, Peterhans E, Wyler R. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am. J. Vet. Res. 1982;43:36–40. [PubMed] [Google Scholar]
- 5.Muylkens B, Thiry J, Kirten P, Schynts F, Thiry E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Veterinary research. 2007;38:181–209. doi: 10.1051/vetres:2006059. [DOI] [PubMed] [Google Scholar]
- 6.Stratham J, Randall L, Archer S. Reduction in daily milk yield associated with sub-clinical bovine herpes virus infection. Vet. Record. 2015;177:339. doi: 10.1136/vr.103105. [DOI] [PubMed] [Google Scholar]
- 7.Sayers R, Byrne N, O'Doherty E, Arkins S. Prevalence of exposure to bovine viral diarrhoea virus (BVDV) and bovine herpesvirus-1 (BoHV-1) in Irish dairy herds. Res. Vet. Sci. 2015;100:21–30. doi: 10.1016/j.rvsc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Barrett D, et al. Prevalence of bovine viral diarrhoea virus (BVDV), bovine herpes virus 1 (BHV 1), leptospirosis and neosporosis, and associated risk factors in 161 Irish beef herds. BMC Vet. Res. 2018;14:1–10. doi: 10.1186/s12917-017-1324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Palomo F, et al. Characterization of thymus atrophy in calves with subclinical BVD challenged with BHV-1. Vet. Microbiol. 2015;177:32–42. doi: 10.1016/j.vetmic.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Risalde M, et al. Effects of preinfection with bovine viral diarrhea virus on immune cells from the lungs of calves inoculated with bovine herpesvirus 1.1. Vet. Pathol. 2015;52:644–653. doi: 10.1177/0300985814551579. [DOI] [PubMed] [Google Scholar]
- 11.Cowley D, Clegg TA, Doherty ML, More SJ. Aspects of bovine herpesvirus-1 infection in dairy and beef herds in the Republic of Ireland. Acta Veter. Scand. 2011;53:1–9. doi: 10.1186/1751-0147-53-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Ibeas AM, Power C, McClure J, Sayers RG. Prevalence of BoHV-1 seropositive and BVD virus positive bulls on Irish dairy farms and associations between bull purchase and herd status. Irish Vet. J. 2015;68:1–9. doi: 10.1186/s13620-015-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayers R, Arkins S. Prevalence of exposure to bovine viral diarrhoea virus (BVDV) and bovine herpesvirus-1 (BoHV-1) in Irish dairy herds. Res. Vet. Sci. 2015;100:21–30. doi: 10.1016/j.rvsc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Gunn HM, Wilson B. Observations on outbreaks of respiratory disease in intensively housed feedlot cattle and climatic considerations. Irish Vet. J. 1991;44:41–42. [Google Scholar]
- 15.O’Grady L, O’neill R, Collins DM, Clegg T, More S. Herd and within-herd BoHV-1 prevalence among Irish beef herds submitting bulls for entry to a performance testing station. Irish Vet. J. 2008;61:1–7. doi: 10.1186/2046-0481-61-12-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brock J, et al. Epidemiology of age-dependent prevalence of Bovine Herpes Virus Type 1 (BoHV-1) in dairy herds with and without vaccination. Vet. Res. 2020;51:1–13. doi: 10.1186/s13567-020-00842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power C, McClure J, Sayers RG. Prevalence of BoHV-1 seropositive and BVD virus positive bulls on Irish dairy farms and associations between bull purchase and herd status. Irish Vet. J. 2015;68:1–9. doi: 10.1186/s13620-015-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OIE. Terrestrial Animal Health Code 2021. Chapter 11.8.—Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/2018/en_chapitre_ibr_ipv.htm (2021).
- 19.European Commission. (Official Journal of the European Union, 2020).
- 20.Rushton J, et al. Initiation of global burden of animal diseases programme. The Lancet. 2018;392:538–540. doi: 10.1016/S0140-6736(18)31472-7. [DOI] [PubMed] [Google Scholar]
- 21.Moredun Research Institute. Acting on methane: opportunities for the UK cattle and sheep sectors. In Moredun Research Institute on behalf of Ruminant Health & Welfare. https://ruminanthw.org.uk/actingonmethane/ (2022).
- 22.Government of Ireland. Climate Action Plan 2021. https://www.gov.ie/en/publication/6223e-climate-action-plan-2021/ (2020).
- 23.Barrett, D. et al. BVD seroprevalence in the Irish cattle population as the national BVD programme progresses toward eradication (2022). [DOI] [PMC free article] [PubMed]
- 24.Humphry RW, Cameron A, Gunn GJ. A practical approach to calculate sample size for herd prevalence surveys. Prevent. Vet. Med. 2004;65:173–188. doi: 10.1016/j.prevetmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Kramps J, et al. A simple, specific, and highly sensitive blocking enzyme-linked immunosorbent assay for detection of antibodies to bovine herpesvirus 1. J. Clin. Microbiol. 1994;32:2175–2181. doi: 10.1128/jcm.32.9.2175-2181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergeant, E. Epitools epidemiological calculators. https://epitools.ausvet.com.au/ (2018).
- 27.Reiczigel J, Földi J, Ózsvári L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol. Infect. 2010;138:1674–1678. doi: 10.1017/S0950268810000385. [DOI] [PubMed] [Google Scholar]
- 28.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978;107:71–76. doi: 10.1093/oxfordjournals.aje.a112510. [DOI] [PubMed] [Google Scholar]
- 29.Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011;65:23–35. doi: 10.1007/s00265-010-1029-6. [DOI] [Google Scholar]
- 30.Raftery AE. Bayesian model selection in social research. Sociol. Methodol. 1995;1995:111–163. doi: 10.2307/271063. [DOI] [Google Scholar]
- 31.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. CRC Press; 1994. [Google Scholar]
- 32.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 33.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1986;1986:54–75. [Google Scholar]
- 34.Mooney, C. Z. & Duval, R. D. Bootstrapping: A Nonparametric Approach to Statistical Inference (1993).
- 35.StataCorp. (StataCorp LLC, College Station, TX, 2019).
- 36.Ring SC, et al. Genetic variability in the humoral immune response to bovine herpesvirus-1 infection in dairy cattle and genetic correlations with performance traits. J. Dairy Sci. 2018;101:6190–6204. doi: 10.3168/jds.2018-14481. [DOI] [PubMed] [Google Scholar]
- 37.Byrne AW, et al. Post-mortem surveillance of bovine tuberculosis in Ireland: herd-level variation in the probability of herds disclosed with lesions at routine slaughter to have skin test reactors at follow-up test. Vet. Res. Commun. 2020;44:131–136. doi: 10.1007/s11259-020-09777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones C. Bovine herpesvirus 1 counteracts immune responses and immune-surveillance to enhance pathogenesis and virus transmission. Front. Immunol. 2019;10:1008. doi: 10.3389/fimmu.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brock J, et al. A large-scale epidemiological model of BoHV-1 spread in the Irish cattle population to support decision-making in conformity with the European Animal Health Law. Prevent. Vet. Med. 2021;192:105375. doi: 10.1016/j.prevetmed.2021.105375. [DOI] [PubMed] [Google Scholar]
- 40.Nardelli S, et al. Dynamics of infection and immunity in a dairy cattle population undergoing an eradication programme for Infectious Bovine Rhinotracheitis (IBR) Prevent. Vet. Med. 2008;85:68–80. doi: 10.1016/j.prevetmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Knowles G. A review of the road transport of cattle. Vet. Record. 1999;144:197–201. doi: 10.1136/vr.144.8.197. [DOI] [PubMed] [Google Scholar]
- 42.Brown E, Marshall AH, Mitchell HJ, Byrne AW. Cattle movements in Northern Ireland form a robust network: implications for disease management. Prevent. Vet. Med. 2019;170:104740. doi: 10.1016/j.prevetmed.2019.104740. [DOI] [PubMed] [Google Scholar]
- 43.Rautureau S, Dufour B, Durand B. Vulnerability of animal trade networks to the spread of infectious diseases: a methodological approach applied to evaluation and emergency control strategies in cattle, France, 2005. Transbound. Emerg. Dis. 2011;58:110–120. doi: 10.1111/j.1865-1682.2010.01187.x. [DOI] [PubMed] [Google Scholar]
- 44.Boelaert F, et al. Risk factors for bovine herpesvirus-1 seropositivity. Prevent. Vet. Med. 2005;69:285–295. doi: 10.1016/j.prevetmed.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Animal Health Ireland. BVD Programme Results. Animal Health Ireland. https://animalhealthireland.ie/programmes/bvd/programme-results/ (2022).
- 46.Guelbenzu-Gonzalo MP, Lozano J-M, O'Sullivan P, Lane EA, Graham DA. A herd investigation tool in support of the Irish bovine viral diarrhoea eradication programme. Front. Vet. Sci. 2021;8:694774. doi: 10.3389/fvets.2021.694774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iscaro C, Cambiotti V, Petrini S, Feliziani F. Control programs for infectious bovine rhinotracheitis (IBR) in European countries: an overview. Anim. Health Res. Rev. 2021;22:136–146. doi: 10.1017/S1466252321000116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the data controller DAFM on reasonable request and subject to anonymisation of any personal data compliant with GDPR. For data requests please contact the first author or ohssu@agriculture.gov.ie.


