Abstract
Purpose
To evaluate choroidal thickness and retinal nerve fiber layer (RNFL) thickness in different trimesters using enhanced depth imaging optical coherence tomography (EDI-OCT).
Methods
A prospective comparative study included 45 healthy pregnant women in the first trimester, 45 women in the second, 45 women in the third and 45 healthy non-pregnant women as the control group. Macular choroidal thickness was measured at three locations: The subfoveal, 1 mm temporal, and 1 mm nasal from the fovea with EDI-OCT. Peripapillary choroidal thickness (PPCT) and RNFL thickness parameters were automatically calculated by the Spectralis OCT.
Results
The subfoveal, temporal and nasal macular choroidal thickness were all significantly thicker in the second trimester, compared with those parameters in the first, the third trimesters and the control group (all P < 0.05). The PPCT was significantly increased in the second trimeter compared with the control group at global, temporal, temporal inferior, nasal and nasal inferior positions (all P < 0.05). The RNFL thickness was also significantly increased in pregnant women at nasal superior and nasal inferior quadrants (all P < 0.05).
Conclusions
The choroidal thickness in pregnant women was found to be thicker than the control group, regardless of macular or optic disc location. Findings of RNFL thickening might indicate subclinical involvement of the central nervous system.
Keywords: Choroid, Retinal nerve fiber layer, Optical coherence tomography, Pregnancy
1. Introduction
Pregnancy is a natural state that involves physiological changes in almost all organs of the body. These changes are related to alterations in the vascular, immunologic, metabolic, and hormonal systems.1 The eye is one of the most important organs that is affected by these changes during pregnancy. Several ocular changes have been reported to occur in this period, such as increased corneal thickness and curvature, decreased corneal sensitivity and intraocular pressure.2 Moreover, some pathological changes have also been observed during pregnancy, such as central serous chorioretinopathy (CSC) and eclampsia-associated retinopathy.1
The choroid is a vital structure in the eye that performs physiological and pathological functions by supplying blood and nutrients to ocular tissues. During pregnancy, systemic vascular resistance decreases and hemodynamic changes affect blood pressure, which in turn influence choroidal thickness. It has been reported that the incidence of preeclampsia, a pregnancy complication characterized by high blood pressure and organ damage, is about 5%.3 The ocular symptoms of preeclampsia include retinopathy, optic neuropathy, retinal edema, and so on. The damage to the optic nerves can also lead to visual loss. However, the effect of pregnancy on the retinal nerve fiber layer (RNFL) thickness is unclear at present. With the development of technology, enhanced depth imaging optical coherence tomography (EDI-OCT), a noninvasive, rapid, and high-resolution diagnostic method, is widely used for evaluating choroidal anatomy in detail.4
Choroidal thickness is influenced by blood flow and ocular perfusion pressure. Therefore, it is a useful parameter for indicating physiological and pathological changes in the eye. Several studies have reported on choroidal thickness in pregnant women,5, 6, 7 but their results are controversial. In addition, studies on peripapillary RNFL thickness also show different results during pregnancy. One study reports that RNFL decreased significantly after pregnancy regardless of whether the women had preeclampsia, eclampsia, or were healthy.8 Tok et al. report that the mean RNFL thickness was similar in both preeclampsia and healthy pregnant women.9 Most of the current studies are cross-sectional and lack long-term follow-up. The relationship between the changes in choroidal thickness, RNFL thickness, and pregnancy remains unclear at present.
The purpose of this study was to observe and compare the macular choroidal thickness, PPCT, and RNFL thickness in Chinese pregnant and non-pregnant women. Additionally, we aimed to monitor the ocular complications during each trimester of pregnancy.
2. Methods
2.1. Ethical approval
This prospective study was conducted at the People's Hospital of Zhuji from January 2020 to January 2021, following the tenets of the Declaration of Helsinki and with the approval of the Ethics Committee [NO. 2020 (0015)]. All participants signed an informed consent form before enrolling in the study.
2.2. Methods
The study population consisted of 135 healthy pregnant women and 45 non-pregnant women of reproductive age. The pregnant women were divided into four groups according to their gestational age10: the first trimester group (n = 45) included women with less than 13 weeks of pregnancy, the second trimester group (n = 45) included women from 14 to 28 weeks of pregnancy, and the third trimester group (n = 45) included women with more than 28 weeks of pregnancy. The control group (n = 45) comprised healthy non-pregnant women. In our study, 113 pregnant women were unigravida (gravidity one and parity zero, G1P0) and 22 pregnant women were multipara (gravidity two and parity one, G2P1).
The study included healthy pregnant women without systemic diseases as the experimental group and healthy non-pregnant women as the control group. The participants were aged between 18 and 40 years and had an axial length (AL) of 21 mm–26 mm and a best-corrected visual acuity (BCVA) of at least 20/20 (Snellen chart). The study excluded participants with high intraocular pressure (IOP) (more than 21 mmHg), systemic or ocular diseases that could affect the retinal or choroidal thickness, such as a history of CSC, diabetes mellitus, hypertension, glaucoma, uveitis, and choroidal diseases.1
Diastolic blood pressure and systolic blood pressure were measured for each participant. And all participants underwent a comprehensive ocular examination, including BCVA, slit-lamp examination, IOP measurement by noncontact tonometer (TX-20 P, Canon Tokyo Japan), and dilated funduscopy. BCVA was measured by electronic chart. Axial length was measured using an optical biometer (IOL Master 500, Carl Zeiss Germany). Choroidal thickness was measured by EDI-OCT using spectral domain OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) after pupil dilation. The scans covered the macular and optic nerve regions. Choroidal thickness was defined as the distance from the outer surface of the retinal pigment epithelium to the chorioscleral interface. Macular CT was measured at the fovea and at positions 1 mm temporal and nasal to the fovea (Fig. 1A and B). Only the horizontal scan through the center of the macula was selected for further analysis. Peripapillary choroidal thickness measurement was performed according to Jiang et al. using an EDI 3.4-mm diameter peripapillary circle scan (Fig. 1C).11 Manual segmentation was performed. The inner boundary of RNFL was adjusted to the outer layer of RNFL hyper reflection, and then the outer boundary of RNFL was adjusted to the chorioscleral interface. The distance between the inner and outer boundary of RNFL was peripapillary choroidal thickness. Both peripapillary choroidal thickness and RNFL thickness parameters were automatically divided into seven sections: temporal quadrant, temporal superior quadrant, nasal superior quadrant, nasal quadrant, nasal inferior quadrant, temporal inferior quadrant, and global thickness. All OCT scans were completed in the morning (8:00 a.m. to 12:00 p.m.) to avoid diurnal variations of choroidal thickness.2 All measurements were performed by an experienced ophthalmologist. Only the right eye was selected for further analysis.
Fig. 1.
(A) Optical coherence tomography image from second trimester. The choroidal thickness was measured from the retinal pigment epithelium to the inner surface of the sclera at the subfovea (382 μm), 1 mm temporal (358 μm), and 1 mm nasal to the fovea (370 μm). (B) Optical coherence tomography image from a no-pregnant woman. (C) Representative of peripapillary choroidal thickness. T-temporal, TS- temporal superior, NS-nasal superior, N-nasal, NI-nasal inferior, TI-temporal inferior; G-global.
2.3. Statistical analysis
Statistical analysis was performed using SPSS version 22 (IBM Corp., Armonk, NY, USA). All data are expressed as the mean ± standard deviation. A t-test was used to compare the pregnant and non-pregnant women. One-way analysis of variance (ANOVA) was used to compare the four groups. A P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographics and clinical features
The study included 135 eyes of pregnant women and 45 eyes of age-matched non-pregnant women as the control group. The mean gestational age of the pregnant women was 11.47 ± 1.32, 23.69 ± 3.81, and 31.13 ± 2.46 weeks in the first, second, and third trimesters, respectively. There were no significant differences in the mean age, AL, and IOP among the four groups. Table 1 summarizes the demographic and clinical characteristics of the participants.
Table 1.
Demographic and clinical characteristics for pregnant and control groups.
| Characteristics | first trimester (n = 45) | second trimester (n = 45) | third trimester (n = 45) | control group (n = 45) | F | P† |
|---|---|---|---|---|---|---|
| GA (weeks) | 11.47 ± 1.32 | 23.69 ± 3.84 | 31.13 ± 2.46 | / | ||
| Age (years) | 29.18 ± 3.96 | 29.69 ± 4.61 | 30.18 ± 3.71 | 30.64 ± 6.64 | 0.756 | 0.520 |
| AL (mm) | 24.07 ± 1.02 | 24.04 ± 0.88 | 24.13 ± 1.06 | 24.40 ± 1.02 | 0.986 | 0.401 |
| IOP(mmHg) | 13.00 ± 2.07 | 12.35 ± 2.41 | 12.77 ± 1.92 | 13.02 ± 2.66 | 0.831 | 0.479 |
Values are presented as the mean ± standard deviation. GA: gestational age. AL: axial length. IOP: intraocular pressure. †means analysis of variance.
3.2. Macular choroidal thickness
The mean choroidal thickness in pregnant women was 289.87 ± 57.30 μm at the subfoveal and 293.44 ± 62.18 μm, 265.55 ± 56.45 μm at 1 mm temporal and nasal to the fovea. In comparison, the mean CT in non-pregnant women was 267.47 ± 86.73 μm at the subfoveal and 272.27 ± 76.85 μm, 238.44 ± 89.07 μm at 1 mm temporal and nasal to the fovea. There were statistically significant differences in subfoveal (t = 2.094, P = 0.037) and nasal positions (t = 2.533, P = 0.012) between pregnant women and non-pregnant women. However, no significant difference was found in temporal position (t = 1.714, P = 0.092) (Table 2).
Table 2.
Macular choroidal thickness (μm) in pregnant women and non-pregnant women.
| location | Pregnant women (n = 135) | Non-pregnant women (n = 45) | t | P† |
|---|---|---|---|---|
| Subfoveal | 289.87 ± 57.30 | 267.47 ± 86.73 | 2.094 | 0.037 |
| Temporal | 293.44 ± 62.18 | 272.27 ± 76.85 | 1.714 | 0.092 |
| Nasal | 265.55 ± 56.45 | 238.44 ± 89.07 | 2.533 | 0.012 |
Values are presented as the mean ± standard deviation. †means t-test.
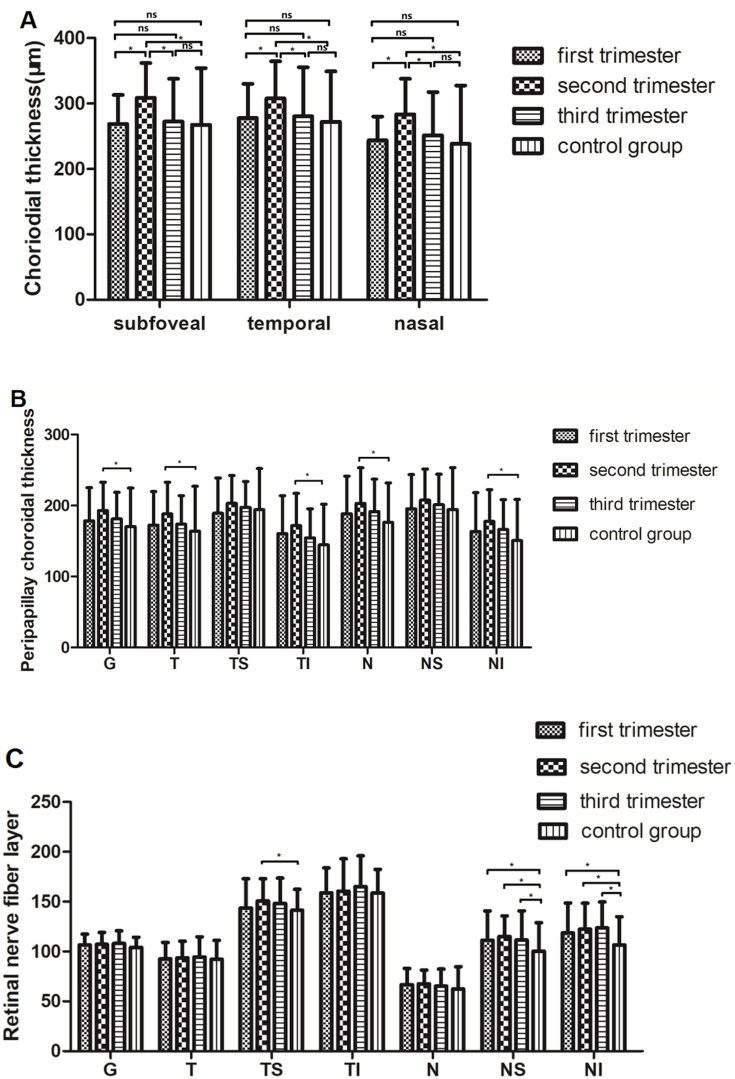
The pregnant women were further divided into three subgroups according to the gestational age (GA). The macular choroidal thickness in subfoveal were 268.87 ± 44.35 μm in the first trimester, 308.98 ± 52.83 μm in the second trimester, 272.64 ± 65.16 μm in the third trimester and 267.47 ± 86.73 μm in non-pregnant women, respectively. The measurements in temporal were 277.69 ± 52.35 μm, 307.82 ± 56.66 μm, 280.44 ± 75.12 μm, 272.27 ± 76.85 μm, respectively. The measurements in nasal were 243.78 ± 36.26 μm, 283.44 ± 54.25 μm, 251.53 ± 65.98 μm and 238.44 ± 89.07 μm, respectively. There were statistically significant differences among the four groups in subfoveal, temporal, and nasal positions (F = 7.268, P < 0.001; F = 4.312, P = 0.006; F = 7.316, P < 0.001, respectively). The mean subfoveal, temporal, and nasal choroidal thickness was significantly greater in the second trimester compared with the first and third trimester (P < 0.001, P = 0.01; P = 0.01, P = 0.02; P = 0.01, P = 0.01, respectively). Fig. 2A shows the distribution of choroidal thickness among each group.
Fig. 2.
Graphs showing the comparative change in choroidal thickness at subfoveal, temporal and nasal (A), peripapillary choroidal thickness in seven positions (B) and retinal nerve fiber layer in seven positions (C). ns means no significant, ∗ means P < 0.05.
3.3. Peripapillary choroidal thickness
Table 3 shows the peripapillary choroidal thickness (PPCT) at different quadrants in pregnant and non-pregnant women. The temporal inferior (TI), nasal (N), and nasal inferior (NI) PCT was significantly thicker in pregnant women than in non-pregnant women (t = 2.440, P = 0.015; t = 2.378, P = 0.018; t = 2.498, P = 0.013, respectively).
Table 3.
Peripapillary choroidal thickness (μm) in pregnant women and non-pregnant women.
| location | Pregnant women (n = 135) | Non-pregnant women (n = 45) | t | P† |
|---|---|---|---|---|
| G | 186.34 ± 41.45 | 170.36 ± 54.28 | 1.846 | 0.070 |
| T | 180.54 ± 44.76 | 163.80 ± 63.39 | 1.670 | 0.101 |
| TS | 198.24 ± 41.57 | 194.09 ± 57.91 | 0.452 | 0.653 |
| TI | 164.57 ± 46.85 | 144.62 ± 57.14 | 2.440 | 0.015 |
| N | 196.41 ± 50.02 | 176.11 ± 55.70 | 2.378 | 0.018 |
| NS | 202.91 ± 44.80 | 194.49 ± 58.89 | 0.897 | 0.374 |
| NI | 171.21 ± 47.05 | 150.64 ± 57.93 | 2.498 | 0.013 |
Values are presented as the mean ± SD. G: global, T: temporal, TS: temporal superior, TI: temporal inferior, N: nasal, NS: nasal superior. †means t-test.
The PPCT at different positions in the first, second, and third trimesters of pregnancy and in non-pregnant women were compared. The global PPCT were 178.47 ± 46.71 μm, 192.84 ± 39.94 μm, 181.22 ± 37.48 μm, and 170.36 ± 54.28 μm in the first, second, third trimesters, and non-pregnant women, respectively. The temporal PPCT were 172.44 ± 47.30 μm, 188.09 ± 44.81 μm, 173.53 ± 40.24 μm, and 163.80 ± 63.39 μm in the corresponding groups, respectively. The temporal superior PPCT were 189.20 ± 49.55 μm, 203.20 ± 39.18 μm, 197.36 ± 36.55 μm, and 194.09 ± 57.91 μm in the corresponding groups, respectively. The temporal inferior PPCT was 160.47 ± 53.11 μm, 171.69 ± 45.51 μm, 154.42 ± 40.89 μm, and 144.62 ± 57.14 μm in the corresponding groups, respectively. The nasal PPCT were 188.36 ± 52.96 μm, 202.91 ± 50.23 μm, 191.44 ± 45.77 μm, and 176.11 ± 55.72 μm in the corresponding groups, respectively. The nasal superior PPCT were 195.33 ± 48.39 μm, 207.60 ± 43.70 μm, 201.11 ± 43.05 μm, and 194.49 ± 58.89 μm in the corresponding groups, respectively. The nasal inferior PPCT was 163.2 ± 54.84 μm, 177.67 ± 44.7 μm, 166.24 ± 42.12 μm, and 150.64 ± 57.93 μm in the corresponding groups, respectively. Peripapillary choroidal thickness was compared among the four groups. Fig. 2B shows the distribution of Peripapillary choroidal thickness among each group. Only Peripapillary choroidal thickness were significantly thicker in the second trimester than in non-pregnant women at global (G), temporal (T), TI, N, and NI positions (all P < 0.01).
3.4. Peripapillary RNFL thickness
The retinal nerve fiber layer (RNFL) thickness was thicker in pregnant women than in non-pregnant women. This difference was significant for the nasal superior (NS) (t = 3.018, P = 0.003) and nasal inferior (NI) (t = 3.368, P = 0.001) quadrants (Table 4).
Table 4.
Peripapillary RNFL thickness (μm) in pregnant women and non-pregnant women.
| location | Pregnant women (n = 135) | Non-pregnant women (n = 45) | t | P† |
|---|---|---|---|---|
| G | 107.14 ± 11.87 | 104.16 ± 10.09 | 1.554 | 0.122 |
| T | 93.56 ± 17.54 | 92.24 ± 18.89 | 0.444 | 0.658 |
| TS | 148.36 ± 24.97 | 141.29 ± 21.07 | 1.750 | 0.081 |
| TI | 161.26 ± 30.30 | 158.67 ± 23.77 | 0.619 | 0.538 |
| N | 66.78 ± 15.27 | 62.47 ± 22.23 | 1.232 | 0.223 |
| NS | 113.31 ± 25.20 | 100.28 ± 28.47 | 3.018 | 0.003 |
| NI | 121.83 ± 27.00 | 106.5 ± 28.31 | 3.368 | 0.001 |
Values are presented as the mean ± SD. G: global, T: temporal, TS: temporal superior, TI: temporal inferior, N: nasal, NS: nasal superior. †means t-test.
The RNFL thickness was compared among different trimesters and non-pregnant women. The global RNFL thickness were 106.47 ± 10.90 μm, 107.09 ± 11.96 μm, 107.93 ± 12.80 μm, and 104.16 ± 10.09 μm in the first, second, third trimesters, and non-pregnant women, respectively. The temporal RNFL thickness were 92.69 ± 16.27 μm, 93.56 ± 16.81 μm, 94.44 ± 20.32 μm, and 92.24 ± 18.89 μm in the corresponding groups, respectively. The temporal superior RNFL thickness were 143.64 ± 29.28 μm, 150.47 ± 22.30 μm, 148.33 ± 25.26 μm, and 141.29 ± 21.07 μm in the corresponding groups, respectively. The temporal inferior RNFL thickness were 158.93 ± 24.94 μm, 160.47 ± 32.58 μm, 165.20 ± 30.72 μm, and 158.67 ± 23.77 μm in the corresponding groups, respectively. The nasal RNFL thickness were 66.67 ± 16.41 μm, 67.62 ± 13.74 μm, 65.22 ± 17.12 μm, and 62.47 ± 22.23 μm in the corresponding groups, respectively. The nasal superior RNFL thickness was 111.31 ± 29.34 μm, 115.13 ± 20.59 μm, 111.64 ± 29.17 μm, and 100.29 ± 28.47 μm in the corresponding groups, respectively. The nasal inferior RNFL thickness was 118.73 ± 29.97 μm, 122.44 ± 26.08 μm, 123.73 ± 26.06 μm, and 106.53 ± 28.31 μm in the corresponding groups, respectively. There were significant differences between the first, second, and third trimesters and non-pregnant women at NS and NI quadrants (all P < 0.05). Additionally, there was a significant difference between the second trimester and non-pregnant women at the temporal superior (TS) quadrant (P = 0.03) (Fig. 2C).
4. Discussion
In our study, we compared the macular choroidal thickness of 135 healthy pregnant women in different trimesters with 45 healthy non-pregnant women. We found that macular choroidal thickness was thicker than that of non-pregnant women, though there was no difference between first trimester and non-pregnant women. This finding was consistent with previous reports.12 However, Azuma et al. reported no significant difference in macular choroidal thickness between healthy pregnant and non-pregnant women.13 They only enrolled pregnant women in the first trimester, which might explain this contradictory result. Unlike their study, we enrolled pregnant women in each trimester and measured macular choroidal thickness (MCT) using EDI-OCT.
Our study showed that the macular choroidal thickness reached the maximum value in the second trimester of pregnancy. This was consistent with the findings of Alizadeh et al. who reported that macular choroidal thickness increased in the subfoveal, temporal, and nasal regions in the second trimester.1 We hypothesized that this was related to the changes in blood volume and vascular resistance during pregnancy. Blood volume gradually increased from the beginning of pregnancy and had a rapid increase in the second trimester, followed by a slow increase. Vascular resistance progressively decreased from the fifth week of gestation, leading to reduced blood pressure.1 The most obvious changes in blood volume and vascular resistance occurred in the middle of pregnancy. Therefore, this might explain the increase of macular choroidal thickness in the second trimester. Pregnancy has been considered as a risk factor for CSC, which commonly develops in the third trimester. One study observed that macular choroidal thickness increased significantly in patients with acute CSC.14 We speculated that the increased macular choroidal thickness observed in the second trimester might be a stimulative factor for the development of CSC in the third trimester.
We measured the PPCT in pregnant women and the control group. The global (G), temporal (T), temporal inferior (TI), nasal (N), and nasal inferior (NI) peripapillary choroidal thickness were significantly thicker in the second trimester than in non-pregnant women (P = 0.006, P = 0.007, P = 0.003, P = 0.004, P = 0.003, respectively). These data on PPCT in pregnant women have not been reported before, and the significance of these findings has yet to be determined. Previous studies on PPCT have only reported some systemic diseases. Vural et al. found that the inferior and nasal peripapillary choroidal thickness decreased in patients with vitamin D deficiency.15 They suggested that a decrease in vitamin D activity in the eye could cause vascular endothelial dysfunction and result in choroidal thinning. Patients with multiple sclerosis (MS) showed PPCT thinning compared with their healthy counterparts in all zones around the optic disc.16 They proposed that the decrease of PPCT might be secondary to a reduction in blood flow due to RNFL atrophy in MS patients. Therefore, changes in choroidal blood volume might result in the dysfunction of photoreceptors, and peripapillary choroidal thickness status was important because of the role of choroidal vascularity in the anterior optic nerve.17 We could not find any publication on peripapillary choroidal thickness measurements for pregnant women. The precise mechanism for increased peripapillary choroidal thickness in most zones during pregnancy in our study is still unclear. We can only speculate whether these pregnant women present with vascular hyperpermeability and dilation of the choroid. This finding might remind us to pay more attention to the underlying development of CSC in these pregnant women.
The RNFL is the inner retinal layer formed by the axons of the retinal ganglion cells, and it is the only central nervous system structure that is visible on fundoscopic examination as the axons converge at the optic disc.9 Therefore, it can detect axonal damage and reflect neuroprotection and neurodegeneration. In our study, we found that the peripapillary RNFL thickness was significantly thicker at the NS and NI quadrants in healthy pregnant women. Tok et al. investigated the effect of severe preeclampsia on RNFL and they found no significant difference between preeclampsia and healthy pregnant women during pregnancy.9 In contrast to their study, Neudorfer's study showed that the RNFL thickness was significantly thicker in preeclamptic women with abnormal retinal findings.18 However, they did not have a control group of healthy pregnant women in their study. To date, we could not find any literature on RNFL measurement in healthy pregnant women. We speculated that RNFL thickening in pregnant women compared with non-pregnant women might be caused by pregnancy-related vasodilation. It might reflect subclinical involvement of the central nervous system in their disease, especially for preeclampsia women. Future studies are needed to test this possibility in depth.
One of the limitations of our study was that we did not evaluate the same pregnant woman from the first trimester to postpartum. A longitudinal study of choroidal thickness could provide more insights into the changes that occur during pregnancy and after delivery. Another limitation of our study was that we did not include patients with preeclampsia, a serious condition that can affect blood pressure and organ function in pregnant women. The effect of preeclampsia on peripapillary choroidal thickness is still unknown and warrants further investigation.
5. Conclusions
Our study found that the macular choroidal thickness increased during pregnancy and reached the maximum value in the second trimester. This implies that we need to pay more attention to the ocular changes of pregnant women in the second trimester. We also found that the peripapillary choroidal thickness and the retinal nerve fiber layer thickness increased in pregnant women compared with non-pregnant women. These findings provide valuable information for interpreting pregnancy-associated ocular disorders and serve as a basis for future studies.
Study approval
This study was conducted at the People's Hospital of Zhuji from January 2020 to January 2021, following the tenets of the Declaration of Helsinki and with the approval of the Ethics Committee [NO. 2020 (0015)]. All participants signed an informed consent form before enrolling in the study.
Author contributions
HW was involved in the study design, analysis, writing the manuscript and agreement to be accountable. HL participated in statistical analysis, revising the manuscript and approval of the manuscript. MR participated in providing resource of pregnant. HF, ND, TW, and FY participated in providing eye examination for pregnant, data recording and collection. JZ participated in conception and design, critical revision, approval of the manuscript and agreement to be accountable. All authors reviewed the results and approved the final version of the manuscript.
Funding
This study was supported by The Medical and health science and technology plan of Zhejiang Province (Grant No.2021RC163 and No.2022RC284).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the support of our colleagues and patients.
Abbreviations
- RNFL
Retinal nerve fiber layer
- EDI-OCT
Enhanced depth imaging optical coherence tomography
- PPCT
Peripapillary choroidal thickness
- CSC
Central serous chorioretinopathy
- AL
Axial length
- BCVA
Best-corrected visual acuity
- IOP
Intraocular pressure
- MS
Multiple sclerosis
- MCT
Macular choroidal thickness
References
- 1.Alizadeh Y., Moravvej Z., Soltani-Moghadam R., et al. Evaluation of choroidal thickness during pregnancy and postpartum: a longitudinal study. J Curr Ophthalmol. 2022;34(3):312–317. doi: 10.4103/joco.joco_42_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benfica C.Z., Zanella T., Farias L.B., et al. Comparative analysis of choroidal thickness in third trimester pregnant women. Int J Retina Vitreous. 2018;29(4):6. doi: 10.1186/s40942-018-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukui A., Tanaka H., Terao N., et al. Changes in choroidal thickness and structure in preeclampsia with serous retinal detachment. J Clin Med. 2023;12(2):609. doi: 10.3390/jcm12020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weill Y., Brosh K., Vineberg T.L., et al. Enhanced depth imaging in swept-source optical coherence tomography: improving visibility of choroid and sclera, a masked study. Eur J Ophthalmol. 2020;30(6):1295–1300. doi: 10.1177/1120672119863560. [DOI] [PubMed] [Google Scholar]
- 5.Acmaz G., Atas M., Gulhan A., et al. Assessment of macular peripapillary nerve fiber layer and choroidal thickness changes in pregnant women with gestational diabetes mellitus, healthy pregnant women, and healthy non-pregnant women. Med Sci Mon Int Med J Exp Clin Res. 2015;21:1759–1764. doi: 10.12659/MSM.893221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sochurek J.A.M., Gembicki M., Grisanti S., et al. Vascular choroidal alterations in uncomplicated third-trimester pregnancy. Tomography. 2022;8(5):2609–2617. doi: 10.3390/tomography8050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J., Wang H., Yu Q., et al. Enhanced depth imaging optical coherence tomography: a new way measuring choroidal thickness in pregnant women. J Ophthalmol. 2017;2017 doi: 10.1155/2017/8296574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arab M., Entezari M., Ghamary H., et al. Peripapillary retinal nerve fiber layer thickness in preeclampsia and eclampsia. Int Ophthalmol. 2018;38(6):2289–2294. doi: 10.1007/s10792-017-0718-9. [DOI] [PubMed] [Google Scholar]
- 9.Tok A., Beyoğlu A. Antenatal and postpartum comparison of HD-OCT findings of macula, retinal nerve fiber layer, ganglion cell density between severe preeclampsia patients and healthy pregnant woman. Hypertens Pregnancy. 2020;39(3):252–259. doi: 10.1080/10641955.2020.1758938. [DOI] [PubMed] [Google Scholar]
- 10.Wu H.F., Ruan X.C., Zhao J.W., et al. Comparison of choroid thickness in healthy pregant women and non-pregant women. Chin J Ocul Fundus Dis. 2020;36(8):620–624. doi: 10.3760/cma.j.cn511434-20190916-00289. [DOI] [Google Scholar]
- 11.Jiang R., Wang Y.X., Wei W.B., et al. Peripapillary choroidal thickness in adult Chinese: the Beijing eye study. Invest Ophthalmol Vis Sci. 2015;56(6):4045–4052. doi: 10.1167/iovs.15-16521. [DOI] [PubMed] [Google Scholar]
- 12.Ataş M., Açmaz G., Aksoy H., et al. Evaluation of the macula, retinal nerve fiber layer and choroid in preeclampsia, healthy pregnant and healthy non-pregnant women using spectral-domain optical coherence tomography. Hypertens Pregnancy. 2014;33(3):299–310. doi: 10.3109/10641955.2013.877924. [DOI] [PubMed] [Google Scholar]
- 13.Azuma K., Okubo A., Suzuki T., et al. Assessment of the choroidal structure in pregnant women in the first trimester. Sci Rep. 2021;11(1):4629. doi: 10.1038/s41598-021-84204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharudin S.N., Saaid R., Samsudin A., et al. Subfoveal choroidal thickness in pre-eclampsia. Optom Vis Sci. 2020;97:81–85. doi: 10.1097/OPX.0000000000001480. [DOI] [PubMed] [Google Scholar]
- 15.Vural E., Hazar L., Çağlayan M., et al. Peripapillary choroidal thickness in patients with vitamin D deficiency. Eur J Ophthalmol. 2021;31(2):578–583. doi: 10.1177/1120672120902025. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Martin E., Jarauta L., Pablo L.E., et al. Changes in peripapillary choroidal thickness in patients with multiple sclerosis. Acta Ophthalmol. 2019;97(1):e77–e83. doi: 10.1111/aos.13807. [DOI] [PubMed] [Google Scholar]
- 17.Moleiro A.F., Godinho G., Madeira C., et al. Peripapillary and subfoveal choroidal thickness in retinal vein occlusions. Clin Ophthalmol. 2022;16:3775–3783. doi: 10.2147/OPTH.S379373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neudorfer M., Spierer O., Goder M., et al. The prevalence of retinal and optical coherence tomography findings in preeclamptic women. Retina. 2014;34(7):1376–1383. doi: 10.1097/IAE.0000000000000085. [DOI] [PubMed] [Google Scholar]