Abstract
N-methyl-d-aspartate (NMDA) receptor (NMDAR) activation mediates glutamate (Glu) toxicity and involves bleomycin (BLM)-induced acute lung injury (ALI). We have reported that bone marrow-derived mesenchymal stem cells (BM-MSCs) are NMDAR-regulated target cells, and NMDAR activation inhibits the protective effect of BM-MSCs on BLM-induced pulmonary fibrosis, but its effect on ALI remains unknown. Here, we found that Glu release was significantly elevated in plasma of mice at d 7 after intratracheally injected with BLM. BM-MSCs were pretreated with NMDA (the selective agonist of NMDAR) and transplanted into the recipient mice after the BLM challenge. BM-MSCs administration significantly alleviated the pathological changes, inflammatory response, myeloperoxidase activity, and malondialdehyde content in the damaged lungs, but NMDA-pretreated BM-MSCs did not ameliorate BLM-induced lung injury in vivo. Moreover, NMDA down-regulated prostaglandin E2 (PGE2) secretion and cyclooxygenase (COX)-2 expression instead of COX-1 expression in BM-MSCs in vitro. We also found that NMDAR1 expression was increased and COX-2 expression was decreased, but COX-1 expression was not changed in primary BM-MSCs of BLM-induced ALI mice. Further, the cultured supernatants of lipopolysaccharide (LPS)-pretreated RAW264.7 macrophages were collected to detect inflammatory factors after co-culture with NMDA-pretreated BM-MSCs. The co-culture experiments showed that NMDA precondition inhibited the anti-inflammatory effect of BM-MSCs on LPS-induced macrophage inflammation, and PGE2 could partially alleviate this inhibition. Our findings suggest that NMDAR activation attenuated the protective effect of BM-MSCs on BLM-induced ALI in vivo. NMDAR activation inhibited COX-2 expression and PGE2 secretion in BM-MSCs and weakened the anti-inflammatory effect of BM-MSCs on LPS-induced macrophage inflammation in vitro. In conclusion, NMDAR activation attenuates the protective effect of BM-MSCs on BLM-induced ALI via the COX-2/PGE2 pathway. Keywords: Acute Lung Injury, BM-MSCs, NMDA receptor, COX-1/2, PGE2.
1. Introduction
Acute lung injury (ALI) is a life-threatening syndrome that causes various acute and chronic lung diseases, leading to high morbidity and mortality [1]. Acute respiratory distress syndrome (ARDS) is the most severe form of ALI and shows alveolar structure injury and uncontrolled i mmation, eventually resulting in acute hypoxemic respiratory failure [2]. The activated and accumulated inflammatory cells in the lungs can produce toxic reactive oxygen species (ROS) and play important roles in the extent of alveolar and epithelial cell damage or destruction and subsequent fibrotic response [1]. Neutrophils and macrophages, as well as their release of inflammatory mediators (interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, IL-8, IL-4, and IL-10), are mandatory in the pathological process of ALI/ARDS [3]. The increased accumulation of neutrophils is associated with the exacerbation of inflammation, and targeting neutrophils can prevent ALI/ARDS in mice [4]. Resident and recruited macrophages play an important role in the clearance and repair of injured tissues, debris, and apoptotic cells, which are, therefore, important for the resolution of inflammation [5].
Glutamate (Glu) is a crucial excitatory neurotransmitter and plays an important role in learning, memory, and development. Excessive Glu induces excitatory toxicity by activating its receptor, which is involved in the pathogenesis of chronic neurodegenerative diseases [6]. N-methyl-d-aspartate (NMDA) receptor (NMDAR) is the principal receptor in mediating Glu toxicity [6]. NMDAR also exists in peripheral tissues, such as the lung [7]. In 1996, Said and colleagues reported that NMDAR-mediated Glu toxicity was also present in the lung and promoted acute oedematous lung injury [8]. In this research, the perfusate containing NMDA, a synthetic agonist that selectively activates NMDAR, caused acute injury in perfused and ventilated rat lungs, and this injury was prevented by MK-801, which is the competitive NMDAR antagonist or a channel blocker. Subsequently, our previous research also showed that intraperitoneal injection of NMDA or Glu caused ALI and that pre-injection of MK-801 could reduce lung damage [9,10]. MK-801 also ameliorated hyperoxia-induced lung injury in neonatal rats [11,12]. Another NMDAR antagonist, memantine, an NMDAR channel-blocker, attenuated BLM-induced ALI [13]. These studies indicate that NMDAR-mediated Glu toxicity is involved in ALI, but the mechanism is still unclear.
Mesenchymal stem cells (MSCs) are adult pluripotential stromal cells that can differentiate into multiple types of cells, such as chondrocytes, myoblasts, and neurocytes, under the stimulation of relevant factors [14]. MSCs are originally isolated from bone marrow (BM) or BM-derived MSCs (BM-MSCs). In preclinical animal models, exogenously administered BM-MSCs can efficiently attenuate lung injury and promote reparative responses [15,16]. BM-MSCs have therapeutic effects in animal models of intrapulmonary ALI and extrapulmonary ALI [[17], [18], [19]]. Some phase I trials have indicated that infusion of allogeneic MSCs is safe and may reduce circulating markers of alveolar epithelial injury in patients with moderate to severe ARDS [[20], [21], [22]]. A larger multi-center phase II trial of human BM-MSCs administration to 60 subjects with moderate-severe ARDS is ongoing [23].
MSCs engraftment can suppress inflammation and deposition of collagen in damaged lungs of BLM-induced mice [24]. Furthermore, MSCs have been observed to display immunomodulatory properties through interaction with a wide range of immune cells and the secretion of multiple paracrine factors to control the inflammatory process, alleviate lung injury, and repair damaged lungs [25,26]. Prostaglandin E2 (PGE2), a subtype of the prostaglandin family, is an important paracrine factor of MSCs as it plays an early regulator of inflammation [27]. PGE2 is synthesized from arachidonic acid (AA) released from membrane phospholipids through sequential enzymatic reactions. Cyclooxygenase-2 (COX-2), known as prostaglandin-endoperoxide synthase, converts AA to prostaglandin H2 (PGH2), and PGE2 synthase isomerizes PGH2 to PGE2 [28]. COX-2 expression and PGE2 biosynthesis (the enzymatic product of COX-2) are crucial factors in the immunomodulatory ability of MSCs, such as reprogramming of host macrophages to increase their interleukin-10 production and alleviating atopic dermatitis by reducing mast cell degranulation [29]. Hence, the protective effect of MSCs partly depends on the secretion of PGE2.
However, the functional status of endogenous BM-MSCs has received less attention. Recently, researchers have found that the adult BM-MSCs circulating in the blood are involved in tracheal regeneration and lung repair [26], and the content of endogenous BM-MSCs in BM has decreased in the BLM-induced model [30]. The endogenous BM-MSCs can localize to the injured lung and assume lung cell phenotypes, and the protection from lung injury and fibrosis also involves the suppression of inflammation and triggering the production of reparative growth factors [15]. These studies indicate that BM stem cells are essential to lung repair and the dysfunction of endogenous BM-MSCs may be one of the potential mechanisms of lung injury. We previously reported that NMDAR expression was present in BM-MSCs and NMDAR activation induced BM-MSCs dysfunction in vitro, thus inhibiting the therapeutic effect of BM-MSCs administration on BLM-induced model in vivo [31]. Our groups also found that NMDAR activation inhibited the protective effect of BM-MSCs on BLM-induced AECs damage [6] and NMDAR blockers attenuated BLM-induced pulmonary fibrosis by inhibiting endogenous BM-MSCs senescence [32]. These researches reveal that BM-MSCs are NMDAR-regulated target cells, and NMDAR-mediated Glu toxicity on BM-MSCs is involved in BLM-induced lung damage. In this study, we investigated the effect of NMDAR activation on BM-MSCs in BLM-induced ALI and the underlying mechanism.
2. Materials and methods
2.1. Animal experiments
Female C57BL/6 mice, weighing about 20 g, were purchased from JingDa Laboratory Animal Company (Changsha, China) and were maintained in 12-h light/12-h dark cycles with free access to food and water.
According to our previous experiments [31], mice were randomly divided into four groups: (1) Con: intratracheal injection of saline and intravenous administration of phosphate-buffered saline (PBS); (2) BLM: intratracheal injection of 3.5 mg/kg BLM (Tokyo, Japan) and intravenous administration of PBS; (3) BLM + MSCs: intratracheal injection of BLM and intravenous administration of BM-MSCs (1 × 106 in 100 μl PBS per mouse). (4) BLM + NMDA-MSCs: intratracheal injection of BLM and intravenous administration of BM-MSCs, which were preconditioned with 3 mM NMDA (Sigma-Aldrich Chemical Company) for 24 h. After anesthetizing, mice were intratracheally injected with 3.5 mg/kg BLM in 50 μl saline. About 1 × 106 normal or NMDA-preconditioned BM-MSCs were washed with PBS, resuspended in 100 μl of PBS, and transplanted into mice via tail vein injection at d 0 after anesthesia awakening. Animals were sacrificed on d 7 after BLM injection under anesthesia. The lung tissues were collected and used for the following assays.
2.2. Glu detection
On d 7, after the BLM challenge, the mice were anesthetized, and the femoral artery blood samples were collected into anticoagulant tubes containing heparin sodium. After centrifugation, the plasma was separated and collected for Glu measurement using a Glu detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
2.3. Histological analysis
The upper lobes of the right lungs were fixed with 4 % paraformaldehyde in 0.1 M PBS (pH 7.0) for 24 h and embedded in paraffin. 6-μm-thick sections were cut and stained with hematoxylin and eosin (H&E). Alveolitis was determined with the H&E-stained sections according to previously published criteria [13]. Briefly, inflammatory infiltration and the area of lesions of lung sections were graded as follows: grade 0, normal tissue; grade 1, <20 % of the slide; 2, 20–50 % of the slide; and 3, >50 % of the slide. The mean score from all examined fields was calculated as the inflammation score.
2.4. Myeloperoxidase (MPO) activity assay
Mice were sacrificed, and lung tissues were collected. According to the manufacturer's instructions, chromometry assessed MPO activity in the lung parenchyma, a neutrophil infiltration marker (Nanjing Jiancheng Bioengineering Institute). The enzyme activity was determined by measuring the optical density change with a multifunctional microplate reader (Thermo Fisher Scientific, USA) at 450 nm. MPO activity was expressed in units per gram weight of wet tissue.
2.5. Malondialdehyde (MDA) content detection
The MDA concentrations were determined by measuring thiobarbituric acid (TBA) reactivity according to a commercially available kit (Nanjing Jiancheng Bioengineering Institute). TBA was added to lung homogenate, and the mixture was centrifuged. The supernatant was obtained and then measured at 532 nm with a multifunctional microplate reader (Thermo Fisher Scientific). The MDA assay was performed following the instructions of the detection kit.
2.6. BM-MSCs and RAW264.7 cells
BM-MSCs derived from C57BL/6 mice were purchased from Cyagen Biosciences, Inc. (Guangzhou, China). BM-MSCs were cultured in a 1:1 mix of Dulbecco's modified Eagle medium/nutrient mixture F-12 (DMEM/F12) containing 10 % fetal bovine serum (FBS) (Cyagen Bioscience, Inc.), 2 mM l-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin, and incubated at 37 °C in a humidified incubator with 5 % CO2.
Mouse monocyte/macrophage cell line, RAW264.7 cells, were purchased from Sigma-Aldrich and cultured in DMEM/F12 medium supplemented with 10 % FBS, 2 mM l-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin.
2.7. Co-culture experiment
The co-culture experiments were performed in 0.4-μm-pore-size transwell system (Corning, Lowell, MA, USA). BM-MSCs (2 × 105 cells/transwell) were cultured in 0.4-μm-pore-size transwells and RAW264.7 macrophages (2 × 105 cells/well) were cultured in 6-well plates. Before co-culture, BM-MSCs were treated with 3 mM NMDA for 24 h and RAW264.7 macrophages were stimulated with 10 ng/ml lipopolysaccharide (LPS, Sigma-Aldrich) for 24 h. After washing the cells and changing the medium, the transwells containing BM-MSCs were placed in 6-well plates containing RAW264.7 macrophages as the lower chamber of the transwell system for another 24 h. During co-culture, RAW264.7 macrophages were simultaneously treated with/without 2 μg/ml PGE2 (Sigma-Aldrich). The cultured supernatants from the lower chamber of the transwell system were collected and frozen at −80 °C for inflammatory factor assessment.
2.8. Primary BM-MSCs isolation and identification
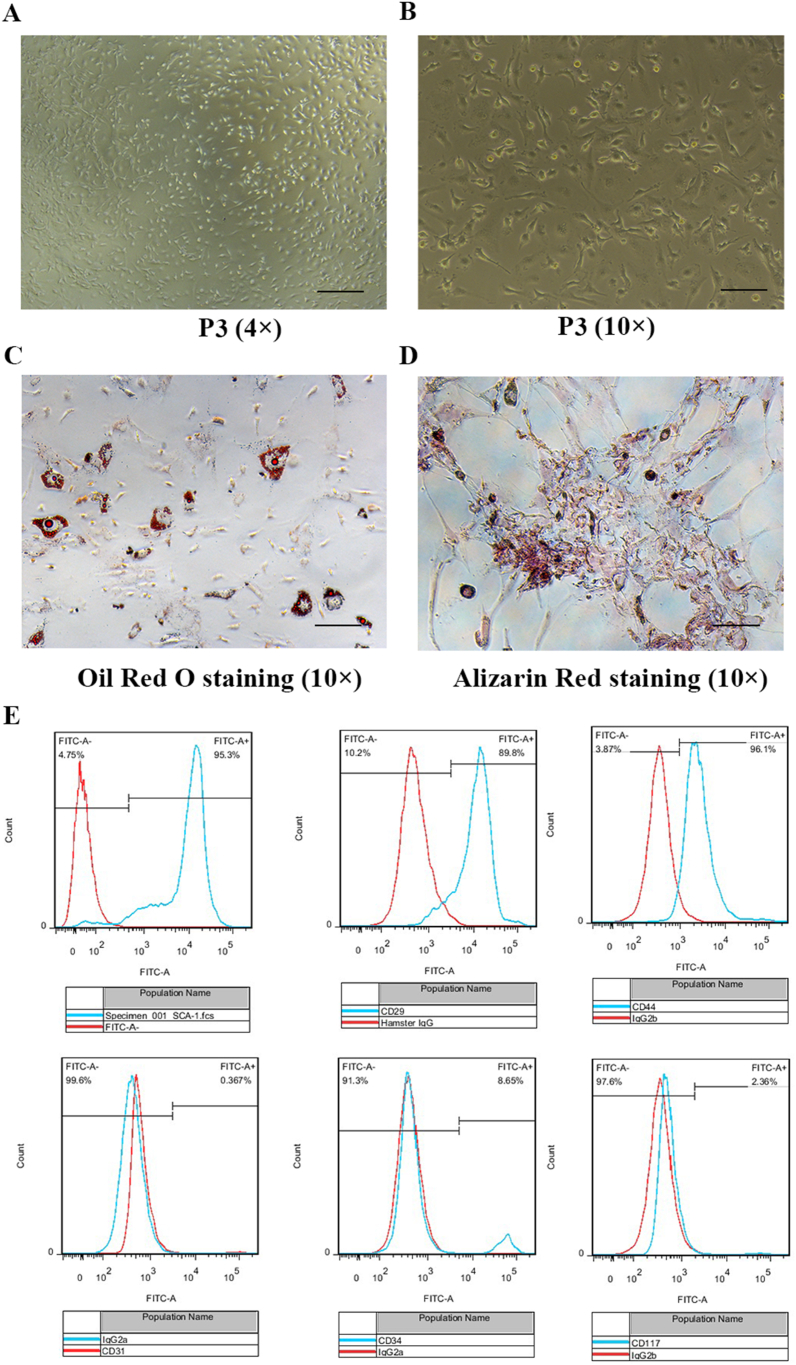
The C57BL/6 mice of the BLM-induced ALI model were anesthetized and disinfected for primary BM-MSCs isolation on d 7. BM cells were obtained from their femur and tibia as previously [31]. The primary BM-MSCs were passaged at times and characterized by their adipogenic and osteogenic differentiation potentials and by flow cytometric analysis of the following surface markers: SCA-1, CD29, CD44, CD31, CD34, and CD117 (Cyagen Biosciences, Inc., Guangzhou, China). The results were analyzed using FlowJo software version 7.2.5 (FlowJo LLC, Ashland, OR, USA) to create the histograms. After analyzed, SCA-1, CD29 and CD44 were positive, whereas CD31, CD34, and CD117 were negative.
For adipogenic and osteogenic differentiation assay as previously [31], the primary BM-MSCs were seeded into 6-well plates and cultured. After differentiation was completed according to the manufacturer's protocols, the cells were stained with Oil Red O (Cyagen Biosciences, Inc.) and Alizarin Red S (Cyagen Biosciences, Inc.), respectively.
2.9. Analysis of cytokines by flow cytometry
Lung tissue homogenates and cell culture supernatants were collected for subsequent analysis by a cytometric bead array (CBA) mouse inflammation kit according to the manufacturer's instructions. The following cytokines were measured using Mouse Cytokine 18-plex assay (LEGENDplex™): IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, TNF-α and IFN-γ (BioLegend, San Diego, CA). The samples were treated according to the manufacturer's instructions and eventually measured using a FACSCanto II station. No signal above the lower detection limit was measured for IL-5 and IL-13; therefore, these cytokines were not plotted. Standard curves and data analysis were analyzed using LEGENDplex v8.0 software (BioLegend).
2.10. Enzyme-linked immunosorbent assay (ELISA)
The lungs were harvested and homogenized at level 5 in a 1 ml ice-cold DMEM. Following homogenization, tissue fragments and intact cells were further fragmented by sonication on ice for 30 s at power level 1. Lipids were then methanol extracted from the homogenate using Sep-Pak cartridges (Waters). The samples were dried under a steady stream of nitrogen to evaporate the solvent and then resuspended in 250 μl DMEM. Mouse PGE2 (eBioscience, San Diego, USA) levels in lung tissue homogenates were measured using an ELISA kit per the manufacturer's instructions.
2.11. Quantitative real-time polymerase chain reaction (RT-qPCR) assay
Total RNA from lungs or BM-MSCs was extracted with TRIzol reagent (Takara, Kusatsu, Japan), and reverse transcription was accomplished with PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara). Gene expression levels were assayed by RT-qPCR using a real-time PCR system (CFX96 Touch™, Bio-Rad Laboratories Inc., Hercules, USA). The levels of mRNA were normalized to β-actin. The data were analyzed using the 2−ΔΔCT method. The following primers were used: β-actin (forward primer, 5′-TTCCAGCCTTCCTTCTTG-3′; reverse primer, 5′-GGAGCCAGAGCAGTAATC-3′), IL-1β (forward primer, 5′-GCCCATCCTCTGTGACTCAT-3′; reverse primer, 5′-AGGCCACAGGTATTTTGTCG-3′), COX-1 (forward primer, 5′-CAGACGACCCGCCTCATCCTCATAG-3′; reverse primer, 5′-GCCTCAACCCCATAGTCCACCAACA-3′), COX-2 (forward primer, 5′-AGGTCATTGGTGGAGAGGTG-3′; reverse primer, 5′-CCTGCTTGAGTATGTCGCAC-3′).
2.12. Western blot analysis
Western blot analysis was performed to measure the protein expression levels. After treatment, BM-MSCs were washed with pre-cooling PBS and lysed in RIPA buffer (Beyotime, Shanghai, China) containing 1 mM PMSF (Beyotime) on ice. Then, they were collected for centrifugation at 12,000×g for 30 min at 4 °C. Protein concentration was determined by bicinchoninic acid protein assay kit (Beyotime) according to the manufacturer's protocol. Next, 50 μg of protein samples were separated by 12 % SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Merck Millipore, MA, USA). After blocking in 5 % non-fat milk, the membranes were incubated with the anti-COX-1 (1:1000; Abcam, Cambridge, USA), anti-COX-2 (1:1000; Abcam), anti-NMDAR1 (1:1000; Abcam) and anti-β-actin (1:7500; Sigma-Aldrich) antibodies at 4 °C overnight. Then, the membranes were incubated with horseradish peroxidase-labeled goat anti-rabbit or mouse secondary antibody at room temperature for 1 h. Finally, proteins were detected with enzyme-linked chemiluminescence (ECL) (Merck Millipore) and semi-quantified by ChemiDoc XRS System (Bio-Rad Laboratories, Inc.). The data were analyzed with Image Lab software (Bio-Rad Laboratories, Inc.). β-actin was used as the control for equal loading of samples.
2.13. Statistical analysis
The data are expressed as the mean ± SEM. Data comparisons were analyzed with Student's t-test or one-way ANOVA, followed by Student–Newman–Keuls multiple comparison test using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA, USA). P-values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Glu was increased in the plasma of BLM-induced ALI mice
BLM is widely utilized to induce experimental pulmonary injury and fibrosis for animal models. In this study, we used intratracheal instillation of BLM, the standard route of administration, to induce an animal model of ALI in C57BL/6 mice. NMDAR-mediated Glu toxicity plays an important role in ALI [7]. Our previous study also demonstrated that the Glu level in bronchoalveolar lavage fluid (BALF) is elevated in BLM-induced ALI mice at d 3 and 7 [13]. The Glu level was increased from endogenous BM cells at d 3 and 14 after the BLM challenge [31]. The first week is a phase of acute lung inflammation, and the inflammatory responses reach a peak at d 7 post BLM challenge and then gradually subside [34]. In this study, we collected the femoral artery blood and isolated the plasma at d 7 after the BLM challenge for Glu detection. The data showed that the Glu level in peripheral plasma was significantly higher in the BLM group than in the control group (Fig. 1).
Fig. 1.
Glu level was elevated in the plasma of BLM-induced ALI mice.
The femoral artery blood was harvested at d 7 after intratracheal injection with BLM. The plasma was isolated for Glu content detection. Data represented the mean ± SEM. ***P<0.001 vs. Con group, n = 6–9.
3.2. NMDAR activation eliminated the beneficial effects of BM-MSCs on BLM-induced ALI
BM-MSCs produced by bone marrow have the anti-injury effects in multiple animal models [6]. To explore the significance of Glu increase, we used NMDA to directly pretreat BM-MSCs in vitro to simulate NMDAR activation state, and then transplanted into the BLM-damaged mice. As in our previous study [31], we used 3 mM NMDA to precondition 1 × 106 BM-MSCs for 24 h and injected them into mice through the tail vein on the d after the BLM challenge. The BM-MSCs used were identified before injection, and the results were reported in our paper [31]. The lung tissues were collected at d 7, and used for histopathological changes evaluated by H&E staining. Results showed significant inflammatory alterations characterized by obvious thickening of the alveolar wall, diffuse lung hemorrhage, and infiltration of inflammatory cells into interstitial tissue and alveolar space (Fig. 2B, F), compared with those of the control group (Fig. 2A, E). After BM-MSCs injection, the pathomorphological changes of lung injury were alleviated compared with the BLM group (Fig. 2C, G). However, NMDA-pretreated BM-MSCs injection did not reduce lung injury and inflammation (Fig. 2D, H). The results of the inflammation score were consistent with the histopathological changes (Fig. 2I).
Fig. 2.
Effects of NMDA-pretreated BM-MSCs on BLM-induced ALI. Lungs were harvested on d 7 after intratracheal injection with BLM. (A–H) Representative images of H&E staining (A-D, 20 × , bar = 50 μm; E-H, 40 × , bar = 25 μm). (I) Comparisons of inflammation score values between each group. (J) The activity of MPO in lung homogenates. (K) Content of MDA in lung homogenates. Data represent the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs. Con group; #P <0.05, ##P<0.01 vs. BLM group; &P<0.05, &&P<0.01 vs. BLM + MSCs group, n = 6–9.
Activated neutrophils are implicated in the pathophysiology of BLM-induced ALI, and MPO is an indicator of neutrophil infiltration [35]. MPO activity was significantly higher in the BLM group than in the control group. After BM-MSCs injection, MPO activity was decreased than that in the BLM group. However, NMDA-pretreated BM-MSCs injection did not decrease MPO activity (Fig. 2J).
Oxidative stress has been proven to be an important factor in the development of BLM-induced pulmonary damage [36]. BLM can cause the reaction of a formed pseudonym with oxygen, producing ROS and leading to an inflammatory response [37]. MDA is an index for lipid peroxidation. This study evaluated oxidative stress by detecting the MDA level of lung homogenates. As shown in , BLM treatment resulted in a significant rise of MDA in lung tissues compared with the control group. After BM-MSCs injection, MDA content was lower than that in the BLM group. However, NMDA-pretreated BM-MSCs injection did not decrease MDA content (Fig. 2K).
3.3. Effects of NMDA-pretreated BM-MSCs on the inflammatory cytokines in the lungs of BLM-induced ALI mice
Next, we determined the inflammatory cytokine profile in the lung homogenates. The results showed that the levels of proinflammatory cytokines, IL-1β, IL-2, IL-6, TNF-α, and IFN-γ, in lung homogenates were significantly higher in the BLM group than those in the control group. After BM-MSCs injection, the levels of proinflammatory cytokines (IL-1β, IL-2, IL-6, TNF-α, and IFN-γ) were decreased. However, NMDA-pretreated BM-MSCs injection did not down-regulate proinflammatory cytokines (IL-1β, IL-2, IL-6, TNF-α, and IFN-γ) (Fig. 3A–E).
Fig. 3.
Levels of inflammatory factors in lung homogenates. Lungs were harvested on d 7 after intratracheal injection with BLM for inflammatory factor detection. (A) The level of IL-1β was measured by RT-qPCR assay. (B–G) The levels of IL-2, IL-6, TNF-α, IFN-γ, IL-4, and IL-10 were measured by flow cytometry with a CBA detection kit. (H) The level of PGE2 was measured by ELISA. Data represent the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs. Con group; #P<0.05, ##P<0.01 vs. BLM group; &P<0.05, &&P<0.01 vs. BLM + MSCs group, n = 6–9.
By contrast, the levels of anti-inflammatory cytokines (IL-4 and IL-10) were significantly decreased in the BLM group compared with those in the control group. After BM-MSCs injection, the levels of IL-4 and IL-10 were increased. However, NMDA-pretreated BM-MSCs injection did not up-regulate the levels of IL-4 and IL-10 (Fig. 3F and G).
PGE2 is a crucial paracrine factor secreted by MSCs and regulates the release of inflammatory cytokines from their corresponding target cells, such as monocytes and macrophages [25]. In in vivo experiments, mice are treated with PGE2 via surgically implanted minipumps after BLM administration, and their pulmonary inflammation and fibrosis are significantly weakened [38]. Therefore, we observed PGE2 levels in the lungs and found that PGE2 was decreased in the BLM group compared with the control group by ELISA (Fig. 3H). After BM-MSCs injection, PGE2 was elevated compared with the BLM group, but NMDA-pretreated BM-MSCs injection did not alleviate the decreased PGE2 induced by the BLM challenge (Fig. 3H).
3.4. NMDAR activation down-regulated COX-2 expression and inhibited PGE2 secretion in BM-MSCs
We investigated the effect and signal mechanism of NMDAR activation on PGE2 secretion in BM-MSCs in vitro. We used 3 or 10 mM NMDA to treat BM-MSCs. NMDA significantly reduced PGE2 secretion compared with the control group (Fig. 4A). COX-1 is generally considered a constitutive enzyme in most cells, whereas COX-2 is a rate-limiting enzyme for controlling PGE2 synthesis under physiological conditions [39]. Therefore, we measured the level of COX-1/2 in BM-MSCs after NMDA treatment by RT-qPCR and Western blot. NMDA decreased the mRNA and protein expression levels of COX-2 instead of COX-1 (Fig. 4B–F). These findings indicated that NMDAR activation inhibited PGE2 production through specific down-regulation of COX-2 expression in BM-MSCs.
Fig. 4.
Effects of NMDAR activation on COX-1/2 expression and PGE2 secretion in BM-MSCs.
After being treated with NMDA for 24 h, the cultured supernatants and total protein of BM-MSCs were collected for further analysis. (A) The level of PGE2 secreted by BM-MSCs was measured with ELISA. (B, C) COX-1/2 mRNA levels were determined by RT-qPCR. (D–F) The protein levels of COX-1 and COX-2 were detected by Western blot. The full, non-adjusted images of Western blot assay were provided in supplementary material (Figs. S1 and S2). Data represented the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs. control group, n = 3–6.
3.5. Increased NMDAR1 and decreased expression of COX-2 were observed in primary BM-MSCs of BLM-induced ALI mice
We isolated the primary BM-MSCs from the femur and tibia of BLM-induced ALI models. BM-MSCs were phenotypically identified by their typical fibroblast-like appearance (Fig. 5A and B). The differentiation abilities of BM-MSCs were tested at P3. BM-MSCs could differentiate exclusively into adipocytes and osteoblasts, as determined by Oil Red O and Alizarin Red S staining, respectively (Fig. 5C and D). BM-MSCs were further analyzed for surface biomarkers by flow cytometry. Results showed that BM-MSCs were positive for SCA-1, CD29, and CD44 and negative for CD31, CD34, and CD117 (Fig. 5E). These results revealed that the phenotype of primary BM-MSCs in BLM-induced ALI mice was unchanged.
Fig. 5.
Identification of endogenous BM-MSCs of BLM-induced ALI mice.
On d 7, after the BLM challenge, the endogenous BM-MSCs of BLM-induced ALI mice were isolated and cultured from mice. The phenotypes of endogenous BM-MSCs at P3 were confirmed by microscopy, differentiation potentials, and surface markers. (A, B) BM-MSCs exhibited fibroblast-like phenotype (A, 4 × , bar = 250 μm; B, 10 × , bar = 100 μm). (C, D) BM-MSCs performed multipotent differentiation potentials into adipocytes and osteoblasts, as shown by Oil Red O staining (C, 10 × , bar = 100 μm) and Alizarin Red staining (D, 10 × , bar = 100 μm), respectively. (E) The surface markers were detected by flow cytometry analysis. The positive markers were SCA-1, CD29, and CD44. The negative markers were CD31, CD34 and CD117. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, we used the primary BM-MSCs for further detection. Western blot results showed that the expression of NMDAR1 in the BLM group was markedly higher than in the control group (Fig. 6A, D). We also measured the expression of COX-1/2 in BM-MSCs. Results showed that the expression of COX-2 was significantly decreased, while the expression of COX-1 was unchanged in the BLM group (Fig. 6A–C). These results confirmed the role of NMDAR activation and COX-2 decrease in BLM-induced lung injury in vivo.
Fig. 6.
NMDAR1 and COX-1/2 expression in endogenous BM-MSCs of BLM-induced ALI mice.
On d 7, after the BLM challenge, the endogenous BM-MSCs of BLM-induced ALI mice were isolated and cultured from mice. (A) The protein expression of NMDAR1 and COX-1/2 was determined by Western blot assay in endogenous BM-MSCs at P3. (B–D) Histograms of gray values in the protein expression of NMDAR1 and COX-1/2. The full, non-adjusted images of Western blot assay were provided in supplementary material (Figs. S3–S5). Data represent the mean ± SEM. *P<0.05, **p<0.01 vs. Con group, n = 3.
3.6. Effects of NMDA-pretreated BM-MSCs on the inflammatory cytokines in LPS-stimulated RAW264.7 macrophages
BM-MSCs have been reported to reduce inflammation and attenuate sepsis via regulating host macrophages [29]. Macrophages, typical representatives of innate immunity, engulf invading particles and participate in the lung's early inflammation and late fibrosis process [42]. To further understand the effect of NMDAR activation on the anti-inflammatory actions of BM-MSCs through the PGE2 paracrine effect in vitro, we used the transwell co-culture system to prevent direct physical contact between BM-MSCs and murine macrophages RAW264.7. In this assay, resting RAW264.7 macrophages were stimulated with 10 ng/ml LPS for 24 h to obtain activated macrophages as previously described [43]. BM-MSCs were preconditioned with 3 mM NMDA for 24 h. Then, BM-MSCs were co-cultured with RAW264.7 macrophages for another 24 h. After co-culture with BM-MSCs, LPS-stimulated RAW264.7 macrophages produced less IL-2, IL-6, TNF-α and IFN-γ (Fig. 7A–D) and more IL-4 and IL-10 (Fig. 7E and F), but this effect was eliminated if BM-MSCs were pretreated with NMDA. These results demonstrated that NMDA impaired the anti-inflammatory effect of BM-MSCs on LPS-induced macrophages. However, the decreased anti-inflammatory effect of BM-MSCs induced by NMDA could be rescued by 2 μg/ml PGE2 treatment (Fig. 7A–F). Therefore, these data indicated that NMDAR activation inhibited the anti-inflammatory effect of BM-MSCs by reducing their PGE2 secretion.
Fig. 7.
Cytokine release of macrophages after co-culture with BM-MSCs. 2 × 105 BM-MSCs were treated with 3 mM NMDA, and 2 × 105 RAW264.7 macrophages were treated with 10 ng/ml LPS for 24 h. Then, they were co-cultured for another 24 h in a transwell system; 2 μg/ml PGE2 was used to treat RAW264.7 macrophages during co-culture. The supernatants of RAW264.7 macrophages were collected for cytokine detection by flow cytometry with a CBA detection kit. Data represent the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs. Con group; #P<0.05, ##P<0.01, ###P<0.001 vs. LPS group; &P<0.05, &&P<0.01 vs. LPS + MSCs group, $P<0.05 vs. LPS + MSCs + NMDA group, n = 3–6.
4. Discussion
Glu toxicity, a result of Glu binding to NMDAR, plays a major role in the pathogenesis of many disorders. Recently, the excessive release of endogenous Glu and NMDAR-mediated Glu toxicity have been paid more attention to lung damage. In this study, the elevated Glu level has been observed in the plasma of BLM-induced ALI mice, which is consistent with our group's published data, showing an increased Glu level in BALF of BLM-induced ALI [13] and hyperoxia-induced newborn rat lung injury [44]. Zhao et al. also demonstrated increased levels of Glu, glutamine, and the antioxidant glutathione (GSH) and decreased levels of aspartate and oxidized counterpart GSSG in IPF lungs [45]. Hu et al. found altered cell energy and amino acid metabolism in the silica-induced lung inflammation and fibrosis model [46]. Memantine, an NMDAR channel-blocker, attenuated BLM-induced ALI [13]. All of these studies suggest that an increased release of endogenous Glu is present in animal models of pulmonary injury and fibrosis, and patients with IPF.
In our previous study, Glu release is increased in endogenous BM cells of BLM-induced lung-damaged mice in vivo, each subunit of NMDAR is present in BM-MSCs, and NMDAR activation causes BM-MSCs dysfunction in vitro [31]. Here, we found that the expression of NMDAR1, the necessary subunit for assembling the functional NMDAR, was increased in endogenous BM-MSCs of BLM-induced ALI mice, which is consistent with our previous report [32]. In this previous report, endogenous BM-MSCs senescence is increased in BLM-induced lung injury mice, and memantine can attenuate lung damage by inhibiting endogenous BM-MSCs senescence. These researches reveal that NMDAR-mediated Glu toxicity causes endogenous BM-MSCs dysfunction, which is involved in the pathogenesis of lung injury.
Many studies have shown that exogenous BM-MSCs administration can reduce lung inflammation and improve survival [[47], [48], [49], [50]]. Numerous studies have demonstrated that MSCs orchestrate the repair process by secretome function, consisting of many angiogenic factors, growth factors, chemokines, and anti-inflammatory cytokines [51]. Up to 80 % of the therapeutic effect of adult MSCs is through paracrine factor-mediated actions [52]. In this study, BM-MSCs were pretreated with NMDA to simulate the NMDAR activation state of BM-MSCs and then transplanted into mice with BLM-induced lung injury. Results demonstrated that the administration of BM-MSCs immediately after the BLM challenge protected damaged lungs from BLM-induced ALI, but the administration of NMDA-preconditioned BM-MSCs did not alleviate the lung injuries, as evidenced by aggravation in morphological changes, the increase of proinflammatory cytokines (IL-1β, IL-2, IL-6, TNF-α, and IFN-γ) and the decrease of anti-inflammatory cytokines (IL-4 and IL-10), neutrophil activation (MPO), and oxidative stress (MDA) in the lungs. This part of the study proves that NMDAR activation destroys the protective effect of BM-MSCs against BLM-induced lung injury in vivo. Interestingly, among these inflammatory cytokines, the content of IFN-γ, the typical T helper (Th) 1 cytokine, is the highest, and the content of IL-4, the Th2-related cytokine, is low. The Th cell balance shifts from Th1 dominant in the early stage of inflammation to Th2 dominant during fibrosis development [53]. Therefore, we believe that NMDAR activation attenuated the protection of BM-MSCs in BLM-induced ALI because the balance of pro-inflammation and anti-inflammation was destroyed through a paracrine effect on the host immune cells in the lungs. In this study, we also observed the reduced secretion of PGE2 in the NMDA-pretreated BM-MSCs group in vivo.
In general, M1 macrophages exhibit potent anti-microbial properties reminiscent of Th1 responses, and M2 macrophages promote Th2 type of responses, secrete less proinflammatory cytokines, and play a role in the resolution of inflammation [54]. Alveolar macrophages act as the first defense against airborne particles and microbes. In response to injury, they can be activated and polarised into the classical activation M1 macrophages to release proinflammatory cytokines (TNF-α, IL-1β, and IL-6) for early response [55]. MSCs can act as regulators of the early phases of inflammation and regulate the inflammatory responses mediated by macrophages through proinflammatory signals [56]. BM-MSCs have been reported to reprogram host macrophages, increasing their IL-10 production and decreasing general inflammation and septicemia [29]. In this study, BM-MSCs inhibited the secretion of proinflammatory cytokines (IL-2, IL-6, TNF-α, and IFN-γ) and promoted the secretion of anti-inflammatory cytokines (IL-4 and IL-10) in LPS-stimulated macrophages. We also demonstrated that NMDA-pretreated BM-MSCs did not inhibit LPS-induced macrophage inflammation by inhibiting PGE2 paracrine in the rescue experiments of co-culture systems in vitro, but the mechanism is unknown.
Furthermore, we found that NMDAR activation down-regulated COX-2 expression and PGE2 secretion in BM-MSCs in vitro. MSCs have been reported to produce detectable levels of PGE2, an important paracrine factor of BM-MSCs, and play an important role in regulating inflammation and alveolar macrophages [57]. A previous study has revealed the importance of COX-2/PGE2 signaling on MSCs proliferation, a major characteristic of stem cells that contributes to stemness [28]. The induction of COX-2 and the increased production of PGE2 play key roles in modulating inflammation and governing vascular tone and barrier function in the lung [58]. COX-2 expression and PGE2 levels are reduced in patients with IPF [59,60]. MSCs administration alleviated airway inflammation and emphysema by down-regulation of COX-2 and COX-2-mediated PGE2 production in COPD rat models [61]. Crisostomo et al. found that human MSCs stimulated by TNF-α, LPS, or hypoxia produce growth factors by an NF-κB but not JNK or ERK-dependent mechanism [62]. However, the molecular link between COX-2/PGE2 and NMDAR activation remains to be further established.
5. Conclusions
We reported that NMDA-preconditioned BM-MSCs did not improve lung injury in the BLM-induced ALI model. The down-regulation of COX-2 expression and PGE2 secretion by NMDAR activation played a key role in mediating the damaged protective effect of NMDA-preconditioned BM-MSCs.
Fundings
This study was supported by the National Natural Science Foundations of China (No. 81900070, 81870059, 82070068) and the Changsha Science and Technology (No. Kq1907026).
Ethics statement
The Ethics Committee of the Second Xiangya Hospital, Central South University (approval No: 2,020,297, Changsha, China) approved the experiments, which were performed following the guidelines from the Committee on Research Animal Welfare of Central South University for the care and use of animals. We confirmed all authors’ compliance with all relevant ethical regulations.
Data availability statement
The data associated with the study has not been deposited into a publicly available repository. Data will be made available on request.
CRediT authorship contribution statement
Xiao-Hong Li: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition. Pu Huang: Formal analysis, Data curation. Hai-Peng Cheng: Formal analysis, Data curation. Yan Zhou: Methodology, Investigation. Dan-Dan Feng: Resources, Project administration. Shao-Jie Yue: Software, Resources. Yang Han: Project administration, Conceptualization. Zi-Qiang Luo: Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This research was supported by the National Natural Science Foundations of China (No. 81900070, 81870059, 82070068) and the Changsha Science and Technology (No. Kq1907026).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23723.
Contributor Information
Yang Han, Email: doctorhy26@163.com.
Zi-Qiang Luo, Email: luoziqiang@csu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Mokra D. Acute lung injury - from pathophysiology to treatment. Physiol. Res. 2020;69(Suppl 3):S353–S366. doi: 10.33549/physiolres.934602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang X., Xiu H., Zhang S., Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabaudon M., Blondonnet R., Ware L.B. Biomarkers in acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2021;27(1):46–54. doi: 10.1097/MCC.0000000000000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sercundes M.K., Ortolan L.S., Debone D., Soeiro-Pereira P.V., Gomes E., Aitken E.H., Condino-Neto A., Russo M., D I.L.M., Alvarez J.M., Portugal S., Marinho C., Epiphanio S. Correction: targeting neutrophils to prevent Malaria-Associated acute lung Injury/Acute respiratory distress syndrome in mice. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El K.D., Filep J.G. Modulation of neutrophil apoptosis and the resolution of inflammation through β2 integrins. Front. Immunol. 2013;4:60. doi: 10.3389/fimmu.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng X., Li X., Li C., Yue S., Huang Y., Huang P., Cheng H., Zhou Y., Tang Y., Liu W., Feng D., Luo Z. NMDA receptor activation inhibits the protective effect of BM-MSCs on bleomycin-induced lung epithelial cell damage by inhibiting ERK signaling and the paracrine factor HGF. Int. J. Mol. Med. 2019;44(1):227–239. doi: 10.3892/ijmm.2019.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao Z., Zhou X., Luo Z., Huo H., Wang M., Yu X., Cao C., Ding Y., Xiong Z., Yue S. N-Methyl-D-aspartate receptor excessive activation inhibited fetal rat lung development in vivo and in vitro. BioMed Res. Int. 2016 doi: 10.1155/2016/5843981. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Said S.I., Berisha H.I., Pakbaz H. Excitotoxicity in the lung: N-methyl-D-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1996;93(10):4688–4692. doi: 10.1073/pnas.93.10.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen L., Han J.Z., Li C., Yue S.J., Liu Y., Qin X.Q., Liu H.J., Luo Z.Q. Protective effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta Pharmacol. Sin. 2007;28(3):392–397. doi: 10.1111/j.1745-7254.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 10.Shen L., Li L., She H., Yue S., Li C., Luo Z. Inhibition of pulmonary surfactants synthesis during N-methyl-D-aspartate-induced lung injury. Basic Clin. Pharmacol. Toxicol. 2010;107(3):751–757. doi: 10.1111/j.1742-7843.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Tang F., Yue S., Luo Z., Feng D., Wang M., Qian C., Zhen X., Duan Y. Role of N-methyl-D-aspartate receptor in hyperoxia-induced lung injury. Pediatr. Pulmonol. 2005;40(5):437–444. doi: 10.1002/ppul.20299. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Yue S., Luo Z., Cao C., Yu X., Liao Z., Wang M. N-methyl-D-aspartate receptor activation mediates lung fibroblast proliferation and differentiation in hyperoxia-induced chronic lung disease in newborn rats. Respir. Res. 2016;17(1):136. doi: 10.1186/s12931-016-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Liu Y., Peng X., Liu W., Zhao F., Feng D., Han J., Huang Y., Luo S., Li L., Yue S.J., Cheng Q., Huang X., Luo Z. NMDA receptor antagonist attenuates Bleomycin-Induced acute lung injury. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018;9(1):63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas M., Xu J., Woods C.R., Mora A.L., Spears W., Roman J., Brigham K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005;33(2):145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalu M.M., Moher D., Marshall J., Fergusson D., Mei S.H., Macleod M., Griffin G., Turgeon A.F., Rudnicki M., Fishman J., Avey M.T., Skidmore B., Grimshaw J.M., Stewart D.J., Singh K., McIntyre L. Efficacy and safety of mesenchymal stromal cells in preclinical models of acute lung injury: a systematic review protocol. Syst. Rev. 2014;3:48. doi: 10.1186/2046-4053-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., He H., Liu A., Xu J., Han J., Chen Q., Hu S., Xu X., Huang Y., Guo F., Yang Y., Qiu H. Therapeutic effects of bone Marrow-Derived mesenchymal stem cells in models of pulmonary and extrapulmonary acute lung injury. Cell Transplant. 2015;24(12):2629–2642. doi: 10.3727/096368915X687499. [DOI] [PubMed] [Google Scholar]
- 18.Liang Z.X., Sun J.P., Wang P., Tian Q., Yang Z., Chen L.A. Bone marrow-derived mesenchymal stem cells protect rats from endotoxin-induced acute lung injury. Chin Med J (Engl) 2011;124(17):2715–2722. [PubMed] [Google Scholar]
- 19.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of E. Coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 21.Zheng G., Huang L., Tong H., Shu Q., Hu Y., Ge M., Deng K., Zhang L., Zou B., Cheng B., Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir. Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson J.G., Liu K.D., Zhuo H., Caballero L., McMillan M., Fang X., Cosgrove K., Vojnik R., Calfee C.S., Lee J.W., Rogers A.J., Levitt J., Wiener-Kronish J., Bajwa E.K., Leavitt A., McKenna D., Thompson B.T., Matthay M.A. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-70297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K.D., Wilson J.G., Zhuo H., Caballero L., McMillan M.L., Fang X., Cosgrove K., Calfee C.S., Lee J.W., Kangelaris K.N., Gotts J.E., Rogers A.J., Levitt J.E., Wiener-Kronish J.P., Delucchi K.L., Leavitt A.D., McKenna D.H., Thompson B.T., Matthay M.A. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann. Intensive Care. 2014;4:22. doi: 10.1186/s13613-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu A.R., Liu L., Chen S., Yang Y., Zhao H.J., Liu L., Guo F.M., Lu X.M., Qiu H.B. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J. Cell. Physiol. 2013;228(6):1270–1283. doi: 10.1002/jcp.24282. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 26.Warburton D., Perin L., Defilippo R., Bellusci S., Shi W., Driscoll B. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc. Am. Thorac. Soc. 2008;5(6):703–706. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cell. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 28.Lee B.C., Kim H.S., Shin T.H., Kang I., Lee J.Y., Kim J.J., Kang H.K., Seo Y., Lee S., Yu K.R., Choi S.W., Kang K.S. PGE2 maintains self-renewal of human adult stem cells via EP2-mediated autocrine signaling and its production is regulated by cell-to-cell contact. Sci. Rep. 2016;6 doi: 10.1038/srep26298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemeth K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., Hu X., Jelinek I., Star R.A., Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dygai A.M., Skurikhin E.G., Andreeva T.V., Ermolaeva L.A., Khmelevskaya E.S., Pershina O.V., Krupin V.A., Reztsova A.M., Stepanova I.E., Goldberg V.E. Reactions of the blood system and stem cells in bleomycin-induced model of lung fibrosis. Bull. Exp. Biol. Med. 2011;152(2):173–176. doi: 10.1007/s10517-011-1480-z. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Li C., Tang Y., Huang Y., Cheng Q., Huang X., Zhao F., Hao C., Feng D., Xu J., Han J., Tang S., Liu W., Yue S., Luo Z. NMDA receptor activation inhibits the antifibrotic effect of BM-MSCs on bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;315(3):L404–L421. doi: 10.1152/ajplung.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang P., Zhou Y., Li X.H., Zhang Y.N., Cheng H.P., Fu J.F., Liu W., Yue S., Luo Z.Q. N-methyl-D-aspartate receptor blockers attenuate bleomycin-induced pulmonary fibrosis by inhibiting endogenous mesenchymal stem cells senescence. Ann. Transl. Med. 2022;10(11):642. doi: 10.21037/atm-22-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degryse A.L., Lawson W.E. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am. J. Med. Sci. 2011;341(6):444–449. doi: 10.1097/MAJ.0b013e31821aa000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullane K.M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods. 1985;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 36.Tobwala S., Fan W., Stoeger T., Ercal N. N-acetylcysteine amide, a thiol antioxidant, prevents bleomycin-induced toxicity in human alveolar basal epithelial cells (A549) Free Radic. Res. 2013;47(9):740–749. doi: 10.3109/10715762.2013.819974. [DOI] [PubMed] [Google Scholar]
- 37.Claussen C.A., Long E.C. Nucleic Acid recognition by metal complexes of bleomycin. Chem Rev. 1999;99(9):2797–2816. doi: 10.1021/cr980449z. [DOI] [PubMed] [Google Scholar]
- 38.Dackor R.T., Cheng J., Voltz J.W., Card J.W., Ferguson C.D., Garrett R.C., Bradbury J.A., DeGraff L.M., Lih F.B., Tomer K.B., Flake G.P., Travlos G.S., Ramsey R.J., Edin M.L., Morgan D.L., Zeldin D.C. Prostaglandin E(2) protects murine lungs from bleomycin-induced pulmonary fibrosis and lung dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;301(5):L645–L655. doi: 10.1152/ajplung.00176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brock T.G., McNish R.W., Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J. Biol. Chem. 1999;274(17):11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- 42.Wynn T.A., Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30(3):245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Y., Qin C., Zheng G., Lai D., Tao H., Zhang Y., Qiu G., Ge M., Huang L., Chen L., Cheng B., Shu Q., Xu J. Mesenchymal stem Cell-Educated macrophages ameliorate LPS-Induced systemic response. Mediat. Inflamm. 2016 doi: 10.1155/2016/3735452. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M., Luo Z., Liu S., Li L., Deng X., Huang F., Shang L., Jian C., Yue S. Glutamate mediates hyperoxia-induced newborn rat lung injury through N-methyl-D-aspartate receptors. Am. J. Respir. Cell Mol. Biol. 2009;40(3):260–267. doi: 10.1165/rcmb.2008-0135OC. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y.D., Yin L., Archer S., Lu C., Zhao G., Yao Y., Wu L., Hsin M., Waddell T.K., Keshavjee S., Granton J., de Perrot M. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res. 2017;4(1) doi: 10.1136/bmjresp-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y.B., Lin Z., Feng D.Y., Li X., Chu L., Jiang H.Y., Peng J.W. Silica induces plasminogen activator inhibitor-1 expression through a MAPKs/AP-1-Dependent mechanism in human lung epithelial cells. Toxicol. Mech. Methods. 2008;18(7):561–567. doi: 10.1080/15376510701795470. [DOI] [PubMed] [Google Scholar]
- 47.Ortiz L.A., Gambelli F., McBride C., Gaupp D., Baddoo M., Kaminski N., Phinney D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortiz L.A., Dutreil M., Fattman C., Pandey A.C., Torres G., Go K., Phinney D.G. Interleukin 1 receptor antagonist mediates the anti-inflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu S.H., Liu L.J., Lv B., Che C.L., Fan D.P., Wang L.F., Zhang Y.M. Inhibition of bleomycin-induced pulmonary fibrosis by bone marrow-derived mesenchymal stem cells might be mediated by decreasing MMP9, TIMP-1, INF-gamma and TGF-beta. Cell Biochem. Funct. 2015;33(6):356–366. doi: 10.1002/cbf.3118. [DOI] [PubMed] [Google Scholar]
- 50.Mei S.H., Haitsma J.J., Dos S.C., Deng Y., Lai P.F., Slutsky A.S., Liles W.C., Stewart D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 51.Kusuma G.D., Carthew J., Lim R., Frith J.E. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: Opportunities to engineer the therapeutic effect. Stem Cells Dev. 2017;26(9):617–631. doi: 10.1089/scd.2016.0349. [DOI] [PubMed] [Google Scholar]
- 52.Hsiao S.T., Asgari A., Lokmic Z., Sinclair R., Dusting G.J., Lim S.Y., Dilley R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21(12):2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wynn T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4(8):583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prockop D.J. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cell. 2013;31(10):2042–2046. doi: 10.1002/stem.1400. [DOI] [PubMed] [Google Scholar]
- 57.Spaggiari G.M., Abdelrazik H., Becchetti F., Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 58.Hodges R.J., Jenkins R.G., Wheeler-Jones C.P., Copeman D.M., Bottoms S.E., Bellingan G.J., Nanthakumar C.B., Laurent G.J., Hart S.L., Foster M.L., McAnulty R.J. Severity of lung injury in cyclooxygenase-2-deficient mice is dependent on reduced prostaglandin E(2) production. Am. J. Pathol. 2004;165(5):1663–1676. doi: 10.1016/S0002-9440(10)63423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petkova D.K., Clelland C.A., Ronan J.E., Lewis S., Knox A.J. Reduced expression of cyclooxygenase (COX) in idiopathic pulmonary fibrosis and sarcoidosis. Histopathology. 2003;43(4):381–386. doi: 10.1046/j.1365-2559.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 60.Ozaki T., Moriguchi H., Nakamura Y., Kamei T., Yasuoka S., Ogura T. Regulatory effect of prostaglandin E2 on fibronectin release from human alveolar macrophages. Am. Rev. Respir. Dis. 1990;141(4 Pt 1):965–969. doi: 10.1164/ajrccm/141.4_Pt_1.965. [DOI] [PubMed] [Google Scholar]
- 61.Gu W., Song L., Li X.M., Wang D., Guo X.J., Xu W.G. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci. Rep. 2015;5:8733. doi: 10.1038/srep08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crisostomo P.R., Wang Y., Markel T.A., Wang M., Lahm T., Meldrum D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294(3):C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with the study has not been deposited into a publicly available repository. Data will be made available on request.