Abstract
Inflammatory responses and oxidative stress contribute to the pathogenesis of brain ischemia/reperfusion (IR) injury. Naturally occurring bioflavonoids possess antioxidant and anti-inflammatory properties. The phytochemicals of Juniperus sabina L., known as “Abhal” in Saudi Arabia, have been studied and cupressuflavone (CUP) has been isolated as the major bioflavonoid. This study aimed to investigate the neuroprotective potential of CUP in reducing brain IR damage in rats and to understand probable mechanisms. After 60 min of inducing cerebral ischemia by closing the left common carotid artery (CCA), blood flow was restored to allow reperfusion. The same surgical procedure was performed on sham-operated control rats, excluding cerebral IR. CUP or vehicle was given orally to rats for 3 days prior to ischemia induction and for a further 3 days following reperfusion. Based on the findings of this study, compared to the IR control group, CUP-administered group demonstrated reduced neurological deficits, improved motor coordination, balance, and locomotor activity. Additionally, brain homogenates of IR rats showed a decrease in malondialdehyde (MDA) level, an increase in reduced glutathione (GSH) content, and an increase in catalase (CAT) enzyme activity following CUP treatment. CUP suppressed neuro-inflammation via reducing serum inflammatory cytokine levels, particularly those of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) and enhancing the inflammatory cytokine levels, such as Nuclear factor kappa- B (NF-κB), TANK-binding kinase-1 (TBK1), and interferon beta (IFN-β) in brain tissues. Furthermore, CUP ameliorated the histological alterations in the brain tissues of IR rats. CUP significantly suppressed caspase-3 expression and downregulated the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway as a result of suppressing High mobility group box 1 (HMGB1). To our knowledge, this is the first study to document the neuroprotective properties of CUP. Thus, the study findings revealed that CUP ameliorates IR–induced cerebral injury possibly by enhancing brain antioxidant contents, reducing serum inflammatory cytokine levels, potentiating the brain contents of TBK1 and IFN-β and suppressing the HMGB1/TLR-4 signaling pathway. Hence, CUP may serve as a potential preventive and therapeutic alternative for cerebral stroke.
Keywords: Neuroprotection, Anti-inflammatory, Cerebral stroke, Cupressuflavone
1. Introduction
Stroke is a life-threatening illness affecting the respiratory system and circulatory function, resulting in severe neurological impairment. Globally, it ranks among the top five causes of death (Frank et al., 2022), Stroke is caused by one of two mechanisms: either ischemic or hemorrhagic, with ischemic stroke accounting for more than 80 % of all cases (Zhou et al., 2018). Inadequate blood flow and arterial blood obstruction are characteristics of ischemic stroke, resulting in considerable number of fatalities or disabling conditions worldwide. In a short period, it can cause irreversible ischemic damage to nervous tissue (Kraft et al., 2012). Furthermore, the progressive loss of uninterrupted cerebral blood flow degrades memory and promotes the onset of dementia (Cao et al., 2018).
The pathogenesis of stroke involves a complicated cascade of reactions, and the severity of these reactions is influenced by the length of ischemia and the depth of the ischemic site (Xie et al., 2021). A growing body of research indicates that the blood–brain barrier (BBB) is disrupted, along with neuroinflammation, oxidative stress, mitochondrial dysfunction, and neurotoxicity (Yuan et al., 2021). Within a few hours of ischemia, apoptosis and necrosis have been seen in some cases (Wang et al., 2015). These pathogenic reactions can damage brain tissue. Therefore, early reperfusion of ischemic brain tissue, restoration of normal blood flow and blocking the aforementioned pathogenic processes, such as inflammation and oxidative stress are crucial to treating cerebral ischemia/reperfusion (IR) injury.
Due to the multifunctional complex mechanisms of cerebral ischemic injury, finding efficient medications become censorious, to inspect potential prophylactic agents. Recently, the neuroprotective potential of natural compounds has attracted the attention of researchers. Many natural agents with anti-inflammatory, antioxidant, and anti-apoptotic properties have demonstrated positive effects on brain damage (Mohsenpour et al., 2021). Flavonoids, including bioflavonoids, are the most prevalent plant polyphenols which confer color and flavor to fruits and vegetables (Teixeira, 2002). Bioflavonoids have antioxidant, anti-angiogenic, and anti-inflammatory properties beside the capability to reduce fluid retention and strengthen capillary membranes (Majumdar and Srirangam, 2010). These properties are attributed to their ability to inhibit enzymes that produce free radicals, scavenge free radicals, and chelate iron (Russo et al., 2000). Flavonoids, such as scutellarin, benefit brain injury induced by cerebral IR, owing to their anti-oxidative and anti-inflammatory properties, as well as their capacity to reduce neuronal damage (Wang and Ma, 2018).
Interestingly, the pathogenesis of cerebral ischemia involves free radical-mediated oxidative damage, inflammation, and apoptosis (Yuan et al., 2021). Thus, bioflavonoids may be useful to prevent or treat cerebral ischemia, which can lead to death if left untreated.
Members of the genus Juniperus are evergreen shrubs or trees characterized by needle- or scale-like leaves (Ogren, 2015). J. sabina, known in Saudi Arabia as “Al Abhal, described in folk medicine as abortifacient, diuretic, emetic, emmenagogue and irritant (Abdel-Kader et al., 2017, Lust, 1982, Chiej, 1984). The antimicrobial activity of the Essential oil of the plant reported to express antimicrobial activity correlated to the terpene contents (Akimov et al., 1977). Our phytochemical study of the non-polar fractions of J. sabina resulted in the identification of trans-calamenene, cadalene, epi-cubenol, manool, calamenene-10β-ol, calamenene-10α-ol, 4-epi-abietic acid, sandaracopimaric acid and isopimaric acid (Abdel-Kader et al., 2019) The polar fraction afforded Cupressuflavone (CUP) as the major bioflavonoid in addition to amentoflavone and robustaflavone (Khafagy et al., 2023). The present study aims to explore the neuroprotective benefits of CUP by improving behavioral imbalance, oxidant/antioxidant dysfunction, and histological abnormalities resulting from cerebral IR injury in rats.
2. Materials and methods
Approximately 48 mature male Wistar rats (180–200 g) were obtained from Animal Unit of the National Research Centre, Egypt, after approval of the animal experimentation process from Ethical Committee for Medical Research (approval no. 1416072022). The animals were kept in standard cages at constant room temperature with regular light–dark cycle and free access to food and water. The regulations of Institutional Animal Ethical Committee (IAEC) and National Regulations for Animal Welfare were followed during the experiments.
2.1. Preparation and isolation of CUP
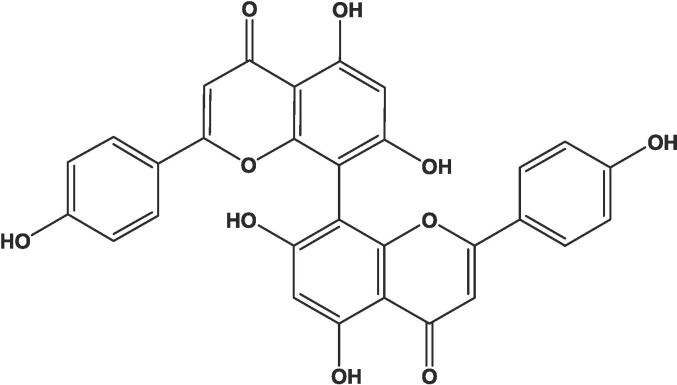
The CUP used in this study was isolated from J. sabina and identified as previously described (Khafagy et al., 2023). In brief the lyophilized aqueous ethanol fraction of 1 kg of the dried aerial parts was reconstituted in water and filtered to yield 37.54 g of water insoluble fraction. Twenty grams of the insoluble fraction were purified over Sephadex LH-20 column (300 g, 5 cm id) eluting with methanol. Fractions 14–28 of the 20 mL fractions collected from the column provided 435 mg of CUP (Fig. 1).
Fig. 1.
Chemical structure of cupressuflavone.
2.1.1. Characterization of CUP
Yellow powder; mp 392 °C Decomp.; UV λmax (MeOH) 329, 276, and 226 nm; 1H- and 13C NMR (CD3OD, δ): (Khafagy et al., 2023). HRESIMS: m/z 539.0972.
(Cal. 539.0978 for C30H18O11) (100, [M + H]+).
2.2. Induction of cerebral IR injury
A left common carotid artery occlusion (CCAO) model was created, as previously mentioned (Das et al., 2018). Prior to surgery, the rats received CUP or vehicle orally for 3 days. One hour after the last dose, rats were anesthetized with an intraperitoneal dose of ketamine (75 mg/kg) and xylazine (5 mg/kg). Subsequently, animals were positioned supinely on a surgery table. Left CCA was dissected following a midline neck incision and occluded using a noninvasive arterial clamp to induce cerebral ischemia. The clamp was withdrawn 60 min after occlusion to allow reperfusion. Control rats underwent sham surgery using the same surgical technique, but without left CCAO. During operation, lamp radiation maintained the animals' body temperatures at 37 ± 0.5 °C. The neck incision was sutured after treatment with 0.5 % bupivacaine. All rats were allowed a 24 h recovery period in individual cages.
2.3. Experimental design
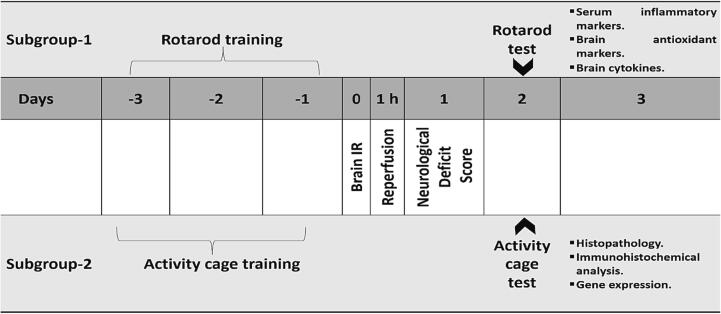
Experimental design is illustrated in Fig. 2. Four groups of 12 rats each were randomly distributed among 48 rats.
Fig. 2.
Schematic representation of the experimental design.
1. Sham group: The identical surgical method as the IR operation was performed on the rats, with the exception of occlusion of the left CCA. The rats were administered vehicle (2 % Tween 80 in sterile saline).
2. IR control group: Rats with CCAO were orally administered the vehicle.
3. IR + CUP-20 group: Rats were subjected to left CCAO and orally administered 20 mg/kg CUP.
4. IR + CUP-40 group: Rats were subjected to left CCAO and orally administered 40 mg/kg CUP.
Cupressuflavone doses were determined based on Al-Sayed et al. (2018). Rats received vehicle (Sham and IR control groups) and CUP (IR + CUP-20 and IR + CUP-40 groups) orally for three days before left CCA ligation, at the initiation of reperfusion, and for a further 3 days after reperfusion.
Twenty-four hours after brain IR injury, the neurological deficit scores of all rats were evaluated. In each group, rats were subdivided to two subgroups. Animals in subgroup-1 (n = 6) were subjected to rotarod experiment and biochemical evaluation, whereas animals in subgroup-2 (n = 6) were subjected to the activity cage experiment, histological evaluation, immunohistochemical assessment, and gene expression evaluation.
2.4. Assessment of neurological deficit score
The neurologic findings were assessed using the following five-point scale (Longa et al., 1989): 0- no neurologic impotence; 1- light focal neurologic impotence (inability to completely expand the left forepaw); 2- moderate focal neurologic impotence (spins to the left side); 3- severe focal impotence (dropping to the left side); 4- rats unable to walk on their own with low grade of consciousness. Animals scoring between 0 and 3 points were included in the study; those receiving 0 or 4 points were excluded (Abdel-Rahman et al., 2020).
2.5. Rotarod test for assessing motor coordination and balance
According to the procedure described by Vijitruth et al. (Vijitruth et al., 2006), motor coordination and balance were evaluated using a rotarod device (Model No. 7750; Ugo Basile, Italy) two days after cerebral IR injury. Three days before inducing cerebral IR damage, all rats in subgroup-1 were trained using an accelerated rotating rod (4–40 rpm, 5 min). Three tests were performed for each rat at 10-min intervals. For each rat, the delay to fall was measured in each trial with a maximum recording period of 5 min, and average of three trials was counted. Walking alongside the rod while in passive rotation was regarded as falling (Abdel-Rahman et al., 2020).
2.6. Activity cage test for assessing motor activity
The rats' spontaneous motor activity was measured two days after brain IR injury using a grid floor activity cage (Model No. 7430; Ugo-Basile, Comerio, Italy). Activity cage software automatically recognizes rat movements disrupted by infrared beams and analyzes the information to provide counts of horizontal movements. Each rat in subgroup-2 was separately placed in the activity cage for 300 s training session 3 days prior to inducing cerebral IR damage, and basal activity counts were recorded. The grid floor was thoroughly cleaned between sessions using 70 % ethyl alcohol. Animals were reintroduced to the activity cage on the test day and total horizontal and vertical activity counts were recorded for 300 s session (Ogaly et al., 2022, Althurwi et al., 2022).
2.7. Blood collection
Two hours following last dose, rats were anesthetized using intraperitoneal injections of ketamine (75 mg/kg) and xylazine (5 mg/kg) two hours after the last treatment. The retro-orbital venous plexus was used to collect blood samples into sampling tubes. Blood samples were centrifuged in a cooled centrifuge (Sigma and Laborzentrifugen, 2 k15, Germany) at 3000 rpm for 10 min to separate the serum.
2.8. Analysis of serum inflammatory biomarkers
The levels of serum inflammatory cytokines such as interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) were estimated using commercially available enzyme linked immunosorbent assay (Biovision, CA, USA) ELISA kits in accordance with manufacturer's instructions.
2.9. Euthanasia and samples of brain tissue
After decapitation of the anesthetized rats, their left hemispheres were immediately dissected on ice plates. To assess the levels of antioxidant biomarkers (GSH, CAT and MDA), and brain cytokines, rat brains of subgroup-1 were recruited, whereas those from subgroup-2 were utilized for histological, immunohistochemical, and gene expression assessment.
2.10. Brain homogenates preparation
For biochemical investigation, brains were gathered and washed using ice-cold saline. Brain tissue homogenate was obtained (10 % w/v) in 0.1 M phosphate buffer (pH 7.4). Brain homogenates were centrifuged in a cooled centrifuge at 10,000 rpm for 15 min. Supernatants that were produced were kept at −80 °C until further examination.
2.11. Determination of brain antioxidant markers
The brain MDA and GSH contents were evaluated following the procedures described by Ruiz-Larrea et al. and Ellman, respectively (Gl, 1959, Ruiz-Larrea et al., 1994). The brain CAT content was determined as described by Chance and Maehly (Chance and Maehly, 1955).
2.12. Determination of brain inflammatory cytokines
TBK1, NF-κB, and IFN-β levels were assessed in brain homogenates using commercial ELISA kits (Biovision, CA, USA).
2.13. Histopathological evaluation of brain tissue
The left-brain hemispheres were kept in neutral-buffered formalin (10 %) for 24 h, stained, and visualized using light microscopy. Serial dilutions with alcohol were prepared to dehydrate the samples, followed by cleaning in xylene, and embedding in paraffin wax for 6 h at 56 °C. Approximately 4–6 μ sections of paraffin wax tissue blocks were sectioned by a microtome. These sections were assembled and deparaffinized on glass slides. Hematoxylin and Eosin staining was used for regular histological investigations (Bancroft and Gamble, 2008).
2.14. Immunohistochemical evaluation of Caspase-3
Deparaffinized and rehydrated brain tissues from each group were used for this assessment. In accordance with the procedures outlined by Abdel-Rahman et al. (Abdel-Rahman et al., 2020), antigen retrieval was carried out using citrate buffer (10 mM, pH 6.0). Brain sections were combined with mouse monoclonal anti-caspase-3 antibodies (Elabscience, Cat# E-AB-63602, Dil.1:50) and incubated for 8 h. Additionally, for negative control slides, the principle antisera were eliminated using bovine serum albumin (Sigma). Unconjugated antibodies were removed from the tissue slices by extensive washing with tris-buffered saline (TBS). After incubation with biotinylated goat anti-rabbit antibodies (Vector Laboratories' Vectastain ABC-HRP kit) for 10 min, the sections were thoroughly rinsed with TBS before being treated with 3,3-diaminobenzidine (DAB). Sections were mounted using DPX and stained using Mayer's hematoxylin. The captured images were analyzed using the ImageJ software. The percentage of immunostained cells that were positive using 10 microscopic fields/group was quantified.
2.15. Quantitative real-time polymerase chain reaction (RT-PCR) analysis of toll-like receptor 4 (Tlr4) and high mobility group box 1 (Hmgb1) genes
Next, quantitative RT-PCR was used to evaluate the effect of CUP on the mRNA expression levels of Tlr4 and Hmgb1 in acute focal cerebral ischemia. Total RNA was purified from the brain, and subsequent reverse transcription and RT-PCR amplification were performed using commercially available RNA, as described previously (Althurwi et al., 2022, Ogaly et al., 2022). The primer sequences used were: Tlr4-forward, 5′- CCGTCACCACATACTGCCTTTA-3′ and Tlr4-reverse, 5′-GCAGTTTGGACTATTGAAATACGAAA-3′ (NM_001109668.1); Hmgb1-forward, 5′- CCTAAGAAGCCGAGAGGCAA-3′ and Hmgb1-reverse, 5′- AAGTTGACAGAAGCATCCGGG-3′ (NM_001409387.1); and GAPDH-forward, 5′- CCTCGTCTCATAGACAAGATGGT-3′ and GAPDH-reverse, 5′- GGGTAGAGTCATACTGGAACATG-3′ (NM_001394060.2). The relative mRNA expressions of the test genes were evaluated using GAPDH as an internal control gene (Livak and Schmittgen, 2001).
2.16. Statistical analyses
Data of the current investigation are presented as M ± SEM. Graph Prism® software was used to statistically validate the results using one-way ANOVA (analysis of variance) followed by Tukey's test to determine intergroup variability. Statistical significance was set as p < 0.05.
3. Results
3.1. Assessment of neurological deficit score
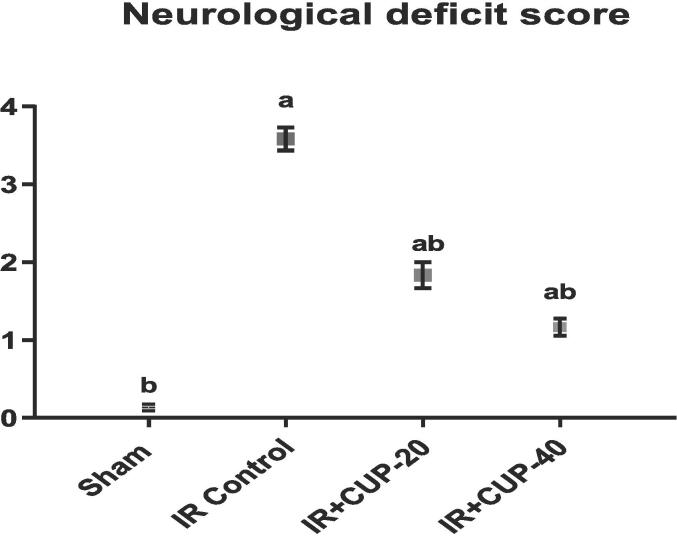
Neurological impotence was evaluated to detect neurological impairments in all groups 24 h after reperfusion (Fig. 3). The sham-operated rats did not exhibit any neurological deficits. However, the neurological deficit score in IR control rats was markedly larger than that in sham animals. In contrast, both doses of CUP markedly reduced neurological deficit scores in IR-injured animals compared to those in the IR control rats (Fig. 3).
Fig. 3.
Evaluation of the neurological deficit score. Results are presented as M ± SEM (n = 12). a Significantly different in comparison with sham group (p ≤ 0.05). b Significantly different in comparison with IR control group (p ≤ 0.05). IR: ischemia/reperfusion; CUP: cupressuflavone.
3.2. Rotarod test for assessing motor coordination and balance
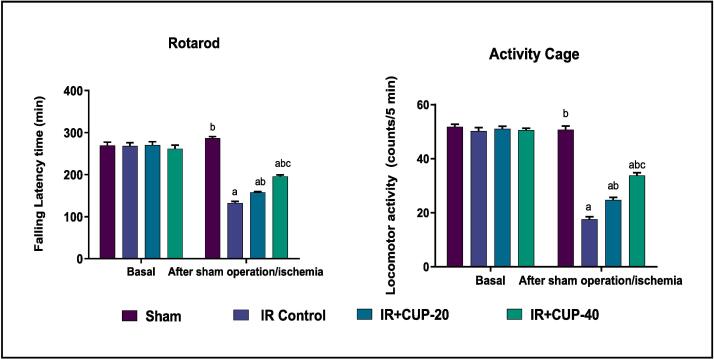
A rotarod test was conducted to assess motor coordination function (Fig. 4). The results revealed that IR control rats had the lowest potential to perform the test as evidenced by their shorter latency to fall off the rotarod under acceleration (132.53 ± 3.78 sec) than that in sham rats (286.72 ± 3.80 sec). In comparison to the IR control rats, those administered CUP demonstrated significantly (p ≤ 0.05) and dose-dependently enhanced rotarod performance.
Fig. 4.
Effect of cupressuflavone (CUP) on motor coordination (rotarod test) and locomotor activity (activity cage test) in IR rats. Results are presented as M ± SEM (n = 6). a Significantly different in comparison with sham group (p ≤ 0.05). b Significantly different in comparison with IR control group (p ≤ 0.05). c Significantly different in comparison with IR + CUP-20 group (p ≤ 0.05). IR: ischemia/reperfusion; CUP: cupressuflavone.
3.3. Activity cage test for assessing motor activity
Motor activities of rats were assessed using activity cage and the results are recorded in Fig. 4. IR control rats demonstrated a significantly lower level of locomotor activity (17.54 ± 1.02 counts/5 min) than that of sham-operated rats (53.04 ± 1.84 counts/5 min). However, 20 mg/kg CUP significantly improved the spontaneous motor activity counts (23.71 ± 1.85 counts/5 min) in the treated rats when compared with those in IR control rats (p ≤ 0.05). Additionally, CUP-40-treated rats revealed a significant upsurge in the locomotor activity (30.84 ± 0.93 counts/5 min).
3.4. Determination of serum inflammatory biomarkers
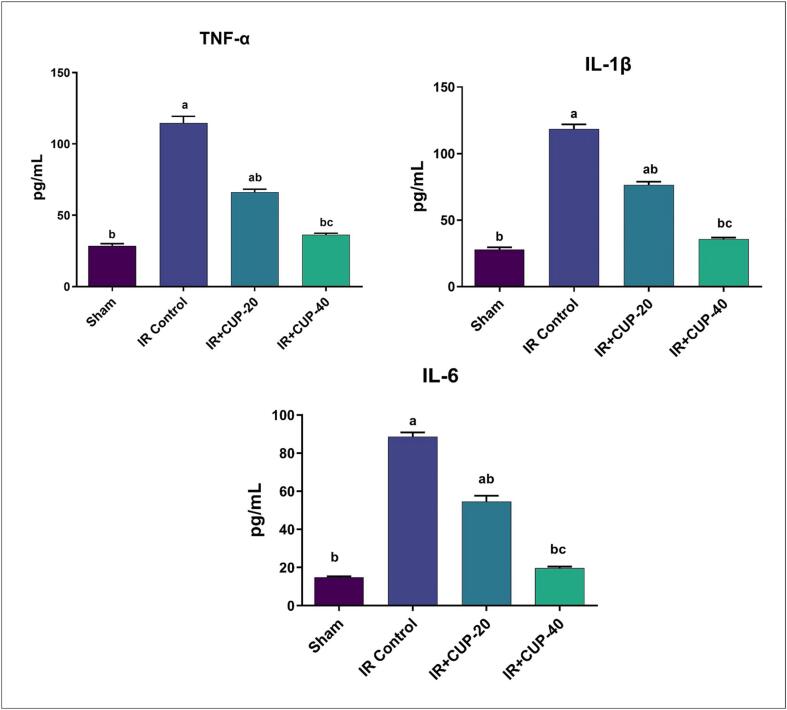
As seen in Fig. 5, IR control rats exhibited larger values of TNF-α, IL-1β, and IL-6 in serum than those in sham-operated rats. Compared to the IR control rats, those treated with CUP (20 and 40 mg/kg) exhibited significantly decreased values of pro-inflammatory cytokines.
Fig. 5.
Effect of Cupressuflavone (CUP) on serum inflammatory biomarkers of IR rats. Values are expressed as Mean ± SEM of six animals in each group. a Significantly different from the values of sham group at p ≤ 0.05. b Significantly different from the values of IR control group at p ≤ 0.05. c Significantly different from the values of IR + CUP-20 group at p ≤ 0.05.
3.5. Determination of brain inflammatory cytokines
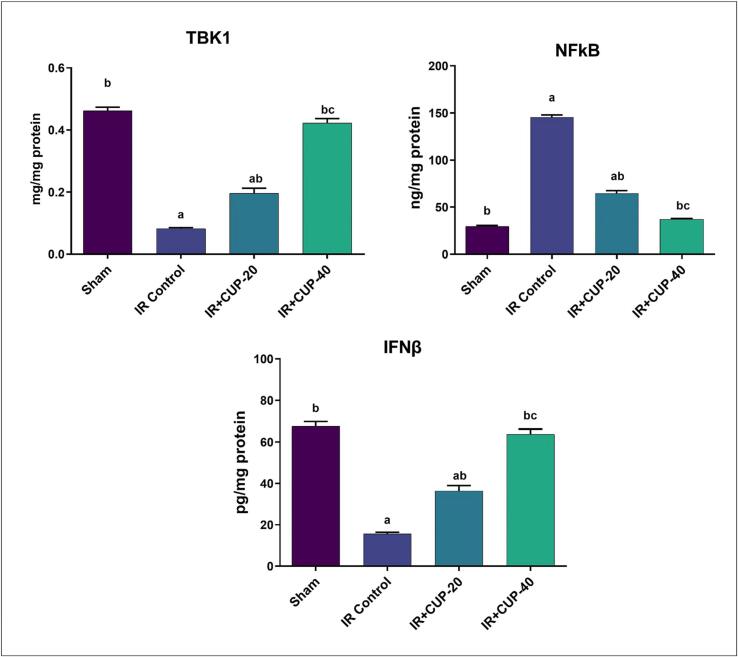
IR control rats showed significantly lower levels of the brain TBK1 and IFN-β contents than those in the sham group (Fig. 6), whereas NF-κB levels were significantly higher in IR control rats than those in sham animals. As outlined in Fig. 6, both doses of CUP considerably and dose-dependently elevated values of the inflammatory biomarkers (TBK1 and IFN-β) in brain while significantly reducing the level of NF-κB.
Fig. 6.
Effect of Cupressuflavone (CUP) on brain inflammatory cytokines of IR rats. Values are expressed as Mean ± SEM of six animals in each group. a Significantly different from the values of sham group at p ≤ 0.05. b Significantly different from the values of IR control group at p ≤ 0.05. c Significantly different from the values of IR + CUP-20 group at p ≤ 0.05.
3.6. Determination of brain antioxidant markers
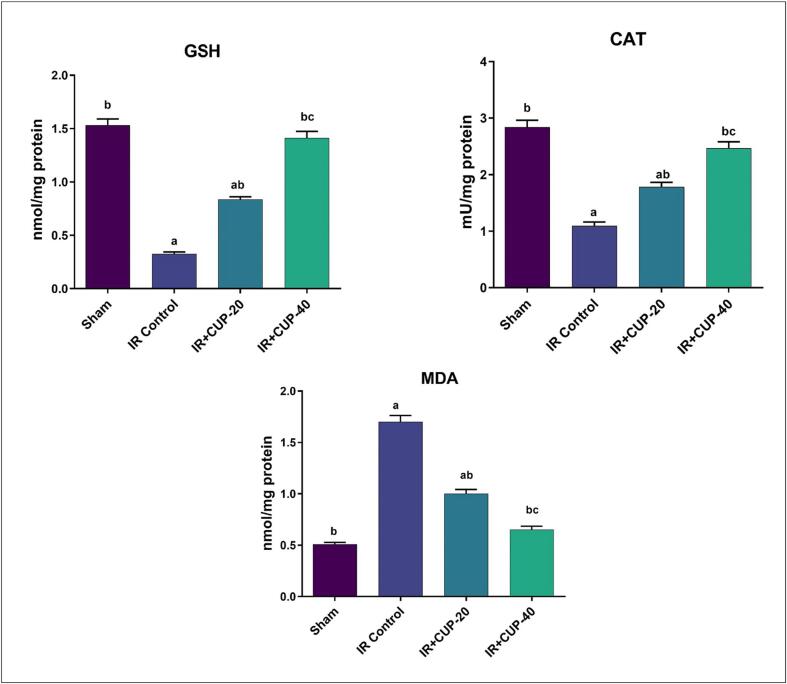
MDA, GSH, and CAT levels were assessed in brain homogenates (Fig. 7). The brains of IR controls showed significantly lower levels of GSH and activity of CAT than those in sham-operated rats. In contrast, the MDA contents were significantly larger in IR control group than those in sham animals. In comparison to IR control rats, those treated with CUP (20 and 40 mg/kg) demonstrated significantly lower MDA levels, increased GSH levels, and increased CAT activity in the brain homogenate (p ≤ 0.05).
Fig. 7.
Effect of Cupressuflavone (CUP) on brain antioxidant markers of IR rats. Values are expressed as Mean ± SEM of six animals in each group. a Significantly different from the values of sham group at p ≤ 0.05. b Significantly different from the values of IR control group at p ≤ 0.05. c Significantly different from the values of IR + CUP-20 group at p ≤ 0.05.
3.7. Histopathological examination of brain tissue
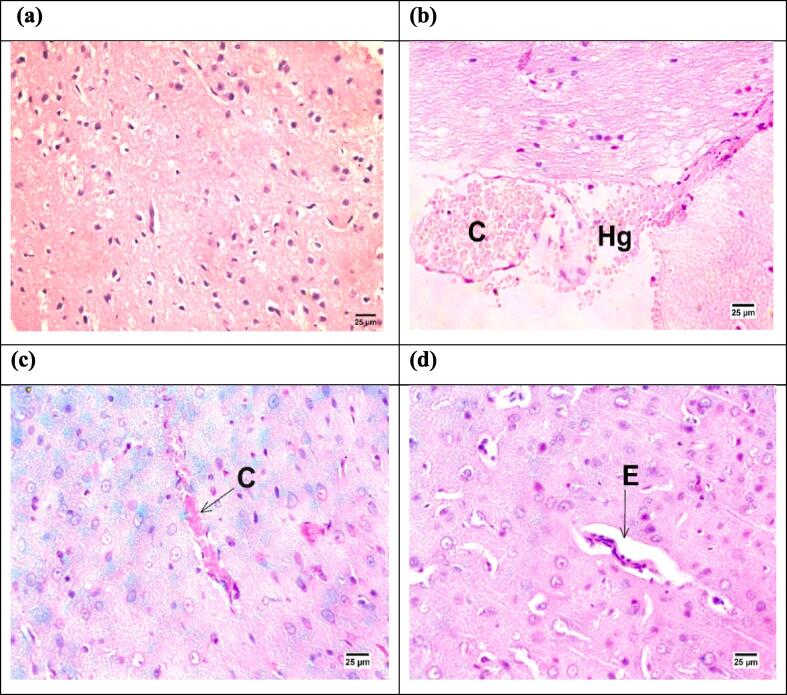
As depicted in Fig. 8, histopathological investigations of brain sections from IR rats revealed hemorrhage with congestion of the cerebral blood vessels. However, brain sections from CUP-treated animals exhibited slight congestion of the cerebral blood capillaries and slight perivascular edema.
Fig. 8.
Histopathological photomicrographs of the Hematoxylin and eosin stained brain sections (×400) from different groups. (a) Sham group showing normal β-cells, (b) IR control group showing hemorrhage [Hg] with congestion of cerebral blood vessels [C]. (c) IR + CUP-20 group showing congestion of cerebral blood vessels [C], and (d) IR + CUP-40 group showing perivascular edema [E] in the cerebrum. IR: ischemia/reperfusion; CUP: cupressuflavone.
3.8. Immunohistochemical examination of Caspase-3 in neurons of the brain
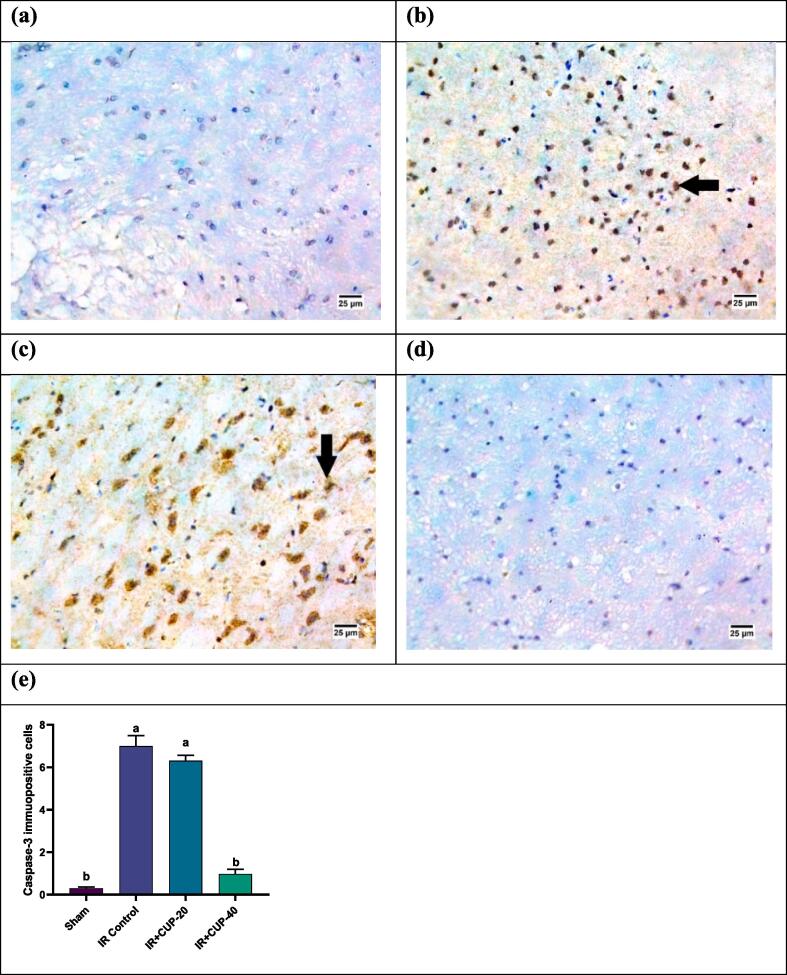
Caspase 3 expression in the brain neurons is shown in Fig. 9. Brain tissue sections from the IR control rats revealed increased expression of Caspase-3 immunopositive cells (Fig. 9-b). Reduced expression of caspase-3 was recorded in CUP-20-treated group (Fig. 9-c), whereas no reaction for caspase 3 was observed in neurons of the brains from CUP-40-treated rats (Fig. 9-d).
Fig. 9.
Photomicrographs of the brain sections from different groups (IHC- peroxidase – DAB). (a) Sham group showing negative reaction for caspase 3 in neurons of the brain, (b) IR control group showing highly positive reaction for caspase 3 in the nuclei of neurons, (c) IR + CUP-20 group showing moderate positive reaction for caspase 3 in the nuclei of neurons, and (d) IR + CUP-40 group showing negative reaction for caspase 3 in neurons of the brain. (e) Bar chart depicting caspase 3 expression in the four groups. All values are expressed as M ± SEM (n = 6); ap ≤ 0.05 versus the sham group, bp ≤ 0.05versus IR control group. IR: ischemia/reperfusion; CUP: cupressuflavone.
3.9. Effect of CUP on Tlr4 and Hmgb1 gene expression
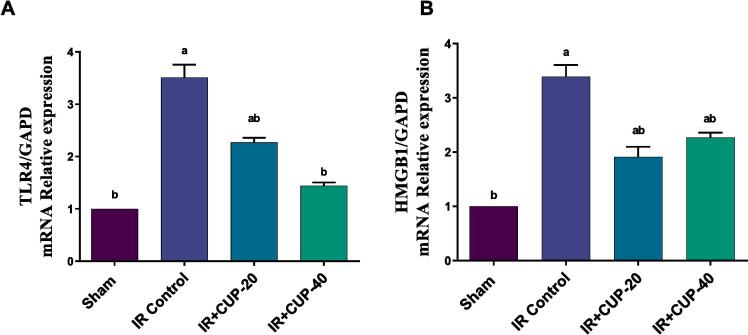
Tlr4 mRNA level in IR control rats were significantly larger than those in sham animals (Fig. 10A). However, CUP treatment ameliorated the IR-induced upregulation of Tlr4 mRNA expression (p < 0.05; Fig. 10A). Similarly, the Hmgb1 mRNA level in IR control rats was markedly higher than that of sham group and was significantly downregulated in CUP treated groups (p ≤ 0.05; Fig. 10B).
Fig. 10.
Effect of CUP on brain Tlr4 and Hmgb1 expression in cerebral ischemic rats. (B) Quantitative analysis of Tlr4 gene expression. (C) Quantitative analysis of Hmgb1 gene expression. Results are presented as M ± SEM (n = 6). a Significantly different in comparison with sham group (p ≤ 0.05). b Significantly different in comparison with IR control group (p ≤ 0.05). IR: ischemia/reperfusion; CUP: cupressuflavone.
4. Discussion
The current investigation investigated the neuroprotective effects of CUP against IR injury induced by unilateral CCAO and reperfusion in rats. Impaired brain circulation is an underlying cause of many neurological and mental ailments. While a progressive loss in sustained cerebral blood flow diminishes memory functions and aids in the onset of dementia A. Furthermore, a sudden disruption of blood supply to particular brain areas may cause stroke (Duncombe et al., 2017).
Experimentally, the induction of cerebral transient hypoperfusion can be achieved through unilateral CCAO, followed by reperfusion, resulting in severe vascular alterations and impaired neuronal activities (Das et al., 2018).
In the current study, the left CCA of rats was blocked for one hour then perfused, causing evident neurological impairments which were detected 24 h after reperfusion in the IR groups. Treatment of IR rats with CUP improved their neurological performance.
Flavonoids and their metabolites can penetrate the BBB, although their ability to do so depends on compound lipophilicity and/or transporters (Faria et al., 2011). CUP, a naturally occurring bioflavonoid, has shown neuroprotective benefits, prevents neurodegeneration via inhibiting cyclin-dependent kinases, and is effective in cases of oxidative stress and amyloid β-peptide-induced cell death in brain (Khafagy et al., 2023).
Ischemia/hypoxia and trauma-induced brain damage are associated with an increase in values of inflammatory cytokines (Saito et al., 1996, Thong-Asa and Tilokskulchai, 2014). In case of ischemic stroke, TNF-α, TGF-β, IL-1β and IL-6 are the most frequently examined cytokines related to inflammation, wherein TNF-α and IL-1β, seem to worsen cerebral injury. Remarkably, a higher level of pro-inflammatory cytokines and lower values of the anti-inflammatory IL-10 are associated with infarctions and defective clinical outcomes (Zhu et al., 2002). In line with these findings, the data from the current study identified significant increase in the serum levels of TNF-α, IL-1β, and IL-6 in IR animals, in comparison with those in sham group. However, compared to the IR group, the CUP-treated groups demonstrated significant decrease in serum values of TNF-α, IL-1β, and IL-6. CUP has potent anti-inflammatory properties, as evidenced by Al-Sayed et al. (Al-Sayed et al., 2018), as evidenced by reduction of paw edema induced by carrageenan. It significantly reduces plasma prostaglandin E2 and TNF-α levels. CUP significantly reduces plasma IL-1β and IL-6. Additionally, CUP demonstrates dose-dependent antiulcerogenic efficacy on stomach ulcers (Koriem et al., 2015).
Because the brain is extremely sensitive to hypoxia, ischemia-induced hypoxia is a major cause of brain IR injury (Chong et al., 2005). Considerable amounts of reactive oxygen species (ROS) are produced during brain ischemia. Consequently, ROS generation modifies vascular reactivity and damages BBB (Wang et al., 2019). Furthermore, ROS cause cell and organelle membrane degradation and impairment by inducing the lipid peroxidation of unsaturated fatty acids (Xing et al., 2018). In addition, brain is particularly vulnerable to oxidative stress due to its lack of antioxidant defense mechanisms, high peroxidizable unsaturated fatty acid content, excessive oxygen consumption per unit weight, high iron concentration, and ascorbate, which is an essential constituent of lipid peroxidation (Gorman et al., 1996). Finally, because of cerebral edema, inflammation, neuronal cell apoptosis, and infarct size expansion, brain tissue damage worsens until death (Wu et al., 2020). Endogenous antioxidant substances found in the human body include free radical-metabolizing enzymes such as CAT and GSH, which scavenge free radicals to prevent oxidative stress.
CUP can exert antioxidant effects by enhancing the efficiency of antioxidant enzymes GSH and SOD (Al-Sayed and Abdel-Daim, 2014). The present results demonstrated that IR animals had significantly lower brain GSH and CAT activity compared to sham-operated animals. Moreover, MDA levels in the brain were markedly higher in IR animals than those in sham-operated group. In contrast, CUP-treated rats showed significantly enhanced GSH brain content and CAT activity, and significantly decreased MDA levels. In addition to its anti-apoptotic activity, Koriem et al. and Sepehrimanesh et al. (Koriem et al., 2015, Sepehrimanesh et al., 2018) reported that CUP presumably suppresses gastrointestinal disorders (gastric ulceration and ulcerative colitis) by enhancing endogenous antioxidant enzymes and eliminating free radicals.
TBK1 is a crucial element in microglial reactivity and is associated with increased neuroinflammatory responses (Rehman et al., 2021). Additionally, TBK1 inhibition enhances neuroprotection in stroke (Kundu et al., 2022). In addition, IFN-β possesses anti-inflammatory capabilities to protect against ischemic stroke. Moreover, NF-κB is crucial for triggering inducible nitric oxide synthase and other inflammatory responses (Kuo et al., 2016). Moreover, the brain levels of TBK1 and IFN-β are significantly lower in IR rats than those in sham-operated rats, although NF-κB levels are significantly higher, supporting the induction of neuroinflammation. Compared to IR control rats, CUP administration in IR rats significant enhanced brain TBK1 and IFN-β, and significantly reduced NF-κB levels.
Caspase-3 predominates in many pathological conditions, such as hypoxic brain injury. Immunostaining of brain sections has revealed a significant increase in caspase-3 expression induced by cerebral IR damage (Abdel-Rahman et al., 2020, Althurwi et al., 2022, Ogaly et al., 2022). In this study, brain tissue sections from the IR-control group showed increased expression of caspase-3 in immunopositive cells. However, moderate caspase-3 expression was observed in CUP-20-treated groups and no expression was detected in the CUP-40-treated rats (Fig. 8). These findings supported the neuroprotective effects of CUP.
HMGB1 is a key member of a family of non-histone DNA-binding proteins called high mobility group box (HMGB) family (Jiang et al., 2012, Tao et al., 2015). HMGB1 acts as a neuroinflammatory mediator, activating microglial cells in neurodegenerative diseases and the post-ischemic brain (Shichita et al., 2014). Under ischemic conditions, HMGB-1 is released from its chromatin-bound state from the nucleus to the extracellular space, where it links to its receptor sites and TLRs. Activation of HMGB-1 induces NF-κB, which eventually aggravates neuroinflammation through targeting several inflammatory cytokines, including TNF-α, IL-1β, and IL-6 (Cai et al., 2016, Chen et al., 2017). In addition, stimulation of NF-κB signaling pathway triggers oxidative stress that follow brain ischemia (Tao et al., 2015).
The HMGB1/TLR4/NF-κB signaling pathway is activated during IR injury in various tissues, which constitutes an important part of the neuroinflammatory response mechanism in post-ischemic tissue injury (Zhu et al., 2018, Zhang et al., 2020).
Quantitative RT-PCR revealed that IR injury significantly upregulated the activation of HMGB1-TLR-4 signaling, as indicated by a marked increase in mRNA expression values of cerebral Hmgb1 and Tlr-4. Compared to the IR group, the CUP-20 and CUP-40- treated groups demonstrated significantly reduced Hmgb1 and Tlr4 upregulation in ischemic brain tissue. Together with the suppressing effect of CUP on NF-κB, our findings suggest that neuroprotective activities of CUP could be partially referred to inhibition of inflammatory cascades by modulating the HMGB1-mediated NF-κB signaling pathway.
5. Conclusion
In summary, to the best of our knowledge, this is the first study to document the neuroprotective properties of CUP. The proposed mechanism of protection includes the enhancement of brain antioxidant contents of GSH and CAT, reduction of serum inflammatory cytokine levels, and potentiation of the brain contents of TBK1 and IFN-β. In addition, the mechanism may involve HMGB1-dependent expression of NF-κB via suppression of the HMGB1/TLR-4 signaling pathway. Thus, CUP may serve as a potential preventive and therapeutic alternative for cerebral stroke.
Funding
The project was funded by Prince Sattam bin Abdulaziz University through the project number (PSAU/2023/01/33345).
CRediT authorship contribution statement
Faisal F. Albaqami: Methodology, Visualization, Data curation, Resources, Investigation, Writing – original draft. Rehab F. Abdel-Rahman: Conceptualization, Methodology, Investigation, Writing – original draft. Hassan N. Althurwi: Methodology, Visualization, Data curation, Investigation, Writing – original draft. Khalid M. Alharthy: Methodology, Visualization, Data curation, Investigation, Writing – original draft. Gamal A. Soliman: Conceptualization, Resources, Methodology. Tariq M. Aljarba: Methodology, Resources, Visualization, Data curation, Investigation, Writing – original draft. Hanan A. Ogaly: Methodology, Investigation, Writing – original draft. Maged S. Abdel-Kader: Conceptualization, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2023/01/33345).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Kader M.S., Alanazi M.T., Bin Saeedan A.S., Al-Saikhan F.I., Hamad A.M. Hepatoprotective and nephroprotective activities of Juniperus sabina L. aerial parts. J. Pharm. Pharmacogn. Res. 2017;5(1):29–39. [Google Scholar]
- Abdel-Kader M.S., Hamad A.M., Alanazi M.T., Alanazi A.H., Ali R., Foudah A.I., Alqarni M.H. Characterization and hepatoprotective evaluation of sesquiterpenes and diterpenes from the aerial parts of Juniperus sabina L. Saudi Pharm. J. 2019;27:920–929. doi: 10.1016/j.jsps.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Rahman R.F., El Awdan S.A., Hegazy R.R., et al. Neuroprotective effect of Crocus sativus against cerebral ischemia in rats. Metab. Brain Dis. 2020;35:427–439. doi: 10.1007/s11011-019-00505-1. [DOI] [PubMed] [Google Scholar]
- Akimov Y.A., Kharchenko G.I., Krylova A.P., Belova N.N. An-timicrobial activity of terpenes from Juniperus sabina L. Appl. Biochem. Microbiol. 1977;13(2):185–188. [PubMed] [Google Scholar]
- Al-Sayed E., Abdel-Daim M.M. Protective role of Cupressuflavone from Cupressus macrocarpa against carbon tetrachloride-induced hepato-and nephrotoxicity in mice. Planta Med. 2014;80:1665–1671. doi: 10.1055/s-0034-1383211. [DOI] [PubMed] [Google Scholar]
- Al-Sayed E., Gad H.A., El-Shazly M., et al. Anti-inflammatory and analgesic activities of cupressuflavone from Cupressus macrocarpa: Impact on pro-inflammatory mediators. Drug Dev. Res. 2018;79:22–28. doi: 10.1002/ddr.21417. [DOI] [PubMed] [Google Scholar]
- Althurwi H.N., Abdel-Rahman R.F., Soliman G.A., et al. Protective effect of beta-carotene against myeloperoxidase-mediated oxidative stress and inflammation in rat ischemic brain injury. Antioxidants. 2022;11:2344. doi: 10.3390/antiox11122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J.D., Gamble M. Elsevier Health Sciences; 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- Cai M., Yu Z., Wang L., et al. Tongxinluo reduces brain edema and inhibits post-ischemic inflammation after middle cerebral artery occlusion in rats. J. Ethnopharmacol. 2016;181:136–145. doi: 10.1016/j.jep.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Cao D., Bai Y., Li L. Common carotid arteries occlusion surgery in adult rats as a model of chronic cerebral hypoperfusion. Bio-Protocol. 2018;8:e2704–e. doi: 10.21769/BioProtoc.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Maehly A. Assay of catalases and peroxidases. Meth. Enzimol. 1955;2:764–775. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Chen C.-B., Liu L.-S., Zhou J., et al. Up-regulation of HMGB1 exacerbates renal ischemia-reperfusion injury by stimulating inflammatory and immune responses through the TLR4 signaling pathway in mice. Cell. Physiol. Biochem. 2017;41:2447–2460. doi: 10.1159/000475914. [DOI] [PubMed] [Google Scholar]
- Chiej R. MacDonald; Edinburgh: 1984. The Macdonald Encyclopaedia of Medicinal Plants. [Google Scholar]
- Chong Z.Z., Li F., Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Das K.K., Yendigeri S.M., Patil B.S., et al. Subchronic hypoxia pretreatment on brain pathophysiology in unilateral common carotid artery occluded albino rats. Indian J. Pharmacol. 2018;50:185. doi: 10.4103/ijp.IJP_312_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe J., Kitamura A., Hase Y., et al. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017;131:2451–2468. doi: 10.1042/CS20160727. [DOI] [PubMed] [Google Scholar]
- Faria A., Pestana D., Teixeira D., et al. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011;2:39–44. doi: 10.1039/c0fo00100g. [DOI] [PubMed] [Google Scholar]
- Frank D., Zlotnik A., Boyko M., et al. The development of novel drug treatments for stroke patients: a review. Int. J. Mol. Sci. 2022;23:5796. doi: 10.3390/ijms23105796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gl E. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gorman A.M., McGowan A., O'Neill C., et al. Oxidative stress and apoptosis in neurodegeneration. J. Neurol. Sci. 1996;139(Suppl):45–52. doi: 10.1016/0022-510x(96)00097-4. [DOI] [PubMed] [Google Scholar]
- Jiang W.-L., Xu Y., Zhang S.-P., et al. Tricin 7-glucoside protects against experimental cerebral ischemia by reduction of NF-κB and HMGB1 expression. Eur. J. Pharm. Sci. 2012;45:50–57. doi: 10.1016/j.ejps.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Khafagy E.-S., Soliman G.A., Shahba A.-A.-W., et al. Brain targeting by intranasal drug delivery: effect of different formulations of the biflavone “Cupressuflavone” from Juniperus sabina L. on the motor activity of rats. Molecules. 2023;28:1354. doi: 10.3390/molecules28031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriem K.M., Gad I.B., Nasiry Z.K. Protective effect of Cupressus sempervirens extract against indomethacin-induced gastric ulcer in rats. Interdiscip. Toxicol. 2015;8:25. doi: 10.1515/intox-2015-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft P., De Meyer S.F., Kleinschnitz C. Next-generation antithrombotics in ischemic stroke: preclinical perspective on ‘bleeding-free antithrombosis’. J. Cereb. Blood Flow Metab. 2012;32:1831–1840. doi: 10.1038/jcbfm.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu N., Kumar A., Corona C., et al. A STING agonist preconditions against ischaemic stroke via an adaptive antiviral Type 1 interferon response. Brain Commun. 2022;4:fcac133. doi: 10.1093/braincomms/fcac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P.C., Scofield B.A., Yu I.C., et al. Interferon-β modulates inflammatory response in cerebral ischemia. J. Am. Heart Assoc. 2016;5:e002610. doi: 10.1161/JAHA.115.002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longa E.Z., Weinstein P.R., Carlson S., et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lust J. Bantam Books; New York: 1982. The Herb Book. [Google Scholar]
- Majumdar S., Srirangam R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J. Pharm. Pharmacol. 2010;62:951–965. doi: 10.1211/jpp.62.08.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsenpour H., Pesce M., Patruno A., et al. A review of plant extracts and plant-derived natural compounds in the prevention/treatment of neonatal hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2021;22:833. doi: 10.3390/ijms22020833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogaly H.A., Abdel-Rahman R.F., Mohamed M.A.E., et al. Thymol ameliorated neurotoxicity and cognitive deterioration in a thioacetamide-induced hepatic encephalopathy rat model; involvement of the BDNF/CREB signaling pathway. Food Funct. 2022;13:6180–6194. doi: 10.1039/d1fo04292k. [DOI] [PubMed] [Google Scholar]
- Ogren T.L. The Allergy-Fighting Garden: Stop Asthma and Allergies with Smart Landscaping. Ten Speed Press; Berkeley, CA, USA: 2015. pp. 131–133. [Google Scholar]
- Rehman R., Tar L., Olamide A.J., et al. Acute TBK1/IKK-ε inhibition enhances the generation of disease-associated microglia-like phenotype upon cortical stab-wound injury. Front. Aging Neurosci. 2021;13:415. doi: 10.3389/fnagi.2021.684171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Larrea M.B., Leal A.M., Liza M., et al. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–388. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Russo A., Acquaviva R., Campisi A., et al. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000;16:91–98. doi: 10.1023/a:1007685909018. [DOI] [PubMed] [Google Scholar]
- Saito K., Suyama K., Nishida K., et al. Early increases in TNF-α, IL-6 and IL-1β levels following transient cerebral ischemia in gerbil brain. Neurosci. Lett. 1996;206:149–152. doi: 10.1016/s0304-3940(96)12460-5. [DOI] [PubMed] [Google Scholar]
- Sepehrimanesh M., Samimi N., Koohi-Hosseinabadi O., et al. Effects of Cupressus sempervirens extract on the healing of acetic acid-induced ulcerative colitis in rat. J. Coloproctol. 2018;38:309–313. [Google Scholar]
- Shichita T., Ito M., Yoshimura A. Post-ischemic inflammation regulates neural damage and protection. Front. Cell. Neurosci. 2014;8:319. doi: 10.3389/fncel.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Sun X., Yin L., et al. Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition. Free Radic. Biol. Med. 2015;84:103–115. doi: 10.1016/j.freeradbiomed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Teixeira S. Bioflavonoids: proanthocyanidins and quercetin and their potential roles in treating musculoskeletal conditions. J. Orthop. Sports Phys. Ther. 2002;32:357–363. doi: 10.2519/jospt.2002.32.7.357. [DOI] [PubMed] [Google Scholar]
- Thong-Asa W., Tilokskulchai K. Neuronal damage of the dorsal hippocampus induced by long-term right common carotid artery occlusion in rats. Iran. J. Basic Med. Sci. 2014;17:220. [PMC free article] [PubMed] [Google Scholar]
- Vijitruth R., Liu M., Choi D.-Y., et al. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson's disease. J. Neuroinflammat. 2006;3:1–16. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li Y., Gao S., et al. Breviscapine injection improves the therapeutic effect of western medicine on angina pectoris patients. PLoS One. 2015;10:e0129969. doi: 10.1371/journal.pone.0129969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Luo J., Li S.-Y. Nano-curcumin simultaneously protects the blood–brain barrier and reduces M1 microglial activation during cerebral ischemia–reperfusion injury. ACS Appl. Mater. Interfaces. 2019;11:3763–3770. doi: 10.1021/acsami.8b20594. [DOI] [PubMed] [Google Scholar]
- Wang L., Ma Q. Clinical benefits and pharmacology of scutellarin: a comprehensive review. Pharmacol. Ther. 2018;190:105–127. doi: 10.1016/j.pharmthera.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Wu L., Xiong X., Wu X., et al. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front. Mol. Neurosci. 2020;13:28. doi: 10.3389/fnmol.2020.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q., Li H., Lu D., et al. Neuroprotective effect for cerebral ischemia by natural products: a review. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.607412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing P., Ma K., Wu J., et al. Protective effect of polysaccharide peptide on cerebral ischemia-reperfusion injury in rats. Mol. Med. Rep. 2018;18:5371–5378. doi: 10.3892/mmr.2018.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Yuan Y., Zheng Y., et al. Anti-cerebral ischemia reperfusion injury of polysaccharides: a review of the mechanisms. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111303. [DOI] [PubMed] [Google Scholar]
- Zhang W., Song J., Li W., et al. Salvianolic acid D alleviates cerebral ischemia-reperfusion injury by suppressing the cytoplasmic translocation and release of HMGB1-triggered NF-κB activation to inhibit inflammatory response. Mediators Inflammat. 2020;2020 doi: 10.1155/2020/9049614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Lu J., Liu W.-W., et al. Advances in stroke pharmacology. Pharmacol. Ther. 2018;191:23–42. doi: 10.1016/j.pharmthera.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Zhu S., Tang S., Su F. Dioscin inhibits ischemic stroke-induced inflammation through inhibition of the TLR4/MyD88/NF-κB signaling pathway in a rat model. Mol. Med. Rep. 2018;17:660–666. doi: 10.3892/mmr.2017.7900. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Yang G.-Y., Ahlemeyer B., et al. Transforming growth factor-β1 increases bad phosphorylation and protects neurons against damage. J. Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]