Abstract
Background
Minimally invasive repair of pectus excavatum (MIRPE) is a popular method for surgical correction of PE, and its impact on quality of life is a growing area of interest. We performed a systematic review and meta-analysis to evaluate the impact of MIRPE on the quality of life of patients.
Methods
This study was registered with PROSPERO under reference number CRD42020222061. A literature search of PubMed, Cochrane Library, EMBASE and Scopus was conducted from the date of inception till November 23, 2020. We included studies which administered one or more questionnaires on patients up to 60 years old, parents or both, to assess the quality of life before and after MIRPE. Studies not written in English, abstracts, articles without primary data, reviews and studies which combined data on PE and other deformities were excluded. Risk of bias was assessed using the Risk of Bias in Non-randomised Studies of Interventions and the Cochrane risk of bias tool. A random-effects meta-analysis was performed to obtain mean differences for key themes of quality of life before and after MIRPE. Responses from the same questionnaires, as well as common themes across different questionnaires, were compared.
Results
Of the 20 studies identified for systematic review, 7 studies that reported the responses of 478 patients were included in the meta-analysis. Patients who underwent MIRPE experienced an increased self-esteem [standardized mean difference (SMD): 1.38, 95% confidence interval (CI): 0.95 to 1.81, P<0.00001] and a smaller degree of chest interference with their social activities (SMD: 0.84, 95% CI: 0.60 to 1.08, P<0.00001). These findings were consistent even after the implanted bar was removed.
Conclusions
MIRPE may be associated with a better quality of life for patients with PE as self-esteem and extent of chest interference with social activities are improved after the procedure. The key limitations of this study are the lack of high-quality evidence due to paucity of randomized trials, and the significant heterogeneity in reported outcomes due to variations in the questionnaires and timepoints of administration.
Keywords: Pectus excavatum (PE), funnel chest, minimally invasive repair of pectus excavatum (MIRPE), quality of life
Highlight box.
Key findings
• Minimally invasive repair of pectus excavatum (MIRPE) is associated with a better quality of life for patients with pectus excavatum (PE).
• Self-esteem and the extent of chest interference with social activities are improved after MIRPE, and also after eventual bar removal.
What is known and what is new?
• MIRPE is commonly indicated in patients with reduced exercise capacity due to PE. However, its impact on quality of life is difficult to measure.
• Our study found a consistent improvement in the quality of life of patients after MIRPE, based on analyses of responses to PE-specific questionnaires administered in various studies.
What is the implication, and what should change now?
• In view of the evidence supporting the positive impact of MIRPE on quality of life, patients with PE and their parents should be assured of the effectiveness of MIRPE.
Introduction
Pectus excavatum (PE) is a congenital deformity involving sternal depression into the thoracic cavity. The extent of deformity in PE can be extremely varied. In severe cases, a reduction in exercise capacity as evidenced by chest discomfort and dyspnoea on exertion can present as a result of compromised cardiopulmonary function (1-3). Aside from reduced exercise capacity being a strong indication for surgical repair of PE, many will also offer surgery on account of the condition’s negative effects on physical appearance, self-esteem and social interaction (4,5).
In general, there are two surgical approaches: open and minimally invasive. Historically, the Ravitch procedure involved a thoracic midline vertical incision, cartilage resection, xiphoid excision and a transverse external sternal osteotomy (6). The Nuss procedure, which is a minimally invasive repair of PE (MIRPE), was reported in 1998 (7). It involved placement of a substernal metal bar via lateral thoracic incisions under thoracoscopic guidance. MIRPE eventually gained popularity as it was shown to result in shorter operative times and decreased blood loss (8,9), although a second short surgery is required to remove the implanted bar (10).
According to the World Health Organization (WHO), quality of life is defined as a “state of complete physical, mental and social well-being” (11). Given that body image is a cornerstone of personal satisfaction and interpersonal communication, it is therefore a major determinant of one’s overall well-being and consequently, the quality of life. Although the MIRPE is a well-received procedure, its actual impact on quality of life is difficult to measure. Current evidence on this subject is also scarce. In this study, we aim to test our hypothesis that MIRPE enhances psychosocial well-being in patients, thereby improving their quality of life. The meta-analyses further verified that MIRPE improves important psychosocial aspects of quality of life, such as self-esteem and participation in social activities. We present this article in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) reporting checklist (12) (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1647/rc).
Methods
The study protocol was registered with the international prospective register of systematic reviews (PROSPERO) under reference number CRD42020222061. As the study progressed, the protocol was revised to optimize the quality and quantity of studies included, without any change to the primary outcome of the study. In the interest of maintaining scientific integrity, the authors did not retrospectively alter the initial PROSPERO registration. A comprehensive search was conducted independently by two authors (J.S.M. and J.W.T.) in PubMed, Cochrane, EMBASE and Scopus from the date of inception to November 23, 2020. Combinations and permutations of medical subject headings (MeSH), EMBASE (Emtree) terms (funnel chest, surveys and questionnaires, quality of life) and keywords (pectus excavatum, funnel breast, hollow chest, Ravitch, Nuss, MIRPE, minimally invasive repair, quality of life, QOL, HRQOL, health questionnaire, outcomes, survey, esteem, function) were used. The complete search strategy is depicted in Table S1.
Study selection
Eligible study types were cohort studies, case-control studies and randomized controlled trials. Inclusion criteria comprised studies from any surgical teams which administered one or more assessment questionnaires on patients, parents or both, to assess the quality of life before and after MIRPE. Although MIRPE is typically performed in children and young adults, we noted that middle-aged adults have also undergone PE repair. In order not to miss out on any meaningful data on the impact of MIRPE on quality of life, studies reporting on patients of up to 60 years old at the time of surgery (defined as the upper age limit for middle-aged adults) were included. In addition, manuscripts not written in English, conference abstracts, articles without any primary data, reviews, studies that reported combined data on PE and other deformities were excluded. References of the selected articles were also reviewed to identify additional relevant studies.
Data extraction and management
Two reviewers (J.S.M. and J.W.T.) independently screened the titles and abstracts of the studies, and performed full text reviews of all included studies. The third reviewer (J.K.C.T.) served as the adjudicator in the event of disagreements. Two reviewers (J.S.M. and J.W.T.) extracted relevant data from each study: authors, year of publication, journal of publication, title, study design, number of patients, gender, age of patients at surgery, name of assessment questionnaire, timepoints of quality of life assessment, response rates of patients or parents who underwent quality of life assessment, and the outcomes of these assessments. Data was collected in a customized Excel file belonging to the authors.
Assessment of risk of bias
The Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) was used to evaluate the results of the included non-randomized studies (13). For each domain in the ROBINS-I, an assessment of either a low, moderate, serious or critical risk of bias was made. The Cochrane Risk of Bias assessment tool was used to assess randomized studies (14).
Statistical analyses
Meta-analyses were performed using Review Manager 5.44 (The Cochrane Collaboration, Copenhagen, Denmark). For each assessment questionnaire, values reported as medians were excluded as these results were assumed not to be normally distributed, and thus unsuitable for meta-analysis. Furthermore, not all questionnaires which reported medians provided sufficient information for the mean and standard deviations to be estimated. Finally, the effect of such estimations on the outcome of the meta-analyses was uncertain. A random effects model was used to generate Forest plots. To maximize precision of the analysis, questionnaires that employed different scales in measuring similar domains were evaluated by utilizing the standardized mean difference (SMD). Statistical heterogeneity was assessed by the I2 statistic, with values of ≤25%, 26–75% and >75% being classified as low, moderate, and high heterogeneity respectively (15). For high heterogeneity (I2>75%), sensitivity analysis (backward omission step-wise analysis) was conducted by eliminating one study sequentially to examine each individual study’s impact on I2. P values below 0.05 indicated statistical significance.
Results
Results of literature search
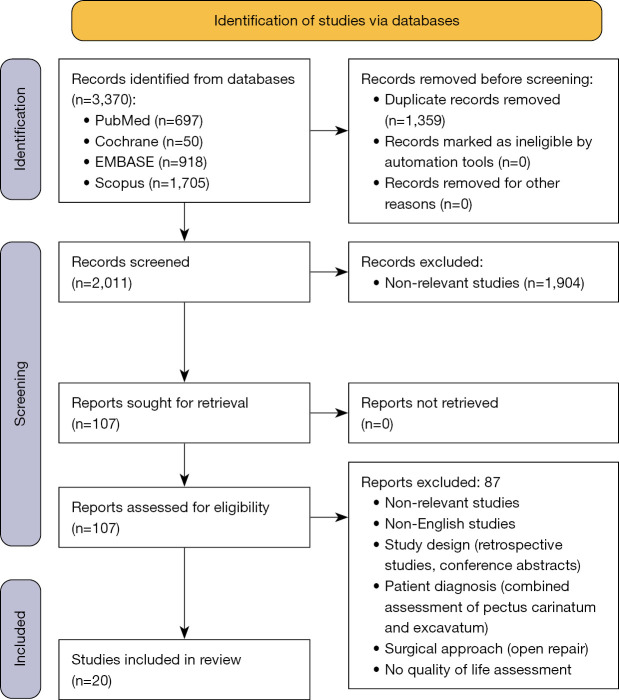
The search identified 3,370 articles, of which 2,011 remained after removal of duplicates. Title and abstract screening resulted in 1,904 excluded articles and the full texts of 107 articles were examined. Among these, 71 articles were excluded as they did not report on quality of life. Out of the remaining articles, 16 were excluded due to the following reasons: lack of at least one questionnaire, lack of assessment either before or after MIRPE, quality of life assessment performed on parties other than patients or parents, combined assessment of pectus carinatum and excavatum, conference abstracts, and non-English articles. Twenty studies were eventually included in the systematic review, of which 8 were included in the meta-analysis (Figure 1).
Figure 1.
PRISMA flow diagram.
Study characteristics
Table 1 shows the characteristics of the 20 included studies (16-35). Eighteen studies were fully prospective while two studies involved a retrospective review of patient records prior to prospective assessment of quality of life. Of the 20 studies, 17 were cohort studies, 2 were case-control studies and 1 was a randomized controlled trial. Fifteen studies were performed in a single institution, whereas the remaining five were multicenter studies. Two studies compared open surgery versus MIRPE, while the other 18 reported only on MIRPE. A total of 2,023 patients underwent MIRPE, and majority were males. Most of the patients underwent surgery as young adults, and only two studies involved patients who were ≥40 years old at the time of surgery. Six studies reported on the number of inserted bars, with a single bar being more common than two bars. The bar(s) was kept in-situ for around 3 years in the seven studies that described it.
Table 1. Characteristics of included studies.
| Author and year | Design | Country (single or multicenter) | Surgery and number of patients | % (male) | Age at surgery (years) | Number of metal bars inserted | Duration of bar in-situ |
|---|---|---|---|---|---|---|---|
| Lawson 2003 (16) | PCS | USA (multicenter) | MIRPE: 19 | NR | 8–18 | NR | NR |
| Roberts 2003 (17) | PCS | Canada (single) | MIRPE: 5 | NR | NR | NR | NR |
| Krasopoulos 2006 (18) | PCS | UK (single) | MIRPE: 20 | 100% | 18 [14–37] | 1 bar: 19 2 bars: 1 |
NR |
| Metzelder 2007† (19) | PCS | Germany (single) | MIRPE: 45 | 65% | 13.5 [6–20] | 1 bar: 45 2 bars: 0 |
31 [23–39] months |
| Lam 2008 (20) | PCS | Canada (single) | MIRPE: 19; open: 24 |
88% | 15.4±2.2 (MIRPE), 15.1±1.9 (open) |
NR | NR |
| Kelly 2008 (21) | PCS | USA (multicenter) | MIRPE: 283; open: 43 |
85% | 4–21 | NR | NR |
| Jacobsen 2010† (22) | PCC | Denmark (single) | MIRPE: 172 | 59% | 16.4±2.5 | NR | NR |
| Hadolt 2011† (23) | PCS | Austria (single) | MIRPE: 17 | 76% | 15.6±2.5 | NR | 3 years |
| Kim 2011† (24) | PCS | Korea (single) | MIRPE: 61 | NR | 6.8±3.2 | NR | 2–3 years |
| Hanna 2013 (25) | PCS | Canada (single) | MIRPE: 73 | 89% | 20 [16–51] | 1 bar: 59 2 bars: 14 |
3 years |
| Kuru 2015† (26) | PCS | Turkey (single) | MIRPE: 80 | 85% | 16.91±4.37 | NR | NR |
| Kuru 2015 (27) | PCS | Turkey (single) | MIRPE: 88 | 85.2% | 18.44±3.93 | NR | NR |
| Lomholt 2016† (28) | PCC | Denmark (single) | MIRPE: 107 | 87% | 15.3±1.8 (M), 13.2±1.9 (F) |
NR | NR |
| Sacco Casamassima 2016 (29) | PCS | USA (single) | MIRPE: 132 | 74.5% | 30.9 [21.8–55.1] | 1 bar: 122 2 bars: 10 |
32.9±16.9 months |
| Gibreel 2016 (30) | PCS | USA (single) | MIRPE: 313 | 79% | 15±3 | NR | 2.4–3.8 years |
| Luo 2017 (31) | PCS | China (single) | MIRPE: 266 | 85.7% | 19.02±4.42 | NR | NR |
| Zuidema 2018† (32) | PCS | Netherlands (multicenter) | MIRPE: 131 | 86.2% | 16.1±2.3 | NR | NR |
| Zuidema 2019 (33) | PCS | Netherlands (multicenter) | MIRPE: 54 | 88.8% | 17.9 [16–29.4] | NR | 3 years |
| Zuidema 2020† (34) | PCS | Netherlands (multicenter) | MIRPE: 108 | 87% | 16±2.20 | 1 bar: 99 2 bars: 9 |
NR |
| de Carvalho 2021 (35) | RCT | Brazil | MIRPE: 30 | 90% | 17±3.3 | 1 bar: 27 2 bars: 3 |
NR |
Data has been presented as a range, mean/median [range] or mean ± SD, depending on what was reported in the original study. †, studies included in meta-analyses. PCS, prospective cohort study; MIRPE, minimally invasive repair of pectus excavatum; NR, not reported; PCC, prospective case-control study; RCT, randomized controlled trial; SD, standard deviation.
Assessment of the risk of bias
Out of the 19 non-randomized studies, 17 were cohort studies and 2 were case-control studies (Table 2). All studies had a low risk of confounding as there was only one intervention received, namely MIRPE. In the selection of participants, five studies were deemed to have a moderate risk of bias as the assessments of quality of life were conducted only after MIRPE. With respect to classification of the intervention and deviations from intended interventions, the risks of bias were low as MIRPE was uniformly performed in all studies. Six studies had a moderate risk of bias due to missing outcome data as the response rate for questionnaires were less than 80%. By nature of their study design, the two case-control studies were assessed to have a moderate risk of bias in the measurement of outcome as the reported quality of life may have been influenced by the researchers’ knowledge of whether the patients belonged to the intervention or control group. All 19 studies were deemed to have a low risk of bias in the selection of the reported results as the questionnaires corresponded to the intended assessment of quality of life.
Table 2. Risk of bias assessment with ROBINS-I for non-randomized studies.
| Author and year | D1 | D2 | D3 | D4 | D5 | D6 | D7 |
|---|---|---|---|---|---|---|---|
| Lawson 2003 (16) | Low | Low | Low | Low | Low | Low | Low |
| Roberts 2003 (17) | Low | Low | Low | Low | Low | Low | Low |
| Krasopoulos 2006 (18) | Low | Low | Low | Low | Low | Low | Low |
| Metzelder 2007 (19) | Low | Moderate | Low | Low | Low | Low | Low |
| Lam 2008 (20) | Low | Moderate | Low | Low | Moderate | Low | Low |
| Kelly 2008 (21) | Low | Low | Low | Low | Low | Low | Low |
| Jacobsen 2010† (22) | Low | Low | Low | Low | Moderate | Moderate | Low |
| Hadolt 2011 (23) | Low | Low | Low | Low | Low | Low | Low |
| Kim 2011 (24) | Low | Low | Low | Low | Moderate | Low | Low |
| Hanna 2013 (25) | Low | Low | Low | Low | Moderate | Low | Low |
| Kuru 2015 (26) | Low | Low | Low | Low | Low | Low | Low |
| Kuru 2015 (27) | Low | Low | Low | Low | Low | Low | Low |
| Lomholt 2016† (28) | Low | Low | Low | Low | Moderate | Moderate | Low |
| Sacco Casamassima 2016 (29) | Low | Moderate | Low | Low | Low | Low | Low |
| Gibreel 2016 (30) | Low | Moderate | Low | Low | Moderate | Low | Low |
| Luo 2017 (31) | Low | Low | Low | Low | Low | Low | Low |
| Zuidema 2018 (32) | Low | Low | Low | Low | Low | Low | Low |
| Zuidema 2019 (33) | Low | Low | Low | Low | Low | Low | Low |
| Zuidema 2020 (34) | Low | Moderate | Low | Low | Low | Low | Low |
D1: bias due to confounding; D2: bias in selection of participants; D3: bias in classification of the intervention; D4: bias due to deviations from intended interventions; D5: bias due to missing outcome data; D6: bias in measurement of outcome; D7: bias in selection of reported result. †, case control studies.
For the single randomized controlled trial, we noted that the randomization was with respect to the stabilizers utilized, and not whether the patients underwent MIRPE. However, as the study design is that of a randomized controlled trial, we used the Cochrane Risk of Bias assessment tool and determined that the study had a low risk of selection, attrition and reporting bias (Figure 2). The study had a high risk of performance and detection bias, which we attributed to the difficulty in achieving blinding in trials that involve surgical procedures.
Figure 2.
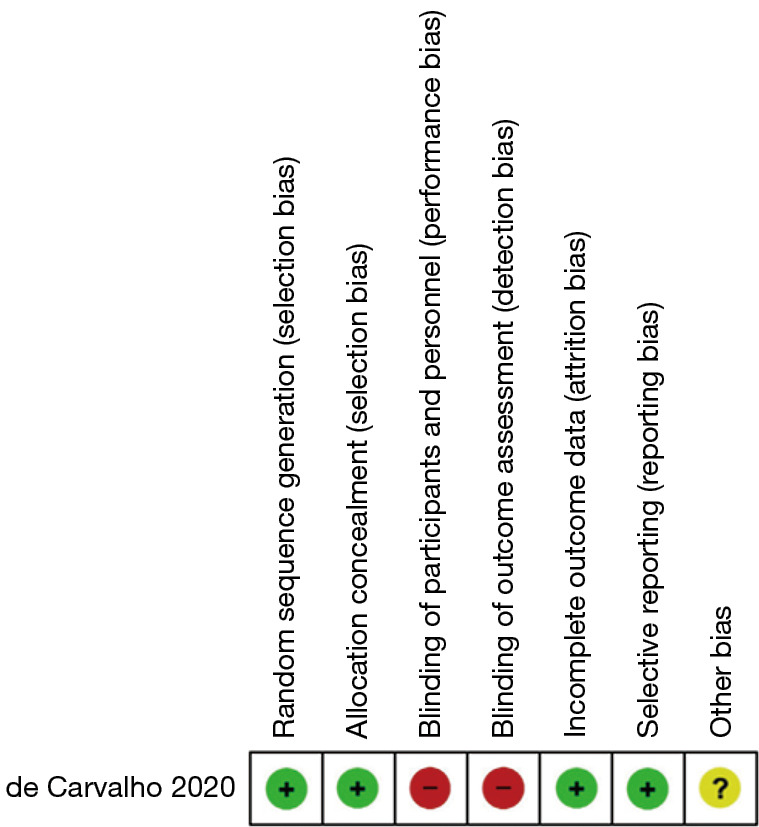
Risk of bias assessment for randomized controlled trial. +, high risk; −, low risk; ?, unclear.
Systematic review of quality of life questionnaires
Of those who underwent MIRPE, a total of 1,623 patients and 727 parents were administered at least one quality of life assessment. There were twelve different questionnaires identified across the included studies (Table 3). However, only three were developed to assess the impact of PE on quality of life, namely the Pectus Excavatum Evaluation Questionnaire (PEEQ), Nuss Questionnaire modified for Adults (NQ-mA) and Single Step Questionnaire (SSQ).
Table 3. QoL assessment.
| Author and year | QoL questionnaire | Number assessed (patients/parents) | Preoperative QoL assessment | Postoperative QoL assessment |
|---|---|---|---|---|
| Lawson 2003 (16) | PEEQ | 19/22 | Yes | 6–12 months |
| Roberts 2003 (17) | KSQOL | 5/5 | No | 6–9 months |
| Krasopoulos 2006 (18) | NQ-mA, SSQ | 20/0 | Yes | 5 months |
| Metzelder 2007 (19) | SSQ | 40/39 | No | 6 months, 2–3 years |
| Lam 2008 (20) | PEEQ, CHQ-CF87 | 23/0 (MIRPE), 11/0 (open) | No | 14.1±11.5 months (MIRPE), 15.3±9.2 months (open) |
| Kelly 2008 (21) | PEEQ | 264/291 | Yes | 12 months |
| Jacobsen 2010 (22) | CHQ-CF87 (patients only), CHQ-PF50 (parents only), NQ-mA, SSQ | 119/119 | No | 6–30 months |
| Hadolt 2011 (23) | OEQ, FBCS, SCL-90-R | 17/17 | Yes | 4 years |
| Kim 2011 (24) | KSQOL | 39/39 | Yes | 5 years |
| Hanna 2013 (25) | SSQ | 51/0 | Yes | Exact timepoint NR |
| Kuru 2015 (26) | NQ-mA | 80/80 | Yes | 6 months |
| Kuru 2015 (27) | NQ-mA | 88/0 | Yes | 6 months |
| Lomholt 2016 (28) | CHQ-CF87 (patients only), CHQ-PF50 (parents only) |
85/85 | Yes | 3 months, 6 months |
| Sacco Casamassima 2016 (29) | SSQ | 39/0 | No | 32.9±16.9 months |
| Gibreel 2016 (30) | PEEQ | 145/0 | No | 7.1 (0.1–15.7) years |
| Luo 2017 (31) | SCL-90 | 266/0 | Yes | 12 months |
| Zuidema 2018 (32) | WHO-QOLbref (23 patients), CHQ-CF87 (82 patients), both (26 patients) |
131/0 | Yes | 6 weeks, 6 months |
| Zuidema 2019 (33) | SF-36 | 54/0 | Yes | 12 months |
| Zuidema 2020 (34) | SSQ | 108/0 | No | 6 weeks, 6 months, 12 months, 24 months |
| de Carvalho 2021 (35) | PEEQ | 30/30 | Yes | 6 months |
QoL, quality of life; PEEQ, Pectus Excavatum Evaluation Questionnaire; KSQOL, Keith-Schalock’s Quality Of Life questionnaire; NQ-mA, Nuss Questionnaire modified for Adults; SSQ, Single Step Questionnaire; CHQ-CF87, Child Health Questionnaire-Child Form 87; CHQ-PF50, Child Health Questionnaire-Parent Form 50; OEQ, Operation Expectation Questionnaire; FBCS, Frankfurter Body Concept Scales; SCL-90-R, Symptom Checklist-90-Revised; SCL-90, Symptom Checklist-90; WHO-QOLbref, World Health Organisation Quality of Life; SF-36, 36-item Short Form survey.
The PEEQ was the pioneer PE-specific questionnaire introduced by Lawson et al. (16). It has both child and parent versions and the responses operate on a 4-point Likert scale. All five studies utilizing the PEEQ suggested that MIRPE had a positive impact on psychosocial and physical well-being, of which three studies demonstrated an improvement from preoperative to postoperative scores. Lawson et al. (16), Kelly et al. (21) and de Carvalho et al. (35) included parents in their studies and reported that their concern about the effects of PE on their children diminished post-MIRPE. Krasopoulos et al. (18) subsequently modified the PEEQ into the NQ-mA to enable summation of scores, with a higher total score indicating a better quality of life. The NQ-mA was otherwise similar to the PEEQ, with only minor changes to direct questions at adults instead of children. The total scores in the four studies which used the NQ-mA all showed a significant increase postoperatively, suggesting that MIRPE had a positive impact on quality of life.
As its name implies, the SSQ provides information on both preoperative and postoperative outcomes despite only a single administration. Krasopoulos et al. (18) also opined that the SSQ can be used to assess satisfaction with MIRPE by its overall score. In all six studies with the SSQ, patients had high levels of satisfaction. In addition, there was an improvement in patients’ self-esteem and the extent which their chests interfered with social activity. Zuidema et al. (34) demonstrated that these improvements were consistent even when the SSQ was administered at four different timepoints post-MIRPE. Although patients experienced at least moderate pain during the hospital stay, Metzelder et al. (19), Hanna et al. (25) and Sacco Casamassima et al. (29) concluded that pain became mild or absent after the metal bar was explanted.
A total of four studies utilized the Child Health Questionnaire-Child Form 87 (CHQ-CF87), of which Jacobsen et al. (22) and Lomholt et al. (28) additionally administered the Child Health Questionnaire-Parent Form 50 (CHQ-PF50) on parents. Both the CF87 and PF50 are generic tools designed to evaluate the physical, emotional, behavioral and social domains of health, with a higher score indicating better well-being. Lomholt et al. (28) and Zuidema et al. (32) demonstrated that the domain scores increased post-MIRPE and this trend was sustained at 6 months postoperatively. Lam et al. (20) showed there was no difference in the CHQ mean scores between MIRPE and open repair, but did not compare scores before and after MIRPE. Similarly, the case-control study by Jacobsen et al. (22) showed higher CHQ scores in the MIRPE group but there was no preoperative comparison.
The assessment questionnaire based on Keith-Schalock’s Quality of Life (KSQOL) model was used in two studies to assess the following: satisfaction, social belonging, empowerment and well-being. Roberts et al. (17) administered the questionnaire on a small group of 5 patients and their parents, both preoperatively and postoperatively, and found improvements in all four domains. In the study by Kim et al. (24) which employed a modified version of the questionnaire, similar results were shown. The Symptom Checklist-90 (SCL-90), which evaluates symptoms of mental disorders, was also utilized in 2 other studies. Luo et al. (31) showed that symptoms decreased after MIRPE while Hadolt et al. (23) only reported that data from the SCL-90 were in the normal range. The World Health Organisation Quality of Life (WHO-QOLbref), Operation Expectation Questionnaire (OEQ), Frankfurter Body Concept Scale (FBCS) and 36-item Short Form survey (SF-36) were each utilized in only one study. In all of these studies, the results suggested that patients who underwent MIRPE experienced an improvement in quality of life.
Despite the diversity of questionnaires, there was consistency in choosing the 6th postoperative month as a suitable timepoint for quality of life assessment. Twelve studies administered the questionnaires at the 6th postoperative month while the rest did so at various time points from the 12th postoperative month. Out of the 20 included studies, only 6 studies did not have a true preoperative questionnaire administration. However, preoperative scores could be inferred if the SSQ was utilized.
Meta-analysis of included studies
The meta-analysis was conducted in two stages: firstly, comparing patient responses for the same questionnaire across multiple studies, and secondly, comparing patient responses to similar themes across different questionnaires. A total of eight studies that utilized five different questionnaires (SSQ, NQ-mA, CHQ-CF87, KSQOL and FBCS) were included in the meta-analysis. The OEQ, SCL-90 and SCL-90-R were excluded as their content were not directly related to quality of life. The PEEQ was also not included as the scoring scale was in an opposite direction to other questionnaires. The reported scores for the SF-36 lacked standard deviation values, and were thus excluded. Of the two studies that utilized the CHQ-PF50, although one reported mean and standard deviation values, the number of parents assessed was unclear, resulting in an unsuitability for meta-analysis of parental scores. We attempted to contact the respective authors to obtain the required data but were unsuccessful.
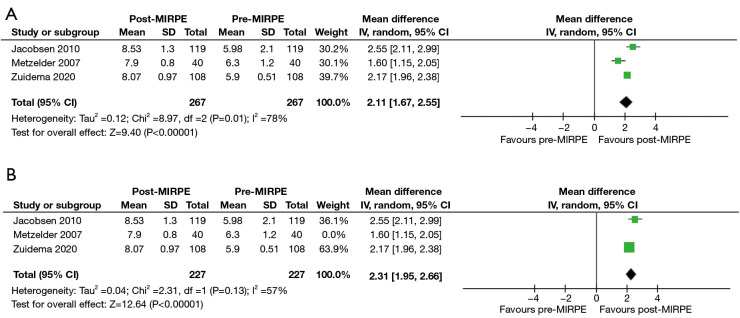
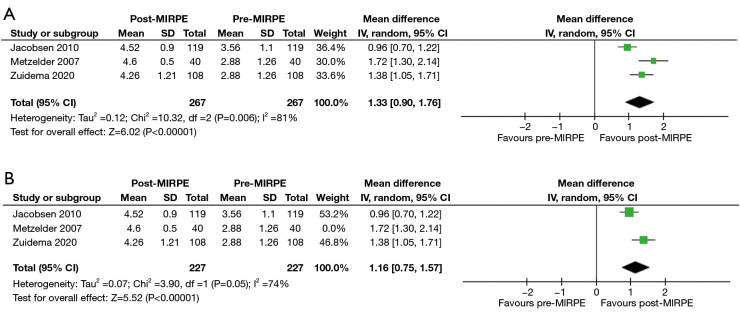
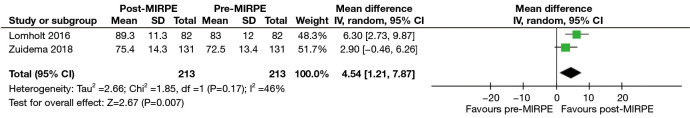
In the initial stage, only the SSQ and CHQ-CF87 were assessed as the mean and standard deviation scores were available in multiple studies. For the SSQ, “self-esteem” and “extent of interference with social activities” were found suitable for analysis due to the availability of preoperative and postoperative scores. It must be noted that preoperative scores are a part of the SSQ, despite it being administered only once postoperatively. Three studies with a total of 267 patients revealed a statistically significant improvement in self-esteem after MIRPE [mean difference (MD): 2.11, 95% confidence interval (CI): 1.67 to 2.55, P<0.00001] (Figure 3A,3B). The I2 statistic was 78% which represented high statistical heterogeneity. Omission of the outlier study by Metzelder et al. (19) resulted in an improved I2 statistic of 57%, with the improvement in self-esteem still remaining statistically significant (MD: 2.31, 95% CI: 1.95 to 2.66, P<0.00001). An improvement in the extent of interference with social activities was also observed post-MIRPE (MD: 1.33, 95% CI: 0.90 to 1.76, P<0.00001) (Figure 4A,4B). Similarly, removal of the study by Metzelder et al. (19) reduced heterogeneity to a moderate level with an I2 statistic of 74%. For the CHQ-CF87 questionnaire, two studies with a total of 213 patients provided data on “self-esteem”, “emotional limitation” and “general health”. A higher self-esteem was observed in patients post-MIRPE (MD: 4.54, 95% CI: 1.21 to 7.87, P=0.007) (Figure 5). However, there was no significant difference in emotional limitation (MD: 4.70, 95% CI: −1.86 to 11.27, P=0.16) (Figure 6) and general health after MIRPE (MD: 0.23, 95% CI: −2.90 to 3.36, P=0.89) (Figure 7). Although I2 statistic was 86% in the meta-analysis for emotional limitation, sensitivity analysis was not feasible as there were only two studies included.
Figure 3.
Forest plots comparing self-esteem in the SSQ in patients who underwent MIRPE. (A) Main forest plot. (B) Forest plot with Metzelder 2007 excluded. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; SSQ, Single Step Questionnaire.
Figure 4.
Forest plots comparing extent of interference with social activities in the SSQ in patients who underwent MIRPE. (A) Main forest plot. (B) Forest plot with Metzelder 2007 excluded. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; SSQ, Single Step Questionnaire.
Figure 5.
Forest plot comparing self-esteem in the CHQ-CF87 in patients who underwent MIRPE. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; CHQ-CF87, Child Health Questionnaire-Child Form 87.
Figure 6.
Forest plot comparing emotional limitation in the CHQ-CF87 in patients who underwent MIRPE. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; CHQ-CF87, Child Health Questionnaire-Child Form 87.
Figure 7.
Forest plot comparing general health in the CHQ-CF87 in patients who underwent MIRPE. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; CHQ-CF87, Child Health Questionnaire-Child Form 87.
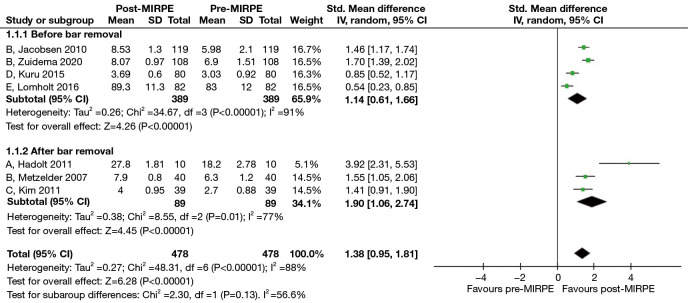
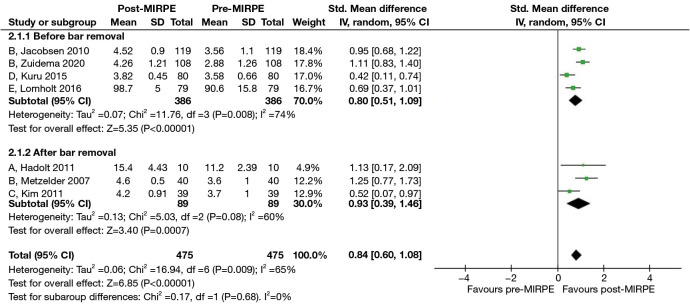
We noted that “self-esteem” and “extent of interference with social activities” were two common themes of quality of life across all the included questionnaires. Therefore, in the second stage, responses that fit within these two themes were pooled to perform a random effects meta-analysis. For authors who likely assessed the same patient population across multiple studies, such as Kuru et al. (27) and Zuidema et al. (34), the most recent study with questionnaire results suitable for meta-analysis was included. Upon analyzing seven studies with a total of 478 patients, we found a statistically significant improvement in self-esteem after MIRPE (SMD: 1.38, 95% CI: 0.95 to 1.81, P<0.00001) and an I2 statistic of 88% which suggested high heterogeneity (Figure 8). To eliminate the presence of the MIRPE bar as a confounder, we segregated the data into before and after bar removal, and repeated the meta-analysis. Based on the results of four studies with a total of 389 patients, self-esteem was still significantly better after MIRPE despite the bar being in-situ (SMD: 1.14, 95% CI: 0.61 to 1.66, P<0.0001). Similar findings were observed in the analysis of patients who had the bar removed (SMD: 1.90, 95% CI: 1.06 to 2.74, P<0.00001), with a significant reduction in I2 to 77%. For the extent of interference with social activities, there was a statistically significant improvement after MIRPE (SMD: 0.84, 95% CI: 0.60 to 1.08, P<0.00001) with an I2 value of 65% (Figure 9). The analysis showed that findings before bar removal (SMD: 0.80, 95% CI: 0.51 to 1.09, P<0.00001) and after bar removal (SMD: 0.93, 95% CI: 0.39 to 1.46, P=0.0007) were similar. It was also observed that statistical heterogeneity was significantly reduced to I2 of 60% when analyzing the subgroup of studies after bar removal.
Figure 8.
Forest plots comparing self-esteem across all questionnaires in patients who underwent MIRPE. A: self-acceptance of one’s body, FBCS; B: self-esteem, SSQ; C: patient’s self-confidence, KSQOL; D: feels shy/self-conscious because of chest, NQ-mA; E: self-esteem, CHQ-CF87. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; FBCS, Frankfurter Body Concept Scale; SSQ, Single Step Questionnaire; KSQOL, Keith-Schalock’s Quality of Life questionnaire; NQ-mA, Nuss Questionnaire modified for Adults; CHQ-CF87, Child Health Questionnaire-Child Form 87.
Figure 9.
Forest plots comparing extent of interference with social activities across all questionnaires in patients who underwent MIRPE. A: acceptance of one’s body by others, FBCS; B: chest interference with social activities, SSQ; C: participation in activities with friends, KSQOL; D: others making fun of him/her because of chest, NQ-mA; E: emotional limitation, CHQ-CF87. MIRPE, minimally invasive repair of pectus excavatum; SD, standard deviation; IV, initialization vector; CI, confidence interval; FBCS, Frankfurter Body Concept Scale; SSQ, Single Step Questionnaire; KSQOL, Keith-Schalock’s Quality of Life questionnaire; NQ-mA, Nuss Questionnaire modified for Adults; CHQ-CF87, Child Health Questionnaire-Child Form 87.
Discussion
PE occurs in about 1 out of every 400 individuals (36). Studies have reported that patients with PE suffer from body image dissatisfaction and thus avoid social situations (37-39). To date, there has been a growing number of studies indicating the positive effects of MIRPE on quality of life. Quality of life is a multi-dimensional concept with variable definitions, and psychosocial health has consistently been cited as a key determinant (40-42). However, interpretation of quality of life is often subjective. Therefore, we performed the first systematic review and meta-analysis aimed at summarizing the most pertinent evidence on the impact of MIRPE on quality of life, with emphasis on objective longitudinal effects as measured through questionnaires.
Studies have suggested that the use of disease-specific measures increases the likelihood of showing the effects of pectus correction (43,44). To the best of our knowledge, only three questionnaires specific to PE, the PEEQ, NQ-mA and SSQ, have been published. In particular, the SSQ has high efficiency in longitudinal assessment, given that it splits responses for certain questions into preoperative and postoperative scores. Although the other included questionnaires were not validated specifically for PE, we noted that self-esteem and participation in social activities were psychosocial domains that would encompass most of the content in all questionnaires.
A consistent improvement from preoperative to postoperative scores for self-esteem and extent of chest interference with social activities was observed in the present study. The results also showed that patients who underwent MIRPE experienced an overall improvement in quality of life. The positive impact of MIRPE can be explained by its immediate elimination of the pectus deformity. With such a dramatic improvement in physical appearance, it is reasonable to expect self-confidence to improve significantly. This would translate into a reduction in avoidance and concealment behaviors, which would then lead to an increased willingness to engage in social situations (45,46). Ultimately, patients would experience an improved quality of life.
In the assessment of quality of life, pain must also be considered due to its deleterious effects on psychosocial outcomes (47,48). Although it has several advantages over open repair, literature has shown that perioperative pain from MIRPE is comparable to that of the former (49,50). It is therefore remarkable that despite the presence of pain, self-esteem and participation in social activities were all shown to have improved post-MIRPE. The reason why postoperative pain did not translate into a reduced quality of life is likely multifactorial. Based on the present study, it cannot be ruled out that postoperative pain from MIRPE is outweighed by chronic musculoskeletal pain. More studies are therefore needed to fully elucidate the relationship between pain and quality of life in pectus patients who undergo MIRPE. These analyses would be of great relevance, particularly in the era of an increasing emphasis on enhancing recovery after surgery.
From a surgical perspective, the MIRPE is best performed in adolescence when cartilages are malleable (51-53). The adolescent period is also when body image is perceived to be of paramount importance due to its implications on peer acceptance, career building and family planning. However, despite being minimally invasive, MIRPE is still associated with complications such as bar displacement, infection and pneumothorax (54). Therefore, it is imperative to present prospective patients and parents with the physical and psychosocial benefits of MIRPE, aside from cosmesis, in order to justify the conduct of the surgery.
The present study has a few limitations. Firstly, although the included studies were prospectively designed, there was a lack of high-quality evidence due to paucity of randomized controlled trials. It is important to note that the study by de Carvalho et al. was only randomized on the type of stabilizers used. Therefore, the included studies are prone to multiple confounders, such as the severity of PE. Additionally, there was significant statistical heterogeneity in the reported outcomes. We posit that this is due to the variability of questionnaires and the timepoints of administration. We attempted to mitigate this by grouping questions under common themes, and performing subgroup analysis stratified by the presence or removal of the implanted bar. The results of the meta-analyses were also subject to selection and responder-recall bias, especially since response rates for the questionnaires were varied. Finally, we propose for future research to focus on the interactions between the psychosocial and physiological outcomes of MIRPE. Although the questionnaires explored physiological outcomes like exercise capacity, comprehensive analysis was not possible due to the lack of relevant data such as pulmonary function tests and echocardiography parameters.
Conclusions
In conclusion, MIRPE is associated with a better quality of life for patients with PE. Furthermore, psychosocial outcomes, specifically self-esteem and extent of chest interference in social activities, are improved after the procedure, and even after eventual bar removal.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1647/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1647/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1647/coif). J.K.C.T. serves as an unpaid editorial board member of Annals of Translational Medicine from November 2022 to October 2024. The other authors have no conflicts of interest to declare.
References
- 1.Zens TJ, Casar Berazaluce AM, Jenkins TM, et al. The Severity of Pectus Excavatum Defect Is Associated With Impaired Cardiopulmonary Function. Ann Thorac Surg 2022;114:1015-21. 10.1016/j.athoracsur.2021.07.051 [DOI] [PubMed] [Google Scholar]
- 2.Casatori L, Pellegrino A, Messineo A, et al. Differential Influence of Physical Activity on Cardiopulmonary Performance and Stroke Volume Assessed at Cardiopulmonary Exercise Test in Pectus Excavatum: A Pilot Study. Front Physiol 2022;13:831504. 10.3389/fphys.2022.831504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaroszewski DE, Farina JM, Gotway MB, et al. Cardiopulmonary Outcomes After the Nuss Procedure in Pectus Excavatum. J Am Heart Assoc 2022;11:e022149. 10.1161/JAHA.121.022149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabbaj R, Burnier M, Kohler R, et al. Minimally invasive repair of pectus excavatum using the Nuss technique in children and adolescents: indications, outcomes, and limitations. Orthop Traumatol Surg Res 2014;100:625-30. 10.1016/j.otsr.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 5.Krasopoulos G, Goldstraw P. Minimally invasive repair of pectus excavatum deformity. Eur J Cardiothorac Surg 2011;39:149-58. 10.1016/j.ejcts.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 6.Ravitch MM. The Operative Treatment of Pectus Excavatum. Ann Surg 1949;129:429-44. 10.1097/00000658-194904000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuss D, Kelly RE, Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. 10.1016/s0022-3468(98)90314-1 [DOI] [PubMed] [Google Scholar]
- 8.Toci GR, Davis TA, Bigelow BF, et al. Analyzing Outcomes of Nuss and Ravitch Repair for Primary and Recurrent Pectus Excavatum in Adults. Ann Thorac Surg 2020;110:272-5. 10.1016/j.athoracsur.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 9.Mao YZ, Tang S, Li S. Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: an updated meta-analysis. J Pediatr Surg 2017;52:1545-52. 10.1016/j.jpedsurg.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 10.Janssen N, Daemen JHT, Ashour O, et al. Nuss bar removal without straightening is a safe technique: a single center experience. J Thorac Dis 2022;14:3335-42. 10.21037/jtd-22-725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Post MW. Definitions of quality of life: what has happened and how to move on. Top Spinal Cord Inj Rehabil 2014;20:167-80. 10.1310/sci2003-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson ML, Cash TF, Akers R, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg 2003;38:916-8. 10.1016/s0022-3468(03)00123-4 [DOI] [PubMed] [Google Scholar]
- 17.Roberts J, Hayashi A, Anderson JO, et al. Quality of life of patients who have undergone the Nuss procedure for pectus excavatum: Preliminary findings. J Pediatr Surg 2003;38:779-83. 10.1016/jpsu.2003.50166 [DOI] [PubMed] [Google Scholar]
- 18.Krasopoulos G, Dusmet M, Ladas G, et al. Nuss procedure improves the quality of life in young male adults with pectus excavatum deformity. Eur J Cardiothorac Surg 2006;29:1-5. 10.1016/j.ejcts.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 19.Metzelder ML, Kuebler JF, Leonhardt J, et al. Self and parental assessment after minimally invasive repair of pectus excavatum: lasting satisfaction after bar removal. Ann Thorac Surg 2007;83:1844-9. 10.1016/j.athoracsur.2006.12.064 [DOI] [PubMed] [Google Scholar]
- 20.Lam MW, Klassen AF, Montgomery CJ, et al. Quality-of-life outcomes after surgical correction of pectus excavatum: a comparison of the Ravitch and Nuss procedures. J Pediatr Surg 2008;43:819-25. 10.1016/j.jpedsurg.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 21.Kelly RE, Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. 10.1542/peds.2007-2723 [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen EB, Thastum M, Jeppesen JH, et al. Health-related quality of life in children and adolescents undergoing surgery for pectus excavatum. Eur J Pediatr Surg 2010;20:85-91. 10.1055/s-0029-1243621 [DOI] [PubMed] [Google Scholar]
- 23.Hadolt B, Wallisch A, Egger JW, et al. Body-image, self-concept and mental exposure in patients with pectus excavatum. Pediatr Surg Int 2011;27:665-70. 10.1007/s00383-011-2854-z [DOI] [PubMed] [Google Scholar]
- 24.Kim HK, Shim JH, Choi KS, et al. The quality of life after bar removal in patients after the nuss procedure for pectus excavatum. World J Surg 2011;35:1656-61. 10.1007/s00268-011-1111-x [DOI] [PubMed] [Google Scholar]
- 25.Hanna WC, Ko MA, Blitz M, et al. Thoracoscopic Nuss procedure for young adults with pectus excavatum: excellent midterm results and patient satisfaction. Ann Thorac Surg 2013;96:1033-6; discussion 1037-8. 10.1016/j.athoracsur.2013.04.093 [DOI] [PubMed] [Google Scholar]
- 26.Kuru P, Dudakli A, Mursaloglu H, et al. How pulmonary function changes after pectus excavatum correction surgery. Asian Cardiovasc Thorac Ann 2015;23:945-9. 10.1177/0218492315596464 [DOI] [PubMed] [Google Scholar]
- 27.Kuru P, Bostanci K, Ermerak NO, et al. Quality of life improves after minimally invasive repair of pectus excavatum. Asian Cardiovasc Thorac Ann 2015;23:302-7. 10.1177/0218492314553442 [DOI] [PubMed] [Google Scholar]
- 28.Lomholt JJ, Jacobsen EB, Thastum M, et al. A prospective study on quality of life in youths after pectus excavatum correction. Ann Cardiothorac Surg 2016;5:456-65. 10.21037/acs.2016.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacco Casamassima MG, Gause C, Goldstein SD, et al. Patient Satisfaction After Minimally Invasive Repair of Pectus Excavatum in Adults: Long-Term Results of Nuss Procedure in Adults. Ann Thorac Surg 2016;101:1338-45. 10.1016/j.athoracsur.2015.09.102 [DOI] [PubMed] [Google Scholar]
- 30.Gibreel W, Zendejas B, Joyce D, et al. Minimally Invasive Repairs of Pectus Excavatum: Surgical Outcomes, Quality of Life, and Predictors of Reoperation. J Am Coll Surg 2016;222:245-52. 10.1016/j.jamcollsurg.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 31.Luo L, Xu B, Wang X, et al. Intervention of the Nuss Procedure on the Mental Health of Pectus Excavatum Patients. Ann Thorac Cardiovasc Surg 2017;23:175-80. 10.5761/atcs.oa.17-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuidema WP, Oosterhuis JWA, Zijp GW, et al. Early Consequences of Pectus Excavatum Surgery on Self-Esteem and General Quality of Life. World J Surg 2018;42:2502-6. 10.1007/s00268-018-4526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuidema WP, Oosterhuis JWA, van der Heide SM, et al. Early cost-utility estimation of the surgical correction of pectus excavatum with the Nuss bar. Eur J Cardiothorac Surg 2019;55:699-703. 10.1093/ejcts/ezy348 [DOI] [PubMed] [Google Scholar]
- 34.Zuidema WP, van der Steeg AFW, van der Heide S, et al. The Outcome of the Single Step Questionnaire in Pectus Excavatum Patients is Phase Dependent. Eur J Pediatr Surg 2020;30:205-9. 10.1055/s-0039-1681025 [DOI] [PubMed] [Google Scholar]
- 35.de Carvalho RLC, Tedde ML, de Campos JRM, et al. Quality of life outcomes after minimally invasive repair of pectus excavatum utilizing a new set of metallic bars and stabilizers. J Pediatr Surg 2021;56:545-9. 10.1016/j.jpedsurg.2020.06.036 [DOI] [PubMed] [Google Scholar]
- 36.Scalise PN, Demehri FR. The management of pectus excavatum in pediatric patients: a narrative review. Transl Pediatr 2023;12:208-20. 10.21037/tp-22-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng YL, Lan CC, Wu YK, et al. Poorer sleep quality among adult patients with pectus excavatum in Taiwan: A pilot study. J Thorac Cardiovasc Surg 2019;157:769-780.e1. 10.1016/j.jtcvs.2018.07.050 [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Luo L, Xiao LJ, et al. Psychological trauma of funnel chest in adolescents and the appropriate age for minimally invasive surgery repair. Chin Med J (Engl) 2013;126:2876-80. [PubMed] [Google Scholar]
- 39.Ji Y, Liu W, Chen S, et al. Assessment of psychosocial functioning and its risk factors in children with pectus excavatum. Health Qual Life Outcomes 2011;9:28. 10.1186/1477-7525-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilhena E, Pais-Ribeiro J, Silva I, et al. Psychosocial factors as predictors of quality of life in chronic Portuguese patients. Health Qual Life Outcomes 2014;12:3. 10.1186/1477-7525-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dezutter J, Casalin S, Wachholtz A, et al. Meaning in life: an important factor for the psychological well-being of chronically ill patients? Rehabil Psychol 2013;58:334-41. 10.1037/a0034393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiovitz-Ezra S, Leitsch S, Graber J, et al. Quality of life and psychological health indicators in the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci 2009;64 Suppl 1:i30-7. 10.1093/geronb/gbn020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solans M, Pane S, Estrada MD, et al. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. Value Health 2008;11:742-64. 10.1111/j.1524-4733.2007.00293.x [DOI] [PubMed] [Google Scholar]
- 44.Raat H, Mohangoo AD, Grootenhuis MA. Pediatric health-related quality of life questionnaires in clinical trials. Curr Opin Allergy Clin Immunol 2006;6:180-5. 10.1097/01.all.0000225157.67897.c2 [DOI] [PubMed] [Google Scholar]
- 45.Roberts J, MacMath S, English M, et al. Body Disfigurement and the Quality of Life of Adolescents with Pectus Excavatum: Effects of the Nuss Procedure. Physical Disabilities: Education and Related Services 2006;24:21-46. [Google Scholar]
- 46.Kent G. Understanding the experiences of people with disfigurements: An integration of four models of social and psychological functioning. Psychol Health Med 2000;5:117-29. 10.1080/713690187 [DOI] [PubMed] [Google Scholar]
- 47.Hadi MA, McHugh GA, Closs SJ. Impact of Chronic Pain on Patients' Quality of Life: A Comparative Mixed-Methods Study. J Patient Exp 2019;6:133-41. 10.1177/2374373518786013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niv D, Kreitler S. Pain and quality of life. Pain Pract 2001;1:150-61. 10.1046/j.1533-2500.2001.01016.x [DOI] [PubMed] [Google Scholar]
- 49.Santana L, Driggers J, Carvalho NF. Pain management for the Nuss procedure: comparison between erector spinae plane block, thoracic epidural, and control. World J Pediatr Surg 2022;5:e000418. 10.1136/wjps-2022-000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papic JC, Finnell SM, Howenstein AM, et al. Postoperative opioid analgesic use after Nuss versus Ravitch pectus excavatum repair. J Pediatr Surg 2014;49:919-23; discussion 923. 10.1016/j.jpedsurg.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 51.Choi S, Park HJ. Complications after pectus excavatum repair using pectus bars in adolescents and adults: risk comparisons between age and technique groups. Interact Cardiovasc Thorac Surg 2017;25:606-12. 10.1093/icvts/ivx162 [DOI] [PubMed] [Google Scholar]
- 52.Ghionzoli M, Martin A, Bongini M, et al. Scoliosis and Pectus Excavatum in Adolescents: Does the Nuss Procedure Affect the Scoliotic Curvature? J Laparoendosc Adv Surg Tech A 2016;26:734-9. 10.1089/lap.2016.0168 [DOI] [PubMed] [Google Scholar]
- 53.Johnson WR, Fedor D, Singhal S. Systematic review of surgical treatment techniques for adult and pediatric patients with pectus excavatum. J Cardiothorac Surg 2014;9:25. 10.1186/1749-8090-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akhtar M, Razick DI, Saeed A, et al. Complications and Outcomes of the Nuss Procedure in Adult Patients: A Systematic Review. Cureus 2023;15:e35204. 10.7759/cureus.35204 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as