Abstract
Background
Women who want to start intrauterine contraception (IUC) during the postpartum period might benefit from IUC insertion immediately after delivery. Postplacental insertion greatly reduces the risk of subsequent pregnancy and eliminates the need for a return visit to start contraception. Without the option of immediate insertion, many women may never return for services or may adopt less effective contraception.
Objectives
Our aim was to examine the outcomes of IUC insertion immediately after placenta delivery (within 10 minutes), especially when compared with insertion at other postpartum times. We focused on successful IUC placement (insertion), subsequent expulsion, and method use.
Search methods
We searched for trials until 1 April 2015. Sources included PubMed (MEDLINE), the Cochrane Central Register of Controlled Trials (CENTRAL), POPLINE, Web of Science, EMBASE, LILACS, ClinicalTrials.gov, and ICTRP. For the original review, the authors contacted investigators to identify other trials.
Selection criteria
We sought randomized controlled trials (RCTs) with at least one treatment arm that involved immediate IUC placement (i.e., within 10 minutes of placenta delivery). Comparison arms could have included early postpartum insertion (from 10 minutes postplacental to hospital discharge) or standard insertion (during a postpartum visit). Trials could also have compared different IUC methods or insertion techniques. Delivery may have been vaginal or cesarean. Primary outcomes were placement (insertion), subsequent expulsion, and method use at study assessment.
Data collection and analysis
For dichotomous outcomes, we used the Mantel‐Haenszel odds ratio (OR) with 95% confidence interval (CI). Earlier studies primarily reported results as life‐table rates. We aggregated trials in a meta‐analysis if they had similar interventions and outcome measures. A sensitivity analysis included studies with moderate or high quality evidence and sufficient outcome data.
Main results
We included 15 trials. Seven studies reported from 2010 to 2014 were added to eight from the original 2001 review. Newer trials compared immediate postplacental insertion versus early (10 minutes to 48 hours) or standard insertion (during the postpartum visit). Of four with full reports, three were small trials. The other three studies had conference abstracts. The eight early trials examined immediate insertion of different devices or insertion techniques. Most studies were published in the 1980s, some with limited reporting.
Our sensitivity analysis included trials with sufficient outcome data and moderate or high quality evidence. Four newer trials comparing insertion times met the inclusion criteria. Two studies used the levonorgestrel‐releasing intrauterine system (LNG‐IUS) after vaginal delivery. The other two trials placed IUC after cesarean section; one used the CuT 380A intrauterine device (IUD) and the other used the LNG‐IUS.
A pilot trial compared immediate insertion versus early or standard insertion. In groups comparing immediate versus early insertion (N = 30), all women had the LNG‐IUS inserted. By six months, the groups had the same expulsion rate and did not differ significantly in IUC use.
For immediate versus standard insertion, we conducted meta‐analyses of four trials. Insertion rates did not differ significantly between study arms. However, the trial from Uganda showed insertion was more likely for the immediate group, although the estimate was imprecise. In the meta‐analysis, expulsion by six months was more likely for the immediate group, but the confidence interval was wide (OR 4.89, 95% CI 1.47 to 16.32; participants = 210; studies = 4). IUC use at six months was more likely with immediate insertion than with standard insertion (OR 2.04, 95% CI 1.01 to 4.09; participants = 243; studies = 4). Study arms did not differ in use at 3 or 12 months in individual small trials.
Authors' conclusions
Recent trials compared different insertion times after vaginal or cesarean delivery. Evidence was limited because studies with full reports generally had small sample sizes. Overall, the quality of evidence was moderate; abstracts and older studies had limited reporting. Ongoing trials will add to the evidence, although some are small. Trials of adequate power are needed to estimate expulsion rates and side effects.
The benefit of effective contraception immediately after delivery may outweigh the disadvantage of increased risk for expulsion. Frequent prenatal visits during the third trimester provide the opportunity to discuss effective contraceptive methods and desired timing for initiation. Clinical follow‐up can help detect early expulsion, as can educating women about expulsion signs and symptoms.
Plain language summary
Intrauterine contraception soon after childbirth
Women have two main choices for intrauterine contraception (IUC): one that releases the hormone levonorgestrel, and one without hormones that contains copper. Beginning IUC use right after childbirth and before hospital discharge can be good for many reasons. The woman knows she is not pregnant, and the time and place are convenient for starting a method that works well. We looked at whether IUC would be more likely to come out on its own if put in right after birth of a baby. For women who wanted IUC but did not have it placed right away, we studied whether they returned later for insertion.
Until 1 April 2015, we ran computer searches for randomized trials of IUC inserted within 10 minutes of placenta (afterbirth) delivery. We wrote to researchers to find more studies. Trials could compare different times for insertion as well as types of IUC and ways to insert the device.
We found 15 trials. Seven recent studies compared IUC insertion right after childbirth versus early insertion (before hospital discharge) or later insertion (weeks after discharge). Four had full reports, although three were small studies. Insertion was as likely to occur when placed right after childbirth as when planned for later placement, except in a study from Uganda. For the four trials overall, the IUC came out more often on its own when placed right after childbirth than when inserted weeks later. Use at six months was more likely with insertion right after childbirth than weeks later. In single studies, the groups did not differ in use at 3 months or 12 months.
Eight older studies looked at types of IUC put in right after childbirth. Changing IUC design did not seem to affect whether IUC stayed in or whether it was used later on. Inserting IUC by hand or with a holding instrument did not seem to make a difference.
We found newer trials with full reports that compared placement times. The studies were of good quality. Ongoing trials will provide additional data, although some sample sizes are small. Larger studies would provide better information on whether the IUC came out on its own and on whether side effects occurred.
Summary of findings
for the main comparison.
| Immediate insertion compared with early insertion for postpartum IUC | |||
|
Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: early insertion (10 minutes to 48 hours post delivery) | |||
| Outcomes | Relative effect (95% CI) | Participants (studies) | Quality of the evidence (GRADE) |
| Expulsion by 6 months | OR 1.00 (95% CI 0.20 to 5.04) |
30 (1 study) | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 0.46 (95% CI 0.04 to 5.75) |
30 (1 study) | ⊕⊕⊕⊝ Moderate |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
2.
| Immediate insertion compared with standard insertion for postpartum IUC | |||
|
Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: standard insertion (at postpartum visit) | |||
| Outcomes | Relative effect (95% CI) | Participants (studies) | Quality of the evidence (GRADE) |
| Placement per protocol | OR 4.07 (95% CI 0.54 to 30.40); I2 = 68% |
243 (4 studies) |
⊕⊕⊕⊝ Moderate |
| Expulsion by 6 months | OR 4.89 (95% CI 1.47 to 16.32) |
210 (4 studies) |
⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 2.04 (95% CI 1.01 to 4.09) |
243 (4 studies) |
⊕⊕⊕⊝ Moderate |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
Background
Description of the condition
During the postpartum period, delay in initiating contraception is common. Effective contraception after childbirth helps lengthen birth intervals and thus improves the health of mothers and infants. Preventing unintended pregnancies helps avoid their financial, psychological, and health costs. A longer birth interval decreases the risk of major maternal complications including death, third‐trimester bleeding, puerperal endometritis, and anemia (Conde‐Agudelo 2000; WHO 2005). To reduce risk for poor maternal and infant health outcomes, the World Health Organization (WHO) has recommended waiting 24 months after a birth before attempting the next pregnancy (WHO 2005).
Intrauterine contraception (IUC) includes the levonorgestrel‐releasing intrauterine system (LNG‐IUS) and copper‐containing intrauterine devices (IUDs). The LNG‐IUS and the CuT 380A IUD provide contraceptive protection similar to that attained with tubal sterilization (Trussell 2011). Compared with sterilization, however, IUC use is simpler, less expensive, and immediately reversible. IUC use is not very common in 'more developed' regions globally, where the most prevalent forms of contraception are condoms and contraceptive pills at 18% each, among women who are married or in union (UN 2011). In 'less developed' areas, female sterilization leads at 21% and is followed by IUC use (15%) and contraceptive pills (7%) (UN 2011). These statistics are influenced by countries with large populations such as India and China, which are dominated by sterilization and IUC use, respectively.
How the intervention might work
Insertion of IUC immediately after delivery has several advantages. It may avoid the discomfort related to standard insertion and bleeding from insertion will be disguised by lochia. The woman is known to be not pregnant, and her motivation for contraception may be high. For women with limited access to medical care, postpartum care before discharge provides an opportunity to discuss contraception. Delay in initiating contraception is common in the postpartum period because of the challenges of caring for a new infant (Teal 2014a). Immediate placement of a long‐acting reversible contraceptive, such as IUC or an implant, results in higher use rates. In the USA, however, most insurance reimbursement policies for delivery‐related care do not allow separate billing for postpartum IUC or implants prior to discharge (Aiken 2014).
Women who delay getting IUC may experience an unintended pregnancy or may never return for the insertion (Allen 2009). An early study from Colombia showed that 95% of women interested in immediate postpartum IUD insertion had it done. Only 45% of those wishing later insertion ultimately had an IUD inserted. Although some women in the latter group may have been ambivalent and later decided against an IUD, the inconvenience and expense of a return visit probably deterred some women (Echeverry 1973). A US database review examined the return rate for IUC insertion when two visits were required (Bergin 2012). Only 54% of the women had IUC inserted; return rates were higher when the IUC order initiated at a gynecologic rather than an obstetric visit. Women delivering at a university hospital in the USA were surveyed about postpartum contraception (Glazer 2011). Nearly a fourth were interested in immediate IUC placement if available. Months later, only 5% were using IUC and nearly a fourth still wanted IUC.
Why it is important to do this review
For the original review in 2001, the randomized controlled trials (RCTs) of immediate postplacental insertion compared different devices or different insertion techniques. Many of the IUDs used in those studies are no longer marketed. None of the trials compared IUC insertion at different time points. In 2010, a conference abstract reported results of a trial of immediate postplacental versus standard insertion. Over subsequent years, several trials have compared insertion times for postpartum IUC. The recent studies have examined immediate IUC insertion after cesarean delivery, as well as after vaginal delivery. Investigators used the LNG‐IUS or the CuT 380A. Such trials could provide further evidence regarding subsequent expulsion and method use after immediate insertion of currently marketed IUC.
Objectives
Our aim was to examine the outcomes of IUC insertion immediately after placenta delivery (within 10 minutes), especially when compared with insertion at other postpartum times. We focused on successful placement (insertion), subsequent expulsion, and method use.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials that had at least one arm with immediate postplacental IUC insertion, defined as within 10 minutes of placenta delivery. Recent trials use the term 'postplacental' rather than 'postpartum' when referring to this timing of insertion.
Types of participants
We included studies of postpartum women of any age.
Types of interventions
Trials were eligible if they examined insertion of any type of IUC within 10 minutes of placenta delivery, either vaginal or cesarean. Comparisons could include the following:
different devices or different insertion techniques
-
immediate postplacental insertion (within 10 minutes of placenta delivery) versus
early postpartum insertion (10 minutes to hospital discharge) or
standard insertion (during a postpartum visit after hospital discharge), often referred to as delayed or interval insertion.
Types of outcome measures
Primary outcomes
Successful placement (insertion), subsequent expulsion, and method use at study assessment
Secondary outcomes
Pregnancy, perforation, infection, and other adverse events
Search methods for identification of studies
Electronic searches
We searched for trials until 1 April 2015. Sources included PubMed (MEDLINE), Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, EMBASE, POPLINE, and LILACS. We also searched for current trials via ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). We have provided search strategies in Appendix 1 and earlier strategies in Appendix 2.
Searching other resources
For the original review, the search began with several review articles. The authors also contacted other investigators to attempt to find studies they might have missed, including unpublished reports. In updating, we examined reference lists of relevant reports and reviews for additional trials or secondary articles.
Data collection and analysis
Selection of studies
We assessed all titles and abstracts identified during the search. Two authors examined the search results for potentially eligible studies. For studies that appeared to be eligible for this review, we obtained and examined the full‐text articles. We resolved discrepancies by discussion.
Data extraction and management
Two authors extracted the data. One author entered the data into Review Manager (RevMan 2014), and a second author checked accuracy. Extracted data included the study characteristics, risk of bias, and outcome data. The authors resolved discrepancies by discussion.
Assessment of risk of bias in included studies
The authors examined trials for methodological quality according to recommended principles (Higgins 2011). We considered factors such as study design, method used to generate the randomization sequence, allocation concealment, blinding, and losses to follow‐up and early discontinuation. We also examined the methods used for outcome assessment.
Measures of treatment effect
When the crude number of events was published for dichotomous outcomes, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% confidence interval (CI). An example is the proportion of women with spontaneous expulsion. We used a fixed‐effect model for analysis within a single study. Fixed and random effects yield the same result if no heterogeneity exists, as when a comparison includes only one study. For meta‐analysis, we used random effects. Even when the interventions were similar across studies, the inclusion criteria could lead to differences in study populations. For the original review, the authors reported results primarily as gross cumulative event rates per 100 women. Most studies had insufficient sample sizes to assess rare events, such as uterine perforation.
Dealing with missing data
If reports were missing information or data needed for analysis, we wrote to the study investigators. However, we limited our requests to studies less than 10 years old, as well as older studies that had a report in the past five years. Investigators are unlikely to have access to data from older studies.
Assessment of heterogeneity
We conducted meta‐analyses of studies with similar intervention and outcome measures. We assessed heterogeneity, or degree of inconsistency, using the I2 statistic (Higgins 2003; Higgins 2011). Values greater than 50% may represent substantial heterogeneity, which could be related to clinical or methodological heterogeneity. However, the meta‐analyses we were able to conduct did not show such heterogeneity.
Data synthesis
We applied principles from GRADE (Grades of Recommendation, Assessment, Development and Evaluation) to assess the evidence quality and to address confidence in the effect estimates (Balshem 2011; Higgins 2011). Our assessment was based on the quality of evidence from individual studies, which could be rated as high, moderate, low, or very low. We considered the evidence from RCTs to be of high quality initially, then downgraded quality for each of the following: (1) no information on randomization sequence generation or allocation concealment, or one of those was clearly inadequate; (2) no blinding; (3) follow‐up less than six weeks for expulsion, less than three months later for method use, or less than six months for pregnancy; (4) losses greater than 20%; and (5) missing information for both blinding and losses.
Additional tables contain summaries of the various interventions, our quality assessment, and available outcome data. When the interventions and outcomes were similar, we conducted meta‐analysis as noted earlier. We created Summary of findings tables (Guyatt 2011). However, most trials differed in the experimental and comparison interventions. In addition, many reports did not provide sufficient outcome data for analysis, especially those had only a conference abstract. We did not conduct meta‐analysis of those trials and did not include them in the Summary of findings.
Sensitivity analysis
We examined a group of trials that had evidence of moderate or high quality and sufficient outcome data. We identified these studies in Additional tables and discussed the study results in Summary of main results.
Results
Description of studies
Results of the search
In 2014, we added the term 'postplacental' to the PubMed strategy and ran the search without date restriction. Some of the other databases included 'postplacenta' under key words. The database searches into April 2015 produced 247 citations (Figure 1). After 64 duplicates were removed (55 electronically and 8 by hand), we had 183 references. An abstract from a trial substudy, which was not eligible, led us to the original trial, for a total of 184 unduplicated citations. Upon screening titles and abstracts, we discarded 170 studies for not meeting eligibility criteria. Many were not RCTs or did not provide the relevant intervention. We reviewed the full text of 14 items for eligibility and excluded three items from two studies. Information on seven newly included trials came from seven primary reports (full text or abstract) plus four secondary reports or abstracts.
1.
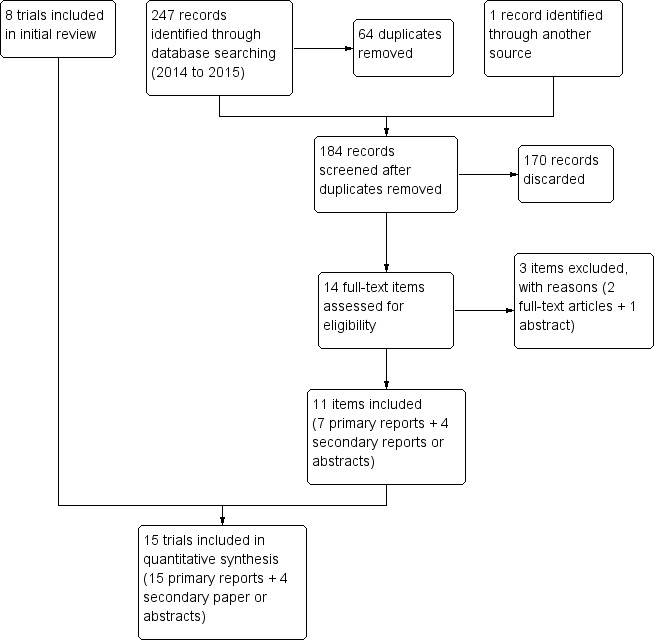
Study flow diagram (2015)
Searches of ClinicalTrials.gov and ICTRP yielded 28 unduplicated studies. We discarded 22 on the basis of title or summary, and added one to Studies awaiting classification and three to Ongoing studies. We excluded two trial listings with reasons.
Included studies
We included 15 RCTs in this review. The seven newer trials compared immediate postpartum insertion versus early or standard insertion and were published from 2010 to 2014. The eight studies from the original review examined immediate insertion of different devices, device modifications, or insertion techniques. Most of the eight early trials were published in the 1980s, except for one that was published in 1996. The interventions are described below and summarized in Table 3.
1. Intervention and quality summary.
| Study | Delivery type | IUC | Inadequate randomization and allocation concealment | No blinding | Loss to follow‐up > 20% | Quality of evidencea |
| Immediate versus early insertion (10 minutes to 48 hours) | ||||||
| Dahlke 2011 | Vaginal | LNG‐IUS | _ | ‐1 | _ | Moderate |
| Ahuja 2014b | Vaginal | CuT 380A | _ | ‐1 | Unclear | Moderate |
| Singh 2014b | Vaginal or cesarean | CuT 380A | ‐1 | Unclear | Unclear | Low |
| Immediate versus standard insertion (weeks) | ||||||
| Chen 2010 | Vaginal | LNG‐IUS (6 to 8 weeks) | _ | _ | _ | High |
| Dahlke 2011 | Vaginal | LNG‐IUS (> 6 weeks) | _ | ‐1 | _ | Moderate |
| Whitaker 2014 | Cesarean | LNG‐IUS (4 to 8 weeks) | _ | _ | ‐1 | Moderate |
| Lester 2015 | Cesarean | CuT 380A (6 weeks) | _ | ‐1 | _ | Moderate |
| Ogburn 2013b | Vaginal or cesarean | CuT 380A (4 to 12 weeks) | ‐1 | Unclear | Unclear | Low |
| Immediate insertion: IUC types, modifications, or insertion techniques | ||||||
| Thiery 1980 | Unclear | Multiload 250 versus CuT 200 | ‐1 | Unclear | _ | Moderate |
| WHO 1980 | Vaginal | Copper 7 versus Lippes Loop D vs Nova‐T‐PP | _ | ‐1 | _ | Moderate |
| Van Kets 1987 | Unclear | Nova‐T‐PP vs Nova T | ‐1 | Unclear | _ | Moderate |
| Lavin 1983 | Unclear | Progestasert vs CuT 200 | _ | Unclear | ‐1 | Moderate |
| Apelo 1985 | Vaginal | IPCS‐52 vs CuT 200 | _ | Unclear | ‐1 | Moderate |
| Cole 1984 | Vaginal |
_ |
Unclear | ‐1 | Moderate | |
| Kisnisci 1985 | Unclear | Delta Loop vs Delta T | _ | Unclear | _ | Moderate |
| Xu 1996 | Vaginal | CuT 380A: hand vs ring‐forceps insertion | _ | _ | _ | High |
aRCTs considered high quality initially, then downgraded for (1) no information on randomization sequence generation or allocation concealment, or one was clearly inadequate; (2) no blinding; (3) losses > 20%; (4) information missing for both blinding and losses. Follow‐up time not shown as all studies met criteria. bNo full report; sources included conference abstracts and clinical trial listings.
The seven recent trials compared immediate versus early or standard insertion.
Two trials examined immediate insertion (within 10 minutes postplacental) versus early postpartum insertion (10 minutes to 48 hours) using the CuT 380A (Ahuja 2014; Singh 2014). Delivery was vaginal in Ahuja 2014, and Singh 2014 included both vaginal and cesarean delivery. Neither study had a full report.
-
Four trials compared immediate versus standard insertion (4 to 12 weeks postpartum).
Two used the CuT 380A (Ogburn 2013; Lester 2015). In Lester 2015, delivery was cesarean, and Ogburn 2013 included women with either vaginal or cesarean birth. Ogburn 2013 did not yet provide a full report.
Two used a levonorgestrel‐releasing intrauterine system (LNG‐IUS) (20 μg) (Chen 2010; Whitaker 2014). In Whitaker 2014, delivery was cesarean.
One trial examined immediate, early, and standard (after six weeks) insertion of the LNG‐IUS after vaginal delivery (Dahlke 2011).
The eight trials from the 2001 review examined different devices, device modifications, or insertion techniques.
A multicenter RCT studied the addition of absorbable chromic sutures tied around the superior arms of conventional IUDs (Cole 1984; Kisnisci 1985). For example, investigators tied two chromic sutures (size No. 2) to the lateral arms of a Copper T 220 C (TCu 220C) with the free ends of the sutures left 0.5 cm long and projecting caudad at a 45‐degree angle. This modification of the TCu 220C was termed the Delta T. Similarly, investigators tied three chromic sutures to the superior bar of a Lippes Loop D, thereafter termed the Delta Loop. Each modification was compared with the conventional device without sutures. The other interventions examined in these two reports were hand versus instrument insertion of the various devices (Cole 1984; Kisnisci 1985).
-
Two early trials focused on progesterone‐releasing IUDs.
Lavin 1983 compared the conventional Progestasert IUD, containing 38 mg progesterone, released into the uterus over a year, with the Copper T 200 (TCu 200). The investigators compared hand versus instrument insertion for each IUD type.
A prototype of a longer‐acting Progestasert contained 52 mg of progesterone (ICPS‐52), and was designed to provide three years of protection. This was compared with the TCu 200 in Apelo 1985.
-
In two trials, investigators modified a Nova T device to have two flexible arms, 2 cm in length, added to the base of the vertical stem; the arms pointed superiorly at a 45‐degree angle. This Nova T Postpartum (Nova‐T‐PP) and standard Nova T were otherwise identical.
Van Kets 1987 compared the modified (Nova‐T‐PP) and standard Nova T.
WHO 1980 compared the Nova‐T‐PP with the Lippes Loop D and with the Copper 7 (Gravigard).
-
Two trials examined other interventions, i.e.,
standard Multiload 250 (ML Cu 250) versus TCu 200 (Thiery 1980); and
hand versus ring‐forceps insertion of a standard CuT 380A (Xu 1996).
Excluded studies
We excluded several trials from the review. Data from Thiery 1983 were included in a larger report (Cole 1984). We also excluded a subgroup analysis of the Cole 1984 data (Chi 1985). Shikary 1987 examined standard postpartum insertions, and Stuart 2015 compared early versus standard insertion. Others were not randomized controlled trials or did not have a relevant outcome measure (Characteristics of excluded studies).
Risk of bias in included studies
Figure 2 shows the overall risk of bias for evidence in this review. Risk of bias by trial can be seen in Figure 3. We also summarized the quality of evidence from each trial in Table 3. Communication with researchers supplemented several of the early reports (WHO 1980; Lavin 1983; Apelo 1985; Kisnisci 1985; Xu 1996).
2.
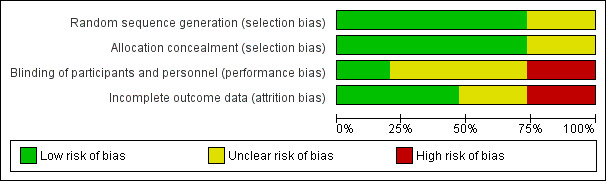
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
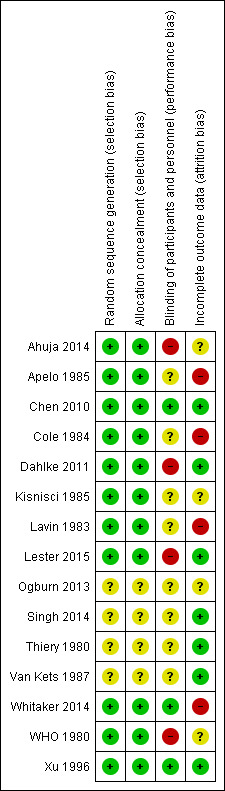
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Of the reports from 2010 to 2014, four provided sample size calculations (Chen 2010; Ahuja 2014; Whitaker 2014; Lester 2015). A pilot study was not powered for the utilization outcome (Dahlke 2011). Those lacking information on sample size calculation were conference abstracts (Ogburn 2013; Singh 2014). For the initial review, only Xu 1996 provided a sample size calculation.
Allocation
Most trials used an appropriate method of generating the randomization sequence and mentioned some type of computer‐generated randomization scheme. Two conference abstracts (Ogburn 2013; Singh 2014) and an older report (Van Kets 1987) made no mention of randomization. Thiery 1980 mentioned a list of random numbers.
Many trials attempted to conceal the upcoming assignment from those involved with the trials, usually by using sequentially numbered, sealed, opaque envelopes. Two conference abstracts (Ogburn 2013; Singh 2014) and two older reports (Thiery 1980; Van Kets 1987) made no mention of allocation concealment.
Blinding
Three trials used some type of blinding: evaluators (Chen 2010), investigator and evaluators (Whitaker 2014), or participants and evaluators (Xu 1996). Four studies did not use any type of blinding, or the clinical trial listing stated that blinding was "not applicable" (WHO 1980; Dahlke 2011; Ahuja 2014; Lester 2015). The other eight made no mention of blinding.
Incomplete outcome data
Loss to follow‐up was high in Whitaker 2014 (33%) and Lavin 1983 (based on reported life‐table rates). Losses were differential across study arms in two trials (Cole 1984; Apelo 1985). In Cole 1984, four of six study groups had high losses, as did one of four in Apelo 1985. One arm in Kisnisci 1985 lost more than 20%. Two conference abstracts provided no information on losses (Ogburn 2013; Ahuja 2014).
Effects of interventions
For trials that compared insertion times, Table 4 provides a summary of insertion rates. A summary of IUC use and expulsion can be found in Table 5. Those tables show sample sizes for trials as well.
2. Immediate versus early or standard insertion: placement summary.
| Study | N | Placement (insertion) per protocol (%) | ||
| Immediate | Early | Standard | ||
| Chen 2010a | 102 | 88 | _ | 90 |
| Dahlke 2011a | 46 | 100 | 100 | 94 |
| Whitaker 2014a | 42 | 95 | _ | 82 |
| Lester 2015a | 68 | 100 | _ | 53 |
| Ogburn 2013 | 156 | 87 | _ | 77 |
| Ahuja 2014 | 263 | _ | _ | _ |
| Singh 2014 | 200 | _ | _ | _ |
aIn sensitivity analysis; sufficient outcome data and evidence of moderate or high quality (Table 3).
3. Immediate versus early or standard insertion: use and expulsion summary.
| Study | N | Use at 6 monthsa (%) | Expulsion by 6 monthsb (%) | ||||
| Immediate | Early | Standard | Immediate | Early | Standard | ||
| Chen 2010c | 102 | 84 | _ | 77 | 22d | _ | 4 |
| Dahlke 2011c | 46 | 87 | 93 | 94 | 27 | 27 | 0 |
| Whitaker 2014c | 42 | 70 | _ | 59 | 20 | _ | 0 |
| Lester 2015c | 68 | 79 | _ | 47 | 3 | _ | 6 |
| Ogburn 2013 | 156 | 57 | _ | 59 | _ | _ | _ |
| Ahuja 2014 | 263 | 89 | 74 | _ | 9 | 24 | _ |
| Singh 2014 | 200 | 84 | 77 | _ | 8 | 11 | _ |
aUse based on women randomized; Ogburn 2013 assessed at 12 months. bExpulsion based on IUC placed (Table 4); Ahuja 2014 assessed at 6 weeks. cIn sensitivity analysis; sufficient outcome data and evidence of moderate or high quality (Table 3). dExcludes 5 women with IUC placed 11 to 15 minutes postplacental; does not significantly affect results.
Immediate postplacental versus early insertion
Three trials compared immediate postplacental versus early postpartum (10 minutes to 48 hours) insertion. In a pilot study, the LNG‐IUS was placed after vaginal delivery (N = 46) (Dahlke 2011). The investigators also studied standard insertion; those results are presented in the next section. Two trials using the CuT 380A had only conference abstracts (Ahuja 2014; Singh 2014), so we presented the results separately below. Both were larger trials (N = 263 and N = 200, respectively).
In Dahlke 2011, all women in the immediate and early insertion groups had the LNG‐IUS placed (inserted) (Analysis 1.1). Of the 15 women with early insertion, 14 had the IUS placed within 30 minutes of placenta delivery. The study arms had the same expulsion rate by six months (Analysis 1.2). Utilization at three or six months did not differ significantly between the study groups (Analysis 1.3; Analysis 1.4), but this pilot study was not powered to detect such a difference.
1.1. Analysis.
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 1 Placement (insertion).
1.2. Analysis.
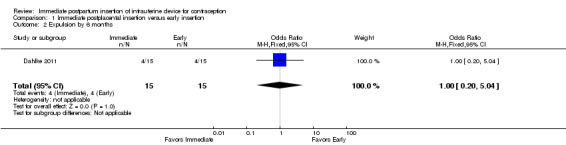
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 2 Expulsion by 6 months.
1.3. Analysis.
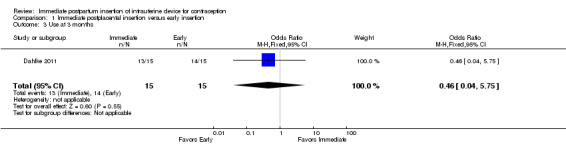
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 3 Use at 3 months.
1.4. Analysis.
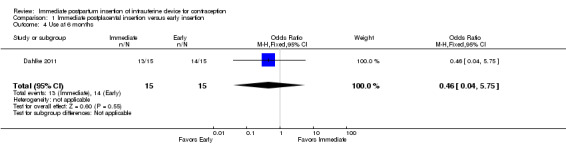
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 4 Use at 6 months.
Two trials used the CuT 380A IUD, but the conference abstracts provided limited data. Ahuja 2014 compared immediate versus early insertion after vaginal delivery, and Singh 2014 included women with either vaginal or cesarean delivery. Expulsion, assessed at six weeks in Ahuja 2014, was lower for the immediate group compared with the early group (9% versus 24%, reported P = 0.0037) (Analysis 1.5). In Singh 2014, the study arms did not differ significantly for expulsion by six months (reported P = 0.06) (Analysis 1.5). Continued use at six months was higher in the immediate group compared with the early group in Ahuja 2014 (reported P = 0.0054), but the groups were not significantly different in Singh 2014 (reported P = 0.13) (Analysis 1.6). No pregnancy occurred in Singh 2014. Removals for medical reasons were 5.2% in the immediate group and 7.4% in the early group (reported P > 0.05) (Singh 2014). No perforation or pelvic inflammatory disease occurred. In Ahuja 2014, complications leading to removals were 2.2% and were reportedly similar for the two groups.
1.5. Analysis.
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 5 Expulsion.
| Expulsion | ||||
|---|---|---|---|---|
| Study | Time frame | Immediate insertion | Early insertion | Reported P |
| Ahuja 2014 | ‐‐‐ | N = 131 | N = 132 | ‐‐‐ |
| Ahuja 2014 | 6 weeks | 24.1% | 9.1% | .0037 |
| Singh 2014 | ‐‐‐ | N = 100 | N = 100 | ‐‐‐ |
| Singh 2014 | 6 months | 8.3% | 10.5% | .06 |
1.6. Analysis.
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 6 Use at 6 months.
| Use at 6 months | |||
|---|---|---|---|
| Study | Immediate insertion | Early insertion | Reported P |
| Ahuja 2014 | 88.9% | 74.1% | .0054 |
| Ahuja 2014 | N = 131 | N = 132 | ‐‐‐ |
| Singh 2014 | 83.5% | 77.1% | .13 |
| Singh 2014 | N = 100 | N = 100 | ‐‐‐ |
Immediate postplacental versus standard insertion
Five trials compared immediate postplacental versus standard insertion. Four had full reports, and the time frames ranged from four to eight weeks postpartum. Two of those trials examined the LNG‐IUS after vaginal delivery (Chen 2010; Dahlke 2011). Dahlke 2011 was a small pilot study (N = 46), and Chen 2010 was mid‐sized (N = 102). Two other trials placed the IUC after cesarean section, using the CuT 380A (N = 68) (Lester 2015) or the LNG‐IUS (N = 42) (Whitaker 2014). Whitaker 2014 was terminated early because of slow recruitment and had a high loss to follow‐up (33% of 42 women randomized). Ogburn 2013 (N = 156) had only a conference abstract to date, so we have presented the results separately below.
Meta‐analysis of the four trials with full reports showed the following.
IUC placement according to the study plan (protocol) was considered successful insertion. The study arms did not differ significantly (Analysis 2.1). The substantial heterogeneity (I2 = 68%) was related to the Uganda study (Lester 2015). Insertion was more likely in the immediate group in that trial, but the CI was very wide. The US trials indicated little difference between the study arms, regardless of whether the IUC was inserted after vaginal or cesarean delivery, or whether the LNG‐IUS or the CuT 380A was used.
For expulsion, the denominator was the number of women with IUC placed according to study plan. Expulsion by six months was more likely with immediate insertion than with standard insertion (OR 4.89, 95% CI 1.47 to 16.32; participants = 210; studies = 4) (Analysis 2.2). However, the wide confidence interval indicates imprecision in the estimate.
For IUC use, the denominator was the number of women randomized. Use at six months was more likely with immediate insertion than with standard insertion (OR 2.04, 95% CI 1.01 to 4.09; participants = 243; studies = 4) (Analysis 2.3). However, the groups did not differ at three months in one small trial (Dahlke 2011) (Analysis 2.4) or at 12 months in another (Whitaker 2014) (Analysis 2.5).
2.1. Analysis.
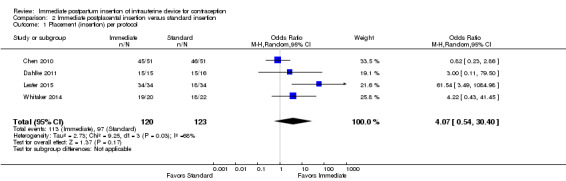
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 1 Placement (insertion) per protocol.
2.2. Analysis.
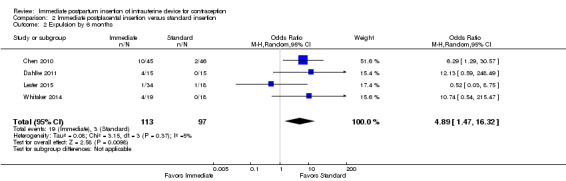
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 2 Expulsion by 6 months.
2.3. Analysis.
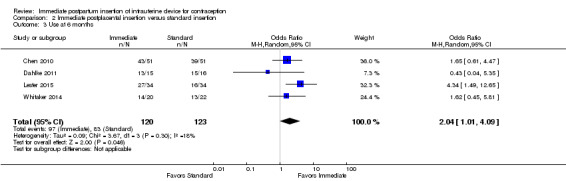
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 3 Use at 6 months.
2.4. Analysis.
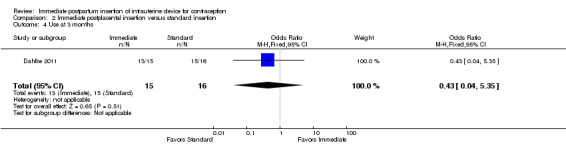
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 4 Use at 3 months.
2.5. Analysis.
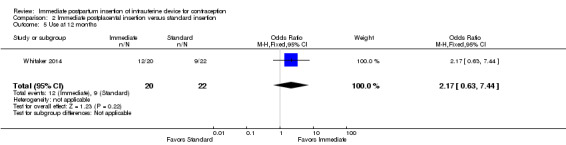
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 5 Use at 12 months.
Other results for these four trials included pregnancies and complications. Two studies found no pregnancies by six months (Chen 2010; Lester 2015). Complications were few. Two trials diagnosed one infection in each randomized arm (Chen 2010; Lester 2015). Two studies found no infection (Dahlke 2011; Whitaker 2014), and two reported no other adverse events (Whitaker 2014; Lester 2015).
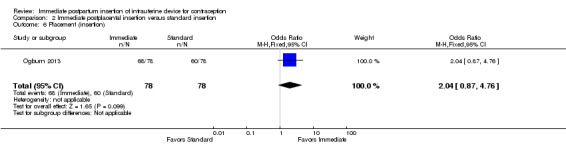
Ogburn 2013, which had only a conference abstract, placed the CuT 380A after either vaginal or cesarean delivery. Immediate postplacental insertion was compared with standard, which ranged from 4 to 12 weeks postpartum. The study arms did not differ significantly for IUC placement (insertion) (Analysis 2.6). The groups were similar for use at 12 months (Analysis 2.7), and no major complication occurred.
2.6. Analysis.
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 6 Placement (insertion).
2.7. Analysis.
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 7 Continued use at 12 months.
| Continued use at 12 months | ||
|---|---|---|
| Study | Immediate insertion | Early insertion |
| Ogburn 2013 | N = 78 | N = 78 |
| Ogburn 2013 | 57% | 59% |
Immediate postplacental insertion: different devices or insertion techniques
The eight trials included from the 2001 review examined immediate insertion of different devices or insertion techniques; none compared insertion times. Seven were published from 1980 to 1987, and one in 1996. The reported life‐table rates mentioned below are also shown in Data and analyses. For these trials, we summarized the results for insertion, use, and expulsion in Table 6.
4. Comparisons of devices or insertion techniques: outcome summary.
| Study | IUC | N | Insertion technique | Placement (insertion) per protocol (reported %) | Use at 6 monthsa | Expulsion by 6 monthsa |
| Life‐table rates (reported) | ||||||
| Thiery 1980 | TCu 200 | 269 | _ | _ | 85.0 | 9.4 |
| Multiload 250 | 293 | _ | _ | 88.8 | 7.4 | |
| WHO 1980b | Copper 7 | 277 | _ | _ | 64.6 | 31.1 |
| Lippes Loop D | 250 | _ | _ | 48.1 | 41.3 | |
| Nova‐T‐PP | 277 | _ | _ | 52.3 | 39.4 | |
| Van Kets 1987 | Nova‐T‐PP | 205 | _ | _ | 87.5 | 5.5 |
| Nova T | 203 | _ | _ | 88.4 | 6.0 | |
| Lavin 1983 | Progestasert | 100 | Hand | _ | 59.9 | 35.8 |
| 100 | Inserter | _ | 57.2 | 35.2 | ||
| TCu 200 | 100 | Hand | _ | 86.3 | 9.0 | |
| 100 | Inserter | _ | 86.1 | 8.1 | ||
| Apelo 1985 | IPCS‐52 mg | 50 | Hand | _ | 57.3 | 39.0 |
| 50 | Inserter | _ | 79.6 | 14.2 | ||
| TCu 200 | 50 | Hand | _ | 81.9 | 14.1 | |
| 50 | inserter | _ | 89.7 | 10.3 | ||
| Cole 1984 | Delta T | 728 | _ | 99.9 | 81.8 | 11.6 |
| TCu 220 | 718 | _ | 99.7 | 81.8 | 11.5 | |
| Delta Loop | 662 | _ | 99.7 | 78.5 | 15.7 | |
| Lippes Loop D | 648 | _ | 99.7 | 73.8 | 21.5 | |
| Delta T | 518 | Hand | 99.7 | 84.2 | 11.2 | |
| 517 | Mechanical | 99.7 | 82.6 | 11.5 | ||
| Kisnisci 1985 | Delta Loop | 122 | _ | 99.2 | 96.3 | 3.7 |
| Delta T | 124 | _ | 100.0 | 90.7 | 7.6 | |
| Percent | ||||||
| Xu 1996 | CuT 380A | 470 | Hand | _ | 81.3 | 13.0 |
| 440 | Ring‐forceps | _ | 79.1 | 12.5 | ||
aLavin 1983 reported use and expulsion by 12 months. bWHO 1980 excluded expulsions within 48 hours.
The addition of chromic sutures to conventional IUDs had little impact on clinical outcomes (Cole 1984) (Analysis 3.1 to Analysis 5.4). Virtually all participants received an IUD (Analysis 3.1; Analysis 4.1; Analysis 5.1). Women were randomly assigned to either a Delta Loop or a Lippes Loop D. The only significant finding noted by the investigators was a lower rate of expulsion with the Delta Loop at six months (15.7 versus 21.5 per 100 women). Gross six‐month continuation rates with the two devices were 78.5 and 73.8 per 100 women, respectively. Follow‐up rates were 62.7 and 66.1. Among women randomized to either the Delta T or the TCu 220C, the rates of expulsion at six months were 11.6 and 11.5 per 100 women, respectively. Six‐month continuation rates were 81.8 per 100 women for both groups, and follow‐up rates were 71.0 and 70.9. The technique of insertion for the Delta Loop device (hand versus inserter or forceps) had no significant impact on expulsion or continuation rates. Follow‐up rates were 84.2 for hand insertion and 83.0 for instrument insertion.
3.1. Analysis.
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 1 Insertion per protocol.
| Insertion per protocol | ||
|---|---|---|
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 99.7% | 99.7% |
5.4. Analysis.
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Life‐table rates per 100 women for follow‐up (6‐month) | ||
|---|---|---|
| Study | Delta Loop Hand insertion | Delta Loop Instrument insertion |
| Cole 1984 | 84.2% | 83.0% |
4.1. Analysis.
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 1 Insertion per protocol.
| Insertion per protocol | ||
|---|---|---|
| Study | Delta T | TCu 220 C |
| Cole 1984 | 99.9% | 99.7% |
5.1. Analysis.
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 1 Insertion per protocol.
| Insertion per protocol | ||
|---|---|---|
| Study | Delta Loop Hand Insertion | Delta Loop Instrument Insertion |
| Cole 1984 | 99.7% | 99.7% |
Kisnisci 1985 compared the Delta Loop and the Delta T (Analysis 6.1 to Analysis 6.6). These results from one center involved in Cole 1984 were published separately. Nearly all participants received an IUD. Expulsion rates per 100 women at 12 months were 3.7 for the Delta Loop and 7.6 for the Delta T. The 12‐month pregnancy rates were 2.1 and 0, respectively. The two devices had similar 12‐month rates of removal for pain or bleeding: 1.1 and 1.0 per 100 women. Likewise, continuation rates were comparable: 93.3 and 90.7 per 100 women, respectively. Follow‐up rates were 78.9% for the Delta Loop and 86.7% for the Delta T. The investigators noted that the groups did not differ significantly in the termination or event rates.
6.1. Analysis.
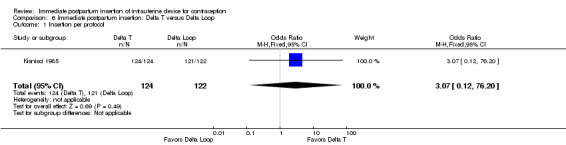
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 1 Insertion per protocol.
6.6. Analysis.
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 6 Life‐table rates per 100 women for follow‐up (12‐month).
| Life‐table rates per 100 women for follow‐up (12‐month) | ||
|---|---|---|
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 86.7% | 78.9% |
The TCu 200 was superior to the Progestasert for immediate postpartum insertion for the outcomes in this review (Lavin 1983) (Analysis 7.1 to Analysis 7.5). The Progestasert had significantly higher expulsion rates than the TCu 200, and the differences were independent of whether the devices had been introduced by hand or with an inserter. The 12‐month expulsion rates for hand insertion and instrument insertion were 9.0 and 8.1 for the TCu 200, and 35.8 and 35.2 for the Progestasert. The 12‐month continuation rates were reported to be significantly higher for the TCu 200 groups (86.3 when introduced by hand and 86.1 for inserter) than for the Progestasert groups (59.9 and 57.2, respectively). At 12 months, follow‐up rates were 69.7 and 67.7 for the TCu200 and Progestasert instrument‐insertion groups, respectively, versus 76.1 and 77.4 for the respective hand‐insertion groups; significance levels were not provided.
7.1. Analysis.
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 1 Hand insertion: Life‐table rates per 100 women for expulsion (12‐month).
| Hand insertion: Life‐table rates per 100 women for expulsion (12‐month) | ||
|---|---|---|
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 9.0 | 35.8 |
7.5. Analysis.
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 5 Life‐table rates per 100 women for follow‐up (12‐month).
| Life‐table rates per 100 women for follow‐up (12‐month) | |||
|---|---|---|---|
| Study | Insertion method | TCu 200 | Progestasert |
| Lavin 1983 | Hand | 76.1 | 77.4 |
| Lavin 1983 | Instrument | 69.7 | 67.7 |
The TCu 200 also appeared to perform better than the prototype three‐year progesterone device (IPCS‐52) (Apelo 1985) (Analysis 8.1 to Analysis 8.9). The 12‐month rates for expulsion when hand‐introduced were 39.0 for IPCS‐52 and 19.9 for TCu 200. The comparable rates for the inserter‐introduced devices were 14.2 for the IPCS‐52 and 10.3 for the TCu 200. The 12‐month continuation rates per 100 women for the IPCS‐52 were 57.3 for hand insertion and 77.1 for instrument insertion. For the TCu 200, the continuation rates were 73.8 for hand inserted and 84.9 for instrument inserted. The 36‐month continuation rates were 52.3, 55.8, 67.7, and 62.9, respectively. The investigators tested for statistical significance after 36 months. The life‐table rates for expulsion at 36 months were 39.0 for the hand‐introduced IPCS‐52, 24.2 for the inserter‐introduced IPCS‐52, 19.9 for the hand‐introduced TCu 200, and 13.1 for the inserter‐introduced TCu 200. Investigators reported that expulsion for the hand‐inserted IPCS‐52 was significantly greater than for the hand‐inserted or instrument‐inserted TCu 200. Removals for bleeding and pain were uncommon with both devices and did not change between 12 and 36 months. For pooled data, the researchers noted that the TCu 200 had a significantly lower expulsion rate than the IPCS‐52, and instrument insertions had a significantly lower expulsion rate than hand insertions. At 12 months, follow‐up rates appeared lower for the TCu 200 group with hand insertion (71.1%) than for the group with instrument insertion (99.1%). For the IPCS‐52, both insertion groups had 83% follow‐up. At 36 months, when statistical testing was done, follow‐up ranged from 55% to 67% across the groups.
8.1. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month) by device and insertion method.
| Life‐table rates per 100 women for expulsion (12‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 19.9 | 10.3 | 39.0 | 14.2 |
8.9. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 9 Life‐table rates per 100 women for follow‐up (36‐month) by device and insertion method.
| Life‐table rates per 100 women for follow‐up (36‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 55.6% | 63.9% | 66.7% | 54.8% |
The modified Nova T was no better than the standard Nova T (Van Kets 1987) or the Copper 7 (WHO 1980).
-
A multicenter trial compared the Nova‐T‐PP, Lippes Loop D, Copper 7, and Lippes Loop (WHO 1980) (Analysis 9.1 to Analysis 9.4).
Spontaneous expulsion rates at six months for all three IUDs exceeded the predetermined stopping rules, so the trial terminated early. The 12‐month expulsion rates per 100 women were 41.3 for the Nova‐T‐PP, 44.1 for the Lippes Loop, and 34.8 for the Copper 7. The investigators stated that expulsion for the Copper 7 was significantly lower than for the Lippes Loop; no statistical testing was mentioned. Study procedures specified that women with expulsion within 48 hours after insertion were discontinued from the study and were excluded from the life‐table analysis. Early expulsion was significantly higher in the Lippes Loop group (reported P < 0.01).
Corresponding 12‐month pregnancy rates were 5.6, 12.1, and 7.2 per 100 women.
Total 12‐month discontinuation rates were high with all devices: 53.1, 60.9, and 47.7 per 100 women. The discontinuation rate at 12 months was significantly higher for the Lippes Loop (60.9) than for the Copper 7 (47.7) (reported P < 0.01).
Loss to follow‐up was 17.8% for Nova‐T‐PP, 16.2% for Lippes Loop, and 16.0% for Copper 7.
Outcomes with the modified and standard Nova T were more favorable in a single‐center trial (Van Kets 1987) than in a multicenter trial (WHO 1980). The 12‐month spontaneous expulsion rates per 100 women were low with both the Nova‐T‐PP (6.2) and standard Nova T (6.6). The 12‐month pregnancy rates were also low with both devices: 0.6 and 0.0, respectively. Continuation rates at 12 months were 87.4 for the Nova‐T‐PP and 78.2 for the Nova T. Loss to follow‐up was 6.2 for both groups at 12 months and 18 months. The investigators stated that none of these differences was statistically significant (Analysis 10.1 to Analysis 10.4).
9.1. Analysis.
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Life‐table rates per 100 women for expulsion (12‐month) | |||
|---|---|---|---|
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 41.3 | 44.1 | 34.8 |
9.4. Analysis.
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 4 Loss to follow‐up (unclear time frame).
| Loss to follow‐up (unclear time frame) | |||
|---|---|---|---|
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 17.8% | 16.2% | 16.0% |
10.1. Analysis.
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Life‐table rates per 100 women for expulsion (12‐month) | ||
|---|---|---|
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 6.2 | 6.6 |
10.4. Analysis.
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 4 Life‐table rates per 100 women for loss to follow‐up (12‐month).
| Life‐table rates per 100 women for loss to follow‐up (12‐month) | ||
|---|---|---|
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 6.2 | 6.2 |
Thiery 1980 noted that results for immediate insertion of the ML Cu 250 and the TCu 200 were similar (Analysis 11.1 to Analysis 11.4). Twelve‐month rates of expulsion per 100 women were 9.9 for the ML Cu 250 and 11.2 for the TCu 200. Corresponding pregnancy rates were 2.4 and 0.5. The 12‐month continuation rates were 77.3 and 77.2, respectively. For 12 months, rates for loss to follow‐up were 1.6 for the ML Cu250 and 2.0 for the TCu 200. At 24 months, the differences between the devices in pregnancy and expulsion rates reportedly showed "borderline" significance.
11.1. Analysis.
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Life‐table rates per 100 women for expulsion (12‐month) | ||
|---|---|---|
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 11.2 | 9.9 |
11.4. Analysis.
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 4 Life‐table rates per 100 women for loss to follow‐up (12‐month).
| Life‐table rates per 100 women for loss to follow‐up (12‐month) | ||
|---|---|---|
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 2.0 | 1.6 |
Xu 1996 showed hand versus instrument insertion of the CuT 380A IUD to be comparable. The study groups did not differ significantly for expulsion by six months (Analysis 12.1), nor for continuation at six months (Analysis 12.2). No pregnancies, perforations, or infections occurred. Loss to follow‐up by six months was 3% for the hand‐insertion group and 7% for the instrument‐insertion group.
12.1. Analysis.
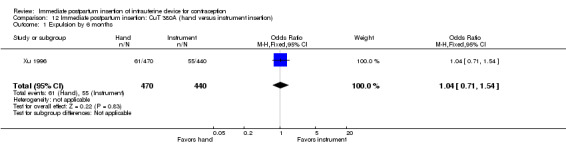
Comparison 12 Immediate postpartum insertion: CuT 380A (hand versus instrument insertion), Outcome 1 Expulsion by 6 months.
12.2. Analysis.
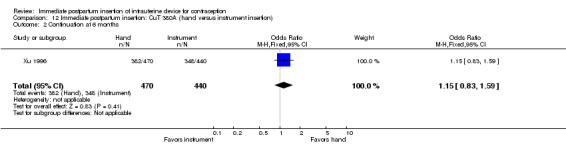
Comparison 12 Immediate postpartum insertion: CuT 380A (hand versus instrument insertion), Outcome 2 Continuation at 6 months.
Discussion
Summary of main results
Sensitivity analysis
In this synthesis, we included trials with sufficient outcome data and evidence of moderate or high quality (Table 3). We focused on the trials that compared IUC insertion times (Table 4; Table 5). Four of these newer trials met the criteria; three used the LNG‐IUS and one used the CuT 380A.
A pilot trial examined immediate postplacental versus early insertion of the LNG‐IUS, and also examined standard insertion. All women in the immediate and early groups received the IUC (N = 30). Expulsion and use data can be found in Table 1. Expulsion rates were the same for both groups by six months. The study arms did not differ significantly for use at six months.
Data on immediate versus standard insertion came from meta‐analysis of four trials (N = 243). Results provide the basis for Table 2.
For rates of insertion (IUC received), the study arms were not significantly different. However, the Uganda study showed that insertion was more likely for the immediate group, although the estimate was imprecise. The three US studies indicated little difference between the groups.
Expulsion by six months was more likely for the immediate group, but the confidence interval was wide. Two trials examined the LNG‐IUS after vaginal delivery. The other two placed the IUC after cesarean section; one used the CuT 380A and the other, the LNG‐IUS.
IUC use at six months was based on the women randomized. Use was more likely for women with immediate insertion than for those with standard insertion. Two studies inserted the IUC after vaginal delivery and two after cesarean section. In individual small trials, the study arms did not differ in use at 3 or 12 months.
Other studies
Three newer trials comparing insertion times used the CuT 380A (Table 3). The conference abstracts lacked design information and outcome data. However, these trials were larger than those with full reports. Two trials examined immediate versus early insertion. Expulsion by six weeks in one study was reported to be lower for the immediate group compared with the early group, and continued use at six months was higher in the immediate group. In the other trial with early insertion, the study arms did not differ significantly in expulsion or continuation at six months. The third trial studied immediate versus standard insertion. Insertion rates did not differ significantly between the study arms, nor did continued use at 12 months.
Eight early studies showed that modifications of IUDs for immediate postpartum insertion were not helpful (Table 6). Modifications included the addition of absorbable sutures to the superior arms of T‐shaped IUDs and Lippes Loops, and the addition of plastic arms to the vertical stem of a Nova T. The choice of insertion technique (hand versus instrument) also appeared to be clinically unimportant. Only one trial noted any significant difference (Apelo 1985), but a later trial with better methods found no substantial differences (Xu 1996).
Overall completeness and applicability of evidence
Three of the seven newer trials examined different insertion times for the LNG‐IUS and four compared insertion times for the CuT 380A. Three placed the IUC after vaginal delivery, two after cesarean delivery, and two after either type of delivery. The eight trials from the 2001 review compared different devices or insertion techniques with immediate insertion rather than different insertion times. Several of those trials examined immediate insertion after vaginal delivery, but others did not specify the type of delivery. Many of the IUDs examined in the earlier studies are no longer widely used. Overall, limited data were provided on safety (e.g., perforation and infection) and on other adverse events.
Immediate IUC insertion is common in many areas of the world. Of the trials comparing insertion times, four were conducted in the USA, two in India, and one in Uganda. The early trials of immediate insertion were conducted in Eastern and Western Europe, South America, and Asia. Three were multisite trials. Non‐randomized studies also provide evidence of how common immediate IUC insertion has become; some are discussed below (Agreements and disagreements with other studies or reviews). Feasibility studies show that IUC insertion may be acceptable in some settings, but conducting an RCT may not. In a study of early versus standard insertion in Malawi, nearly the number planned were recruited but less than half were randomized (Bryant 2013). In Kampala, Uganda, more than half the women screened opted out of the feasibility study for intra‐cesarean insertion (Lester 2015). Surveys of the women who opted out, as presented in a conference abstract, indicated that 41% wanted to ask their husband first, 16% did not like IUC, and 15% said their husband did not like IUC (Lester 2015).
Insertion of hormonal IUC may be delayed because of concerns about the effect on lactation. Another Cochrane review examined hormonal contraceptives and lactation (Lopez 2015). Two included studies did provide data on lactation with LNG‐IUS use. In a secondary analysis from Chen 2010 (Chen 2011), women who had immediate postpartum insertion stopped exclusive breastfeeding earlier than women who had standard insertion. The trial was not designed to measure this effect. The pilot study (Dahlke 2011) stated that breastfeeding at six months did not differ significantly across the immediate, early, and standard groups, although the standard group had the lowest rate. Most progestin‐only methods are considered category 3 for less than six weeks postpartum (WHO 2009). Category 3 means that the theoretical or proven risks usually outweigh the advantages of using the method. The method is not usually recommended unless more appropriate methods are unavailable or unacceptable. In the USA, for the first month postpartum, progestin‐only methods are category 2, meaning that the advantages of using the method generally outweigh the risks (CDC 2011).
Quality of the evidence
We summarized our assessment of the evidence quality (Table 3). Risk of bias is also summarized in Figure 2 and is shown by study in Figure 3. Overall, we considered the evidence to be of moderate quality. About a fourth of the reports lacked information on the randomization process and allocation concealment. Several trials had no blinding or no mention of blinding. The timing of insertion made blinding of participants unfeasible. Assessors could have been blinded, but the outcomes were often expulsion and IUC use. Standards for reporting trials were developed in the late 1990s, and the CONSORT statement was widely adopted in 2010 (Schulz 2010). The three recent trials for which we had only a conference abstract lacked information needed to assess risk of bias. For two of those trials, the investigators communicated that full reports were in progress.
Agreements and disagreements with other studies or reviews
Immediate postpartum insertion of IUC involves trade‐offs. Expulsion rates appear higher with postplacental and early postpartum insertion than with standard insertion. The net effect of these expulsions is not clear from published studies, for example, if detected and another contraceptive is started, accidental pregnancies might be avoided. In the two more recent studies that assessed pregnancy, none was reported. The earlier studies had varied pregnancy rates. Insertion of IUC immediately after delivery is convenient for both the woman and the clinician. Resumption of ovulation can be unpredictable after delivery, and IUC provides highly effective contraception during the puerperium.
Non‐randomized studies provide some information about expulsion and IUC use or continuation after immediate or early insertion. Over a five‐year period in India, 1037 women had a CuT 200B inserted immediately after vaginal (37%) or cesarean (63%) delivery (Shukla 2012). The expulsion rate was 11% by six months and most occurred in the first six weeks. A prospective case series of early LNG‐IUS insertion after vaginal delivery was conducted at a university medical center in the USA. With only 40 women enrolled, the expulsion rate was 38% (Stuart 2012). The results may not be reliable because of the small sample size. In Turkey, 268 women chose the timing for CuT 380A insertion after vaginal or cesarean delivery: immediate, early (up to 72 hours), or standard (Eroglu 2006). Complete expulsion was higher for the immediate (11%) and early (14%) groups compared with the standard insertion group (3.6%) at eight weeks. In Pakistan, 100 women had a CuT 380A or Multiload inserted immediately after vaginal or cesarean delivery (Alam 2014). Complete expulsion occurred in three women by 10 weeks.
Early trials included in this review indicated that immediate postpartum insertion of IUDs was safe and effective. Decades ago, WHO 1980 judged expulsion and pregnancy rates to be excessive for women who underwent immediate IUD insertion. However, variable clinical experience, rather than patient characteristics, may be responsible (Chi 1985; Kisnisci 1985; Thiery 1985). Two systematic reviews examined studies of various designs that compared different insertion times or routes (Kapp 2009; Sonalkar 2014). Evidence from studies of copper‐containing IUDs and the LNG‐IUS indicated that postplacental and early postpartum insertion was safe compared with standard insertion, but expulsion rates were higher.
Expulsion rates may be lower for postplacental insertion after cesarean delivery than after vaginal delivery (Kapp 2009; Sonalkar 2014). Recent studies examined immediate insertion after cesarean delivery but were uncontrolled. A study in Turkey examined the safety and efficacy of immediate insertion of the CuT 380A during cesarean section (Celen 2011). At six months, the cumulative expulsion rate was 10.6 per 100 women and the continuation rate was 81.6%. A feasibility study of immediate insertion after cesarean delivery assessed expulsion and acceptability among 90 women in the USA (Levi 2012). Among the 60% who returned for a six‐week visit or provided a six‐month phone interview, no expulsions were identified by examination or ultrasound nor were reported by the women. The investigators emphasized the importance of providing effective contraception before discharge, given that only 48% returned for the six‐week postpartum visit. A pilot study in Dakar, Senegal, examined cesarean insertion of IUC in 46 women (Gueye 2013). The expulsion rate at three months was 2.2%. In India, 564 women had intra‐cesarean insertion, and the expulsion rate was 9% by six months (Mishra 2014).
Authors' conclusions
Implications for practice.
Recent trials compared different times for postpartum IUC insertion after vaginal or cesarean delivery. However, the evidence is limited because only a few studies had full reports, and most were small trials. Expulsion appeared more likely for immediate postplacental than for standard insertion. Later IUC use did not differ for postplacental insertion compared with standard insertion.
Some early trials of immediate postpartum insertion reported expulsion rates higher than those reported in the literature for standard insertion. The IUC modifications were not helpful in reducing expulsion rates. Insertion of IUC by hand or by instrument appeared to be equally successful.
Counseling women is difficult when evidence from randomized controlled trials is limited. The benefit of providing highly effective contraception immediately after delivery may outweigh the disadvantage of increased risk of expulsion. Clinical follow‐up is important in detecting early expulsion, as is educating women to recognize the signs and symptoms of expulsion (Dahlke 2011). Frequent prenatal visits in the third trimester provide the opportunity to discuss effective contraceptive methods and desired timing for initiation (Teal 2014a).
Implications for research.
Although we found seven new trials, the evidence is still limited. Of the four new studies with full reports, three had small sample sizes. Another three trials provided preliminary results in conference abstracts. These trials were larger than those with full reports and may provide more definitive results. However, they show few significant differences between study arms. Overall, the quality of the evidence was moderate. The abstracts and the older studies provided less information on design and outcomes.
More data are becoming available on comparisons of immediate postplacental versus early or standard insertion. Ongoing trials and one recently completed study will add to the evidence, although some are small. Several address placement after vaginal delivery, for which expulsion rates are high with immediate insertion. Trials of adequate power are needed for more accurate estimation of expulsion and use rates, as well as perforations and other adverse events. Adequate follow‐up is also important for assessing those main outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 7 May 2015 | New citation required and conclusions have changed | 7 new trials compared immediate insertion versus early or standard insertion; 8 earlier trials compared immediate insertion of different devices or insertion techniques |
| 1 April 2015 | New search has been performed | Search updated |
| 26 February 2015 | Amended | Moved 1 trial from Ongoing studies to Studies awaiting classification |
| 28 January 2015 | Amended | Updated terminology in Types of interventions to define 'early' and 'standard' insertion |
| 12 January 2015 | Amended | Updated wording of Objectives and Types of outcome measures to better reflect focus of this new version |
| 4 August 2014 | New search has been performed | 7 new Included studies and 4 Ongoing studies |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 17 February 2010 | New citation required but conclusions have not changed | Preliminary results from a new trial added (Chen 2010). Also, trial in progress identified (Gilliam 2013) |
| 16 February 2010 | New search has been performed | Searches updated |
| 15 April 2008 | Amended | Converted to new review format |
| 30 January 2001 | New citation required and conclusions have changed | Substantive amendments made |
Acknowledgements
Carol Manion of FHI 360 assisted with the literature searches for the initial review and updates.
Appendices
Appendix 1. Search 2015
MEDLINE via PubMed (to 1 April 2015)
(postpartum OR postplacental OR puerperium OR postcesarean OR delivery OR cesarean section) AND (iud* OR iucd* OR IUS OR (intrauterine AND (device* OR system))) AND insert* Article types: Clinical trial
CENTRAL (12 January 2015)
Title, Abstract, Keywords: (postpartum OR post‐partum OR postplacental) OR ((delayed OR interval OR immediate OR early) AND insertion) AND Title, Abstract, Keywords: IUD* OR IUS OR (intrauterine AND (device* OR system)) NOT Record Title: aspiration OR abortion* OR evacuation Publication year: 2009 to 2015 in Trials
Web of Science (27 September 2014)
TOPIC: (IUD OR IUS OR (intrauterine AND (device OR system))) AND TOPIC: (insertion) AND TOPIC: (postpartum OR post‐partum OR postplacental OR puerperium) Refined by: DOCUMENT TYPES: ( ARTICLE OR MEETING ABSTRACT OR PROCEEDINGS PAPER ) Timespan: 2009 to 2014
EMBASE (10 September 2014)
((iud OR IUCD OR IUS) and insertion) AND (postpartum OR puerperium) Study types: randomized controlled trial OR clinical trial Publication year: 2009 to 2014
POPLINE (10 September 2014)
Keyword: IUD AND Keyword: insertion AND Keyword: Puerperium OR postpartum OR postcesarean AND Keyword: Clinical Trials Years: 2009 to 2014
LILACS (to 2 February 2015)
intrauterine device OR intrauterine systeme or dispositivos intrauterinos or dispositivos intra‐uterinos [Words] and childbirth or parto or delivery, obstetric or parto obstetrico or postpartum or posparto or pos‐parto or puerperium or puerperio or cesarian section or cesarean [Words] and insertion or insertions or inserted or insert [Words]
ClinicalTrials.gov (12 January 2015)
Search terms: (immediate OR postplacental OR post‐placental OR delayed OR interval) AND insertion Interventions: IUD OR IUS OR (intrauterine AND (device OR system)) Title: NOT (abortion OR termination OR pain OR lidocaine) First received: 01 January 2010
ICTRP (12 January 2015)
delayed OR immediate* OR early in the Title AND (intrauterine device OR intrauterine system OR IUD OR IUS) in the Intervention Recruitment status: All Date of registration: 01 January 2010 to 12 January 2014
Appendix 2. Previous search
MEDLINE via PubMed (to January 2010)
(postpartum OR puerperium OR postcesarean OR delivery OR cesarean section) AND (iud* OR iucd* OR intrauterine devices OR intrauterine device) AND insert*
EMBASE (to February 2010)
(iud? (3n)insertion? OR iucd? (3n)insertion) AND (postpartum OR puerperium) AND (trial? OR study).
POPLINE (to February 2010)
1) IUD(kw) AND insertion(kw) AND (postpartum(tw) OR puerperium(tw) OR postcesarean(tw))
2) iud(kw) AND (clinical trials(kw) OR clinical research(kw)) AND (postpartum(tw) OR puerperium(tw) OR postcesarean(tw)).
LILACS (to February 2010)
intrauterine devices or dispositivos intrauterinos or dispositivos intra‐uterinos [Words] and childbirth or parto or delivery, obstetric or parto obstetrico or postpartum or posparto or pos‐parto or puerperium or puerperio or cesarian section or cesarean [Words] and insertion or insertions or inserted or insert [Words].
CENTRAL (to February 2010)
1) postpartum OR post‐partum in Title, Abstract or Keywords AND IUD* OR intrauterine device* in Title, Abstract or Keywords
2) intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion) in Title, Abstract or Keywords
ClinicalTrials.gov (to February 2010)
(intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion)
ICTRP (to February 2010)
(intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion)
Data and analyses
Comparison 1. Immediate postplacental insertion versus early insertion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Placement (insertion) | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Expulsion by 6 months | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.20, 5.04] |
| 3 Use at 3 months | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.75] |
| 4 Use at 6 months | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.75] |
| 5 Expulsion | Other data | No numeric data | ||
| 6 Use at 6 months | Other data | No numeric data |
Comparison 2. Immediate postplacental insertion versus standard insertion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Placement (insertion) per protocol | 4 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 4.07 [0.54, 30.40] |
| 2 Expulsion by 6 months | 4 | 210 | Odds Ratio (M‐H, Random, 95% CI) | 4.89 [1.47, 16.32] |
| 3 Use at 6 months | 4 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [1.01, 4.09] |
| 4 Use at 3 months | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 5.35] |
| 5 Use at 12 months | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.63, 7.44] |
| 6 Placement (insertion) | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.87, 4.76] |
| 7 Continued use at 12 months | Other data | No numeric data |
Comparison 3. Immediate postpartum insertion: Delta Loop versus Lippes Loop D.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Insertion per protocol | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) | Other data | No numeric data |
3.2. Analysis.
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Life‐table rates per 100 women for expulsion (6‐month) | ||
|---|---|---|
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 15.7 | 21.5 |
3.3. Analysis.
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Life‐table rates per 100 women for continuation (6‐month) | ||
|---|---|---|
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 78.5 | 73.8 |
3.4. Analysis.
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Life‐table rates per 100 women for follow‐up (6‐month) | ||
|---|---|---|
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 62.7% | 66.1% |
Comparison 4. Immediate postpartum insertion: Delta T versus TCu 220 C.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Insertion per protocol | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) | Other data | No numeric data |
4.2. Analysis.
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Life‐table rates per 100 women for expulsion (6‐month) | ||
|---|---|---|
| Study | Delta T | TCu 220 C |
| Cole 1984 | 11.6 | 11.5 |
4.3. Analysis.
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Life‐table rates per 100 women for continuation (6‐month) | ||
|---|---|---|
| Study | Delta T | TCu 220 C |
| Cole 1984 | 81.8 | 81.8 |
4.4. Analysis.
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Life‐table rates per 100 women for follow‐up (6‐month) | ||
|---|---|---|
| Study | Delta T | TCu 220 C |
| Cole 1984 | 71.0% | 70.9% |
Comparison 5. Immediate postpartum insertion: Delta Loop (hand versus instrument insertion).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Insertion per protocol | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) | Other data | No numeric data |
5.2. Analysis.
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Life‐table rates per 100 women for expulsion (6‐month) | ||
|---|---|---|
| Study | Hand insertion | Instrument insertion |
| Cole 1984 | 11.2 | 11.5 |
5.3. Analysis.
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Life‐table rates per 100 women for continuation (6‐month) | ||
|---|---|---|
| Study | Hand insertion | Instrument insertion |
| Cole 1984 | 84.2 | 82.6 |
Comparison 6. Immediate postpartum insertion: Delta T versus Delta Loop.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Insertion per protocol | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.12, 76.20] |
| 2 Life‐table rates per 100 women for expulsion (12‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for pregnancy (12‐month) | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for continuation (12‐month) | Other data | No numeric data | ||
| 6 Life‐table rates per 100 women for follow‐up (12‐month) | Other data | No numeric data |
6.2. Analysis.
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 2 Life‐table rates per 100 women for expulsion (12‐month).
| Life‐table rates per 100 women for expulsion (12‐month) | ||
|---|---|---|
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 7.6 | 3.7 |
6.3. Analysis.
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 3 Life‐table rates per 100 women for pregnancy (12‐month).
| Life‐table rates per 100 women for pregnancy (12‐month) | ||
|---|---|---|
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 0 | 2.1 |
6.4. Analysis.
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 4 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month).
| Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) | ||
|---|---|---|
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 1.0 | 1.1 |
6.5. Analysis.
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 5 Life‐table rates per 100 women for continuation (12‐month).
| Life‐table rates per 100 women for continuation (12‐month) | ||
|---|---|---|
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 90.7 | 93.3 |
Comparison 7. Immediate postpartum insertion: TCu 200 versus Progestasert.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hand insertion: Life‐table rates per 100 women for expulsion (12‐month) | Other data | No numeric data | ||
| 2 Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month) | Other data | No numeric data | ||
| 3 Hand insertion: Life‐table rates per 100 women for continuation (12‐month) | Other data | No numeric data | ||
| 4 Instrument insertion: Life‐table rates per 100 women for continuation (12‐month) | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for follow‐up (12‐month) | Other data | No numeric data |
7.2. Analysis.
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 2 Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month).
| Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month) | ||
|---|---|---|
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 8.1 | 35.2 |
7.3. Analysis.
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 3 Hand insertion: Life‐table rates per 100 women for continuation (12‐month).
| Hand insertion: Life‐table rates per 100 women for continuation (12‐month) | ||
|---|---|---|
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 86.3 | 59.9 |
7.4. Analysis.
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 4 Instrument insertion: Life‐table rates per 100 women for continuation (12‐month).
| Instrument insertion: Life‐table rates per 100 women for continuation (12‐month) | ||
|---|---|---|
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 86.1 | 57.2 |
Comparison 8. Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg.
8.2. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 2 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) by device and insertion method.
| Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 5.5 | 0 | 3.2 | 5.2 |
8.3. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 3 Life‐table rates per 100 women for continuation (12‐month) by device and insertion method.
| Life‐table rates per 100 women for continuation (12‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 73.8 | 84.9 | 57.3 | 77.1 |
8.4. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 4 Life‐table rates per 100 women for expulsion (36‐month) by device and insertion method.
| Life‐table rates per 100 women for expulsion (36‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 19.9 | 13.1 | 39.0 | 24.2 |
8.5. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 5 Life‐table rates per 100 women for continuation (36‐month) by device and insertion method.
| Life‐table rates per 100 women for continuation (36‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 67.7 | 62.9 | 52.3 | 55.8 |
8.6. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 6 Life‐table rates per 100 women for expulsion (36‐month) by device pooled.
| Life‐table rates per 100 women for expulsion (36‐month) by device pooled | ||
|---|---|---|
| Study | TCu 200 (pooled) | IPCS‐52 (pooled) |
| Apelo 1985 | 16.4 | 31.3 |
8.7. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 7 Life‐table rates per 100 women for expulsion (36‐month) by method pooled.
| Life‐table rates per 100 women for expulsion (36‐month) by method pooled | ||
|---|---|---|
| Study | Hand (pooled) | Inserter (pooled) |
| Apelo 1985 | 29.2 | 18.5 |
8.8. Analysis.
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 8 Life‐table rates per 100 women for follow‐up (12‐month) by device and insertion method.
| Life‐table rates per 100 women for follow‐up (12‐month) by device and insertion method | ||||
|---|---|---|---|---|
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 71.1% | 99.1% | 82.8% | 82.5% |
Comparison 9. Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Life‐table rates per 100 women for expulsion (12‐month) | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for discontinuation (12‐month) | Other data | No numeric data | ||
| 4 Loss to follow‐up (unclear time frame) | Other data | No numeric data |
9.2. Analysis.
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Life‐table rates per 100 women for pregnancy (12‐month) | |||
|---|---|---|---|
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 5.6 | 12.1 | 7.2 |
9.3. Analysis.
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 3 Life‐table rates per 100 women for discontinuation (12‐month).
| Life‐table rates per 100 women for discontinuation (12‐month) | |||
|---|---|---|---|
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 53.1 | 60.9 | 47.7 |
Comparison 10. Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Life‐table rates per 100 women for expulsion (12‐month) | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for loss to follow‐up (12‐month) | Other data | No numeric data |
10.2. Analysis.
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Life‐table rates per 100 women for pregnancy (12‐month) | ||
|---|---|---|
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 0.6 | 0 |
10.3. Analysis.
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 3 Life‐table rates per 100 women for continuation (12‐month).
| Life‐table rates per 100 women for continuation (12‐month) | ||
|---|---|---|
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 87.4 | 78.2 |
Comparison 11. Immediate postpartum insertion: TCu 200 versus ML Cu 250.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Life‐table rates per 100 women for expulsion (12‐month) | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for loss to follow‐up (12‐month) | Other data | No numeric data |
11.2. Analysis.
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Life‐table rates per 100 women for pregnancy (12‐month) | ||
|---|---|---|
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 0.5 | 2.4 |
11.3. Analysis.
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 3 Life‐table rates per 100 women for continuation (12‐month).
| Life‐table rates per 100 women for continuation (12‐month) | ||
|---|---|---|
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 77.2 | 77.3 |
Comparison 12. Immediate postpartum insertion: CuT 380A (hand versus instrument insertion).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Expulsion by 6 months | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.54] |
| 2 Continuation at 6 months | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahuja 2014.
| Methods | Location and time frame: New Delhi, India; planned time frame 16 January 2012 to mid‐2013 Design: randomized controlled trial Sample size calculation and outcome of focus: based on expulsion rate 11% in immediate group and 28% in early group, power 80% and 95% CI, attrition rate 25%; total calculated as 215 with 108 in each group |
|
| Participants | General with N: 263 women with vaginal delivery in 1 hospital Inclusion criteria: 15 to 45 years of age; willing to have postpartum CuT insertion and follow‐up until 6 months postpartum Exclusion criteria: contraindications to CuT 380A insertion; cesarean delivery |
|
| Interventions | CuT 380A IUD postplacental insertion Treatment: immediate (within 10 minutes of placenta expulsion); report refers to as "postplacental" Comparison: early (10 minutes to 48 hours post delivery); report refers to as "immediate postpartum" |
|
| Outcomes | Expulsion rate, continuation rate, complication rate, removal rate for pain and bleeding, patient acceptability, failure rate Assessment times: 6 months to 1 year |
|
| Notes | Information from oral presentation (abstract only) and clinical trial listing 5 September 2014: Investigator communicated that final report was in progress |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial listing states "not applicable" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: not specified in abstract |
Apelo 1985.
| Methods | Location and time frame: Manila, Philippines; approximately 1979 to 1982, based on related report (Cole 1984) Design: RCT comparing 4 devices and 2 insertion techniques Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 200 women (50 in each group) Inclusion criteria: no mention Exclusion criteria: no mention |
|
| Interventions | Immediate IUD insertion (within 10 minutes of placenta expulsion) by hand or instrument Treatment: ICPS‐52 (Intrauterine Contraceptive Progestasert System) with 52 mg reservoir of progesterone, releasing 65 μg/d over 3 years Comparison: TCu 200 IUD |
|
| Outcomes | Reported pregnancy, terminations for expulsion and bleeding or pain, and continuation Assessment times: 12‐month life‐table rates reported |
|
| Notes | ICPS‐52 was modification of Progestasert IUD that had 38 mg reservoir of progesterone, designed for 1 year of use Data on Delta Loop versus Lippes Loop D are in Cole 1984 and are not repeated for this report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence according to correspondence with investigator and study files |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered, opaque envelopes according to correspondence with investigator and study files |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: not presented Follow‐up (reported 12‐month) as % women not previously terminated: ICPS‐52, 83% for hand or instrument insertion; TCu 200, 71% for hand insertion and 99% for instrument insertion |
Chen 2010.
| Methods | Location and time frame: Pittsburgh, PA (USA), from May 2007 to October 2008 Design: randomized controlled trial Sample size calculation and outcome of focus: expected 40% of standard insertion participants would not return for insertion, 4% of inserted IUDs would be discontinued in 6 months because of removal requests, and expulsion would be 11% immediate group and 2% standard group; estimated proportions continuing IUD at 6 months were 0.850 immediate and 0.564 standard; 92 participants (46 per group) for 80% power to detect 34% difference in use at 6 months and significance level 0.05. Increased to 51 per group to allow 10% loss to follow‐up. Expected 40% would not be eligible for insertion because of post‐enrollment ineligibility criteria, increasing the total to 168. |
|
| Participants | General with N: 124 randomized; 102 remained eligible after randomization Inclusion criteria: 18 years and older; ≥ 24 0/7 weeks pregnant at enrollment; anticipates vaginal delivery; desires LNG system for postpartum contraception Exclusion criteria: scheduled cesarean section; allergy to polyethylene or levonorgestrel or other contraindication to LNG‐IUD use; exposure to or treatment for gonorrhea, chlamydia, or trichomoniasis during the pregnancy without subsequent negative test of cure; leiomyomata > 3 cm diameter; uterine anomaly (other than repaired septate uterus); current cervical cancer or carcinoma in situ; desires repeat pregnancy within 1 year of delivery Post‐enrollment ineligibility: cesarean delivery; clinical diagnosis of chorioamnionitis; sexually transmitted infection without negative test of cure; rupture of membranes for > 24 hours; postpartum hemorrhage; rupture of membranes at < 34 weeks gestation; participant no longer desires IUD; precipitous delivery so investigators could not begin within 10 minutes; investigators not notified of labor and delivery |
|
| Interventions | LNG‐IUS insertion Treatment: immediate (within 10 minutes postplacental) Comparison: standard (6 to 8 weeks postpartum) |
|
| Outcomes | Insertion, IUD use, continuation of IUD, expulsion, pregnancy (tested), safety (infection and perforation), pain with insertion, quality of life, contraceptive use Assessment times: visit 6 to 8 weeks post delivery, phone interview at 3 months, and visit at 6 months |
|
| Notes | Among women randomized to immediate insertion, 5 had IUD inserted from 11 to 15 minutes postplacental. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study staff performing 3‐ and 6‐month evaluations were blinded to randomization assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 12% (5 immediate and 7 standard) Ineligible after randomized as per study criteria: 18% each group |
Cole 1984.
| Methods | Location and time frame: 15 sites in 13 countries from April 1979 through June 1982 Design: RCT with 3 comparisons Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 3791 women Inclusion criteria: normal vaginal delivery and consented to immediate postpartum insertion of IUD Exclusion criteria: no mention |
|
| Interventions | IUD insertion within 10 minutes of placenta delivery
|
|
| Outcomes | Reported pregnancy, terminations for expulsion, and continuation Assessment time: 1, 3, and 6 months after insertion |
|
| Notes | Only RCT data are included in this review; report also summarizes data from non‐randomized studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence (from correspondence or study files) |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered, opaque envelopes (from correspondence or study files) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: not presented Follow‐up (reported 6‐month) as % women not previously terminated: Lippes Loop D 66.1, Delta Loop 62.7, TCu 220 C 70.9, Delta T 71.0, Delta Loop hand 84.2, Delta Loop instrument 83.0 |
Dahlke 2011.
| Methods | Location and time frame: Portsmouth, VA (USA), from August 2009 to January 2010 Design: RCT, pilot study Sample size calculation: Discussion notes study was not powered to detect difference in utilization at 6 months. Pilot study was planned to determined feasibility of later trial. |
|
| Participants | General with N: 53 women in a single medical center Inclusion criteria: desired LNG‐IUS (Mirena, Bayer) for postpartum contraception at presentation for admission to labor and delivery and had no contraindication Exclusion criteria: congenital or acquired uterine anomaly including fibroids that distort uterine cavity; known or suspected uterine or cervical neoplasia or unresolved abnormal Pap smear; untreated acute cervicitis or known vaginitis including bacterial vaginosis or other lower genital tract infection; acute liver disease or liver tumor; hypersensitivity to any product component; and known or suspected carcinoma of breast Intrapartum exclusion: postpartum hemorrhage (blood loss > 500 mL); chorioamnionitis; cesarean delivery |
|
| Interventions | LNG‐IUS insertion
|
|
| Outcomes | Insertion, utilization rate, pain at time of placement, breastfeeding status, blood loss, intrauterine infection rate, expulsion rate Assessment times: 3 and 6 months post delivery by phone (n = 36) or record review (n = 7) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sealed, numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding according to ClinicalTrials.gov listing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 2% (1 in standard group) Excluded after randomization because of cesarean section, as per trial criteria: 13% (2 each from immediate and early, 3 from standard) |
Kisnisci 1985.
| Methods | Location and time frame: Ankara, Turkey, from November 1979 to August 1980 Design: RCT comparing 2 modifications of existing IUDs Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 246 women Inclusion criteria: no mention Exclusion criteria: no mention |
|
| Interventions | Immediate IUD insertion (within 10 minutes of placenta delivery) Treatment: Delta Loop Comparison: Delta T |
|
| Outcomes | Reported pregnancy, expulsion, removal for bleeding or pain, and continuation rates Assessment times: 1, 3, 6, 12 months |
|
| Notes | 1 center from Cole 1984 that reported separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: not presented Follow‐up (reported 12‐month) as % women not previously terminated: Delta Loop 78.9, Delta T 86.7 |
Lavin 1983.
| Methods | Location and time frame: Santiago, Chile, from November 1978 to February 1980 Design: RCT comparing 2 standard IUDs Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 400 women Inclusion criteria: no mention Exclusion criteria: no mention |
|
| Interventions | Immediate IUD insertion (within 10 minutes of placenta); hand or instrument insertion Treatment: Progestasert IUD contained 38 mg progesterone, releasing 65 μg/d over 1 year Comparison: TCu 200 |
|
| Outcomes | Reported pregnancy, expulsion, and continuation rates Assessment times: 12‐month life‐table rates |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence (from correspondence with investigator and study files) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes (from correspondence with investigator and study files) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up: not presented Follow‐up (reported 12‐month life‐table rates): TCu hand, 76.1; TCu instrument, 69.7; Progestasert hand, 77.4; Progestasert instrument, 67.7 |
Lester 2015.
| Methods | Location and time frame: Kampala, Uganda, from February 2011 to December 2011 Design: RCT; pilot study to determine feasibility of study Sample size calculation and outcome of focus: based on literature and postpartum return rates at Mulago, 40% do not return for delayed insertion and 10% discontinue in 6 months. 90% immediate group and 54% delayed group would use IUD at 6 months. Sample 30 per group for 84% power (β = 0.16) to detect difference of 36% between groups, using significance level 0.05 |
|
| Participants | General with N: 68 randomized in national referral hospital Inclusion criteria: pregnant at enrollment; scheduled for cesarean delivery > 8 hours after consent; desires copper IUD; age ≥ 18; English or Luganda speaking; willing to be accompanied home from hospital and to have address recorded; willing to have home visit at 6 months postpartum if no return for scheduled visit Exclusion criteria: allergy to copper; pelvic tuberculosis; severe thrombocytopenia; positive test for gonorrhea or Chlamydia; leiomyomata or other anomaly that prevents IUD placement; cervical cancer or carcinoma in situ; desire for repeat pregnancy < 12 months; chorioamnionitis; preterm birth < 34 weeks; diagnosis of AIDS (HIV not exclusion criterion); fetal demise; hemorrhage; ruptured uterus; eclampsia; evidence of severe anemia Post‐enrollment exclusion criteria: participant no longer desires CuT 380A or
|
|
| Interventions | CuT 380A IUD inserted after cesarean section Treatment: immediate postplacental (within 10 minutes) Comparison: standard (6 weeks) |
|
| Outcomes | IUD insertion and use at 6 months post delivery, time to IUD expulsion, time to pregnancy; safety measures (infection, uterine perforation), vaginal bleeding, satisfaction Assessment times: delivery, 6‐week postpartum visit, phone at 3 months, clinic visit at 6 months |
|
| Notes | Information obtained from full report as well as ClinicalTrials.gov. Results from related abstract are also mentioned (see Averbach 2012). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted‐block randomization scheme with block sizes of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes; opened after delivery of baby and placenta, given no intraoperative exclusion criteria developed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 10% (7/68); immediate 15% (5/34) and delayed 6% (2/34) |
Ogburn 2013.
| Methods | Location and time frame: no mention; investigator at university in Albuquerque, NM (USA) Design: randomized trial Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 156 women Inclusion criteria: term vaginal or cesarean delivery Exclusion criteria: no mention |
|
| Interventions | Paragard IUD (CuT 380A) after vaginal or cesarean delivery Treatment: immediate (within 10 minutes of placenta delivery) Comparison: standard (4 to 12 weeks) |
|
| Outcomes | IUD insertion and continuation, reasons for discontinuation, complications Assessment times: 6‐week IUD check and contacted 3, 6, and 12 months after insertion |
|
| Notes | Information from conference abstract only 30 August 2014: Investigator communicated they are working on full report for submission in 1 to 2 months. They had not yet registered the trial at ClinicalTrials.gov. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: no mention |
Singh 2014.
| Methods | Location and time frame: no mention (investigator in Agra, India) Design: randomized trial Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 200 women with term pregnancy Inclusion criteria: desires CuT 380A insertion after vaginal or cesarean delivery Exclusion criteria: severe anemia; prolonged rupture of membranes (> 18 hours); unresolved postpartum hemorrhage; extensive genital trauma; coagulopathies; distorted uterine cavity or medical disorder during pregnancy |
|
| Interventions | CuT 380A IUD insertion after vaginal or cesarean delivery Treatment: immediate postplacental (within 10 minutes) Comparison: early postpartum (10 minutes to 48 hours) |
|
| Outcomes | Efficacy, rate of expulsion, side effects, continuation, pregnancy Assessment times: 6 weeks, 3 months, 6 months postpartum |
|
| Notes | Information from conference poster and abstract; no clinical trial listing found; unable to obtain information from investigator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 3% immediate; 5% standard |
Thiery 1980.
| Methods | Location and time frame: Gent, Belgium, from 1976 to 1978 Design: RCT Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 562 women requesting an IUD Inclusion criteria: no contraindication to IUD use Exclusion criteria: no mention |
|
| Interventions | "Immediately after delivery of placenta" Treatment: Multiload 250 IUD Comparison: TCu 200 IUD |
|
| Outcomes | Reported pregnancy, expulsion, removal for bleeding or pain, removal for other medical reasons, and continuation Assessment times: gross cumulative event rates shown for 6, 12, and 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "List of randomized numbers" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up (reported gross cumulative event rates):
|
Van Kets 1987.
| Methods | Location and time frame: Gent, Belgium, from October 1983 to June 1986 Design: RCT Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 408 women Inclusion criteria: no mention Exclusion criteria: no mention |
|
| Interventions | IUD insertion within 10 minutes of delivery of placenta Treatment: Postpartum Nova T (Nova‐T‐PP) Comparison: Nova T |
|
| Outcomes | Reported pregnancy, expulsion, and continuation rates Assessment times: 1, 3, 9 months and annually up to 3 years |
|
| Notes | Limited details about methods of trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up (reported gross cumulative event rates): 12‐month and 24‐month (same), Nova‐T‐PP 6.2 (95% CI 3.0 to 11.4); Nova‐T 6.2 (95% CI 3.0 to 11.3) |
Whitaker 2014.
| Methods | Location and time frame: Chicago, IL (USA), from May 2007 to July 2010; Evanston, IL (USA), from April 2009 to January 2011 Design: randomized safety and efficacy study Sample size calculation and outcome of focus: assumed 60% of standard insertion would return, 20% of each group would discontinue, estimated 80% postplacental group and 48% standard group would use IUD in 12 months; 46 per group for 90% power to detect difference at alpha = 0.05; assumed 15% loss to follow‐up; planned 108 participants to have 92 participants complete trial; outcome of focus was use at 12 months |
|
| Participants | General with N: 42 women in 2 hospitals (20 postplacental and 22 standard group) Inclusion criteria: pregnant; ≥ 18 years; planning scheduled cesarean delivery; desires LNG‐IUS for contraception; speaks English Exclusion criteria: allergy to polyethylene or levonorgestrel or other contraindication to LNG‐IUS use; positive test for gonorrhea, chlamydia, or trichomoniasis during pregnancy without treatment and subsequent test of cure confirming negative result; leiomyomata distorting uterine cavity; uterine anomaly precluding IUS placement; current cervical cancer or carcinoma in situ; desires repeat pregnancy in 12 months; history of postabortal or postpartum sepsis; randomized to postplacental IUD placement and could not undergo insertion within 10 minutes because of hemorrhage or surgical complication Exclusion at delivery: clinical evidence of infection; prolonged rupture of membranes; or no longer desiring LNG‐IUS |
|
| Interventions | LNG‐IUS after cesarean delivery Treatment: immediate postplacental insertion (within 10 minutes) Comparison: standard insertion, 4 to 8 weeks after delivery |
|
| Outcomes | Use of LNG‐IUS at 12 months after delivery; proportion with IUS inserted per protocol, expulsion, satisfaction with IUD, complications Assessment times: 3 and 6 months after delivery by telephone and 12 months after delivery in clinic (by phone for those with IUD inserted off protocol expelled without replacement or removed) |
|
| Notes | Results also in ClinicalTrial.gov posting Slow recruitment, early termination, small N (42 women) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked‐randomization scheme, stratified by site |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research staff members conducting follow‐up assessments were blinded to participant allocation. ClinicalTrials.gov listing states "single blind (investigator)" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 33% (30% immediate; 36% standard) |
WHO 1980.
| Methods | Location and time frame: Hungary, Belgium, Brazil, United Kingdom, Chile, and Germany Design: RCT at 6 centers Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N: 841 healthy women Inclusion criteria: aged 16 to 40 years; having vaginal delivery; willing to rely on IUD for fertility regulation Exclusion criteria: recent history of pelvic inflammatory disease; sexually transmitted infection treated during current pregnancy; congenital abnormality of uterus or vagina; known or suspected genital tract malignancy; uterine fibroids; or evidence of infection during labor |
|
| Interventions | IUD inserted immediately after expulsion of placenta
Insertions were done with standard or modified IUD inserter or by manual placement at fundus. |
|
| Outcomes | Reported expulsion (partial or complete), pregnancy, removal for bleeding or pain, and other medical removal Assessment times: 6 and 12 months; follow‐up to 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by computer‐generated table of numbers (from communication with investigators) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes with method indicator card (from communication with investigators) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial was stopped prematurely because expulsion rates with all devices exceeded predetermined 20% limit. Women with expulsion within 48 hours after insertion were excluded from life‐table analysis (part of protocol). Loss to follow‐up (reported; time frame unclear): Postpartum T, 17.8%; Lippes Loop, 16.2%; Copper 7, 16% |
Xu 1996.
| Methods | Location and time frame: Shanghai, China, from October 1993 to October 1994 Design: RCT at 13 centers Sample size calculation and outcome of focus: 439 per group (hand versus instrument insertion) for study power of (alpha = 0.05, 1 ‐ beta = 0.9, and f = 0.10); 500 planned to account for attrition; expulsion was outcome of interest |
|
| Participants | General with N: 910 healthy women in antenatal care Inclusion criteria: age 20 to 40 years; no contraindications for IUD use; willing to rely solely on the IUD for fertility regulation; normal vaginal delivery (no complications during labor and delivery) Exclusion criteria: no mention |
|
| Interventions | Insertion of CuT 380A within 10 minutes after placenta delivery Treatment: insertion by hand Comparison: insertion with ring forceps |
|
| Outcomes | Pregnancy, expulsion, and continuation rates Assessment times: 3 and 6 months |
|
| Notes | Investigator provided women‐months for rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence (from correspondence with investigator) |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered opaque envelopes (from correspondence with investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and evaluators were blinded from knowing the insertion method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up (reported 6‐month): hand insertion 3% and instrument insertion 7% |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chi 1985 | Subgroup analysis of Cole 1984 |
| Elsedeek 2012 | Not an RCT, as women chose the timing for insertion; focus on bleeding |
| Eroglu 2006 | Not an RCT, as women chose the timing for insertion |
| Lara Ricalde 2006 | Participants were randomly assigned to type of IUD but not to time frame for insertion. |
| Letti Müller 2005 | Not an RCT; assignment based on type of delivery (vaginal or cesarean) |
| Shikary 1987 | Although a randomized controlled trial, IUD insertions took place 4 to 6 weeks postpartum. |
| Stauble 2014 | Investigator communicated that trial was stopped in 2011. No report prepared because larger study from Pittsburgh was published as this trial began. ClinicalTrials.gov stated expected completion was 2014. |
| Stuart 2015 | Primary outcome of breastfeeding Insertion within 48 hours of delivery versus 4 to 8 weeks, according to listing in ClinicalTrials.gov |
| Tatum 1996 | Although the title states this was a randomized controlled trial, women were allocated by alternate assignment, a non‐random technique that precluded allocation concealment. |
| Thiery 1983 | Data included in Cole 1984 |
| Turok 2014 | Does not have relevant outcome measure for this review; effect on lactation of immediate versus standard insertion of LNG‐IUS |
Characteristics of studies awaiting assessment [ordered by study ID]
Levi 2014.
| Methods | Location and time frame: Chapel Hill, NC (USA), February 2012 to June 2014 Design: randomized controlled trial, open label Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 112 who deliver by cesarean section at university hospital Inclusion criteria: age 18 to 45 years, pregnant ≥ 24 weeks estimated gestational age, live pregnancy, plans to use IUD postpartum for contraception, plans cesarean delivery, intends to stay in Chapel Hill area ≥ 6 months after birth, fluent in English or Spanish Exclusion criteria: known uterine anomalies, allergies to component of IUD of their choice, known or suspected carcinoma of breast, known acute liver disease or liver tumor, known or suspected uterine or cervical neoplasia, active pelvic inflammatory disease, genital bleeding of unknown etiology, history of solid organ transplantation, positive test for gonorrhea or chlamydia during this pregnancy |
| Interventions | LNG‐IUS or CuT 380A Treatment: immediate (cesarean delivery, postplacental) Comparison: standard (4 to 8 weeks post cesarean) |
| Outcomes | IUD use, IUD expulsion, satisfaction with IUD Assessment times: 4‐ to 8‐week postpartum visit; 3‐ and 6‐month visits |
| Notes | Investigator communicated that manuscript was submitted but not yet published. Trial may be included in next update if it meets eligibility criteria. |
Characteristics of ongoing studies [ordered by study ID]
Dias 2014.
| Trial name or title | Randomized Controlled Trial to Assess the Retention of Immediate Post‐delivery Insertion of an Intrauterine Contraceptive Device versus Interval Intrauterine Contraceptive Device Insertion |
| Methods | Location and time frame: Sri Lanka, November 2014 to May 2015 Design: RCT, no blinding |
| Participants | 86 women Inclusion criteria: planned vaginal delivery, desire for copper IUD insertion Exclusion criteria: contraindications for IUD insertion including infection, hemorrhage, and cesarean delivery |
| Interventions | Copper IUD Treatment: immediate insertion (within 10 minutes of placenta delivery) Comparison: standard insertion (6 to 8 weeks postpartum) |
| Outcomes | Retention of IUD Assessment times: 12 hours after insertion; 6 to 8 weeks postpartum |
| Starting date | 01 November 2014 |
| Contact information | Dr. Tiram Dias, University of Kelaniya Sri Lanka; thiran_dias@yahoo.com |
| Notes |
Harper 2014.
| Trial name or title | Mirena Intrauterine System Timing of Insertion: A Randomized Controlled Trial (MISTIC) |
| Methods | Location and time frame: St. Louis, MO (USA), October 2010 to December 2012. Design: randomized controlled trial, open label Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 53 women, enrolled ≥ 36 weeks gestation at study hospital Inclusion criteria: 14 to 45 years, vaginal delivery at study hospital, sexually active with male partner, no tubal ligation or hysterectomy, not currently using contraception, desire reversible contraception, resides in St. Louis City or County, requests LNG‐IUS for contraception Exclusion criteria: allergy to LNG‐IUS, cesarean delivery, cervical cancer, breast cancer, active liver disease, untreated cervicitis, uterine anomaly or fibroids preventing IUS placement, delivery < 36 weeks, chorioamnionitis, prolonged rupture of membranes (> 18 hours) |
| Interventions | LNG‐IUS insertion Treatment: immediate postplacental (within 10 minutes) Comparison: standard (4 to 6 weeks postpartum) |
| Outcomes | LNG‐IUS in place, IUS expulsion, uterine perforation, intrauterine infection Assessment times: 4 to 6 weeks and 6 months postpartum |
| Starting date | October 2010; expected completion December 2012 |
| Contact information | Lorie M. Harper MD; listed as University of Washington School of Medicine, but more recently at University of Alabama at Birmingham (205) 934‐9999 |
| Notes | ClinicalTrials.gov states trial is completed. No report identified. Unable to obtain further information from investigator. |
Teal 2014b.
| Trial name or title | Comparison of the Levonorgestrel IUD and the Copper IUD Placed in the Immediate Postplacental Period: a Randomized Controlled Trial |
| Methods | Location and time frame: Denver, CO (USA), April 2014 to May 2015 for primary outcome Design: randomized controlled trial, open label Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 300 women with vaginal delivery Inclusion criteria: 18 to 45 years old, desires immediate postpartum IUD, English or Spanish speaking, willing to attend 2 follow‐up visits; willing to be randomized (for randomized arms) Exclusion criteria: multiple gestations, delivery < 35 weeks gestational age, allergy or sensitivity to component of either IUD, cesarean delivery, postpartum hemorrhage, chorioamnionitis, abnormal uterine anatomy, treated for chlamydia or gonorrhea during pregnancy without negative follow‐up test, current cervical cancer or carcinoma in situ, current breast cancer, Wilson's disease |
| Interventions | Immediate (≤ 10 minutes) postplacental placement Treatment: LNG‐IUS Comparison: CuT 380A |
| Outcomes | IUD expulsion rate and comparison of complete, partial, and unrecognized expulsion rates; pregnancy, complications, satisfaction, IUD position in uterus Assessment times: 6 weeks and 3 months postpartum |
| Starting date | April 2014; expected completion May 2016 |
| Contact information | Sandra Cano, MS; 720‐505‐7413; sandra.cano@ucdenver.edu Lisa Goldthwaite, MD; 303‐724‐8217; lisa.goldthwaite@ucdenver.edu Principal investigator: Stephanie Teal, MD |
| Notes | Study also has nonrandom (choice) component |
Contributions of authors
Initial review (2001): David A Grimes (formerly of FHI 360) and Kenneth F Schulz (FHI 360) developed the proposal, conducted the literature search, extracted the data, and performed the analysis. Huib van Vliet and Nancy Stanwood were co‐authors who contributed to the writing and revising of the review. At the time, N Stanwood was with the University of Rochester Medical Center (NY).
2005 to 2010: LM Lopez wrote the plain language summary, entered data from original studies into tables, and reviewed search results. In 2010, she incorporated data from an abstract.
2015: LM Lopez conducted the searches, marked the data for extraction from the included studies, and wrote the results and discussion. A Bernholc entered study characteristics and outcome data, and drafted summary tables. G Stuart and D Hubacher clarified technical and clinical issues in the text, and commented on the responses to the peer reviewers. All authors reviewed and commented on the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
2000 to 2015: Support for conducting the review and updates at FHI 360
-
US Agency for International Development, USA.
2000 to 2010: Support for conducting the review and updates at FHI 360 2014: This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of The Evidence Project, cooperative agreement no. AID‐OAA‐A‐13‐00087. The findings and conclusions are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Declarations of interest
Family Health International, now known as FHI 360, conducted two trials (Cole 1984: Kisnisci 1985). An employee of FHI 360 was involved in two trials (Lavin 1983; Apelo 1985). Authors of this review are employed at FHI 360, but none were involved in those trials.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahuja 2014 {published data only (unpublished sought but not used)}
- Ahuja R, Rahtore A. Continuation rates of postpartum intrauterine contraceptive device (IUCD) insertion: randomised trial of post placental versus immediate postpartum insertion (conference abstract). BJOG: An International Journal of Obstetrics and Gynaecology 2014;121(Suppl s2):1‐2. [Google Scholar]
- Rathore AM, Ahuja R. Continuation Rates of Immediate Post partum CuT Insertion: Randomized Trial of Post Placental versus Early Post Partum Insertion. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4103 (accessed 19 August 2014). [CTRI/2012/04/002543]
Apelo 1985 {published and unpublished data}
- Apelo RA, Waszak CS. Postpartum IUD insertions in Manila, Philippines. Advances in Contraception 1985;1(4):319‐28. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only (unpublished sought but not used)}
- Chen BA, Hayes JL, Hohmann HL, Perriera LK, Reeves MF, Creinin MD. A randomized trial of postplacental compared to delayed insertion of the levonorgestrel‐releasing intrauterine device after vaginal delivery (abstract). Contraception 2009;80(2):205. [Google Scholar]
- Chen BA, Reeves MF, Creinin MD, Schwarz EB. Postplacental or delayed levonorgestrel intrauterine device insertion and breast‐feeding duration. Contraception 2011;84(5):499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstetrics and Gynecology 2010;116(5):1079‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 1984 {published and unpublished data}
- Cole LP, Edelman DA, Potts DM, Wheler RG, Laufe LE. Postpartum insertion of modified intrauterine devices. Journal of Reproductive Medicine 1984;29(9):677‐82. [PubMed] [Google Scholar]
Dahlke 2011 {published data only}
- Dahlke JD, Terpstra ER, Ramseyer AM, Busch JM, Rieg T, Magann EF. Postpartum insertion of levonorgestrel‐‐intrauterine system at three time periods: a prospective randomized pilot study. Contraception 2011;84(3):244‐8. [DOI] [PubMed] [Google Scholar]
Kisnisci 1985 {published and unpublished data}
- Kisnisci H, Champion CB. A study of Delta intrauterine devices in Ankara, Turkey. International Journal of Gynaecology and Obstetrics 1985;23(1):51‐4. [DOI] [PubMed] [Google Scholar]
Lavin 1983 {published and unpublished data}
- Lavin P, Bravo C, Waszak C. Comparison of TCu 200 and Progestasert IUDs. Contraceptive Delivery Systems 1983;4(2):143‐7. [PubMed] [Google Scholar]
Lester 2015 {published data only (unpublished sought but not used)}
- Averbach S, Lester F, Fortin J, Byamugisha J, Goldberg A, Kakaire O. Acceptability of the IUD among women who opted out of a randomized controlled trial of intracesarean insertion of the copper‐T 380A in Kampala, Uganda. Contraception 2012;86:318. [Google Scholar]
- Lester F, Kakaire O, Byamugisha J, Averbach S, Fortin J, Maurer R, et al. Intracesarean insertion of the Copper T380A versus 6 weeks post‐cesarean: a randomized clinical trial. Contraception 2015;91(3):198‐203. [DOI] [PubMed] [Google Scholar]
- Lester F, Kakaire O, Byamugisha J, Goldberg A. Intracesarean insertion of the Copper T 380A vs. 6 week post‐cesarean insertion: A pilot randomized controlled trial. 2011 International Conference on Family Planning. 2011 Nov 29‐Dec 01; Dakar, Senegal. www.conftool.com/fpconference2011/index.php?page=browseSessions&form_session=79, (accessed 19 August 2014).
Ogburn 2013 {published data only (unpublished sought but not used)}
- Ogburn T, Espey E, Leeman L, Singh R, Pereda B, Carr S. A randomized trial of immediate postpartum versus interval insertion of an intrauterine device. Contraception 2013;88(3):455. [Google Scholar]
Singh 2014 {published data only (unpublished sought but not used)}
- Singh N, Singh S, Pathak A. Comparison of immediate postplacental and early postpartum intrauterine device insertion (abstract). BJOG ‐an International Journal of Obstetrics and Gynaecology 2014;121(Suppl s2):7‐8. [Google Scholar]
- Singh N, Singh S, Singh S, Pathak A. Comparison of immediate post‐placental and early postpartum intra‐uterine device insertion (conference poster). RCOG World Congress 2014; 2014 March 28‐30; Hyderabad (India). ePostersOnline.
Thiery 1980 {published data only}
- Thiery M, Pas H, Delbeke L, Kets H. Comparative performance of two copper‐wired IUDs (ML Cu 250 and T Cu 200). Immediate postpartum and interval insertion. Contraceptive Delivery Systems 1980;1(1):27‐35. [PubMed] [Google Scholar]
Van Kets 1987 {published data only}
- Kets H, Thiery M, Pas H, Parewijck W. Immediate postpartum insertion: performance of the Nova‐T‐PP and randomized comparison with the Nova‐T. Advances in Contraception 1987;3(1):63‐9. [DOI] [PubMed] [Google Scholar]
Whitaker 2014 {published data only}
- Whitaker AK, Endres LK, Mistretta SQ, Gilliam ML. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception 2014;89(6):534‐9. [DOI] [PubMed] [Google Scholar]
WHO 1980 {published and unpublished data}
- World Health Organization. Comparative multicentre trial of three IUDs inserted immediately following delivery of the placenta. Contraception 1980;22(1):9‐18. [DOI] [PubMed] [Google Scholar]
Xu 1996 {published and unpublished data}
- Xu J‐X, Rivera R, Dunson TR, Zhuang L‐Q, Yang X‐L, Ma G‐T, et al. A comparative study of two techniques used in immediate postplacental insertion (IPPI) of the Copper T 380A IUD in Shanghai, People's Republic of China. Contraception 1996;54(1):33‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chi 1985 {published data only}
- Chi I‐C, Wilkens L, Rogers S. Expulsions in immediate postpartum insertions of Lippes Loop D and Copper T IUDs and their counterpart delta devices ‐‐ an epidemiological analysis. Contraception 1985;32(2):119‐34. [DOI] [PubMed] [Google Scholar]
Elsedeek 2012 {published data only}
- Elsedeek MS. Puerperal and menstrual bleeding patterns with different types of contraceptive device fitted during elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2012;116:31‐4. [DOI] [PubMed] [Google Scholar]
Eroglu 2006 {published data only}
- Eroglu K, Akkuzu G, Vural G, Dilbaz B, Akin A, Taskin L, et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early postpartum period with interval period: 1 year follow‐up. Contraception 2006;74(5):376‐81. [DOI] [PubMed] [Google Scholar]
Lara Ricalde 2006 {published data only}
- Lara Ricalde R, Menocal Tobías G, Ramos Pérez C, Velázquez Ramírez N. Random comparative study between intrauterine device Multiload Cu375 and TCu 380a inserted in the postpartum period [Estudio comparativo al azar entre los dispositivos intrauterinos Multiload Cu375 y TCu 380A, colocados durante el posparto]. Ginecología y Obstetricia de México 2006;74(6):306‐11. [PubMed] [Google Scholar]
Letti Müller 2005 {published data only}
- Letti Müller AL, Lopez Ramos JG, Martins‐Costa SH, Palma Dias RS, Valério EG, Hammes LS, et al. Transvaginal ultrasonographic assessment of the expulsion rate of intrauterine devices in the immediate postpartum period: a pilot study. Contraception 2005;72(3):192‐5. [DOI] [PubMed] [Google Scholar]
Shikary 1987 {published data only}
- Shikary ZK, Betrabet SS, Patel ZM, Patel S, Joshi JV, Toddywala VS, et al. Transfer of levonorgestrel (LNG) administered through different drug delivery systems from the maternal circulation into the newborn infant's circulation via breast milk. Contraception 1987;35(5):477‐86. [DOI] [PubMed] [Google Scholar]
Stauble 2014 {published data only}
- Mary E. Stauble, MD. Safety and Expulsion of Delayed Versus Immediate Postpartum Intrauterine Device Placement (IUD). http://clinicaltrials.gov/ct2/show/NCT01598662 (accessed 19 August 2014).
Stuart 2015 {published data only}
- Stuart G. Postpartum levonorgestrel‐releasing intrauterine system and breastfeeding (PPIUD1). http://clinicaltrials.gov/show/NCT01555931 (accessed 13 August 2014).
- Stuart GS, Lesko C, Stuebe A, Bryant A. Breastfeeding and postpartum insertion of the levonorgestrel intrauterine system: a randomized trial. Obstetrics and Gynecology 2014;123(Suppl 1):15S. [NCT01555931] [Google Scholar]
- Stuart GS, Lesko CR, Stuebe AM, Bryant AG, Levi EE, Danvers AI. A randomized trial of levonorgestrel intrauterine system insertion 6 to 48 hours compared to 6 weeks after vaginal delivery; lessons learned. Contraception 2014;91(4):284‐8. [DOI] [PubMed] [Google Scholar]
Tatum 1996 {published data only}
- Tatum HJ, Beltran RS, Ramos R, Kets H, Sivin I, Schmidt FH. Immediate postplacental insertion of GYNE‐T 380 and GYNE‐T 380 Postpartum intrauterine contraceptive devices: Randomized study. American Journal of Obstetrics and Gynecology 1996;175(5):1231‐5. [DOI] [PubMed] [Google Scholar]
Thiery 1983 {published data only}
- Thiery M, Laufe L, Parewijck W, Pas H, Kets H, Deron R, et al. Immediate postplacental IUD insertion: a randomized trial of sutured (Lippes Loop and TCu 220C) and non‐sutured (TCu 220C) models. Contraception 1983;28(4):299‐313. [DOI] [PubMed] [Google Scholar]
Turok 2014 {published data only}
- Turok D. BLIS ‐ Breastfeeding Levonorgestrel IUD Study. http://clinicaltrials.gov/show/NCT01990703 (accessed 13 August 2014). [NCT01990703]
References to studies awaiting assessment
Levi 2014 {published data only}
- Levi E. Immediate Postplacental Insertion of IUDs at Time of Cesarean Delivery: A Randomized Clinical Trial. http://clinicaltrials.gov/ct2/show/NCT01539759 (accessed 03 September 2014).
References to ongoing studies
Dias 2014 {published data only}
- Dias T. Randomised controlled trial to assess the retention of immediate post‐delivery insertion of an intrauterine contraceptive device versus interval intrauterine contraceptive device insertion. http://trials.slctr.lk/trials/231 (accessed 12 January 2015).
Harper 2014 {published data only (unpublished sought but not used)}
- Harper LM. Mirena Intrauterine System Timing of Insertion: A Randomized Controlled Trial (MISTIC). http://clinicaltrials.gov/ct2/show/NCT01272960 (accessed 19 August 2014).
Teal 2014b {published data only}
- Teal S. Comparison of the Levonorgestrel IUD and the Copper IUD Placed in the Immediate Postplacental Period: A Randomized Controlled Trial. http://clinicaltrials.gov/show/NCT02067663 (accessed 19 August 2014).
Additional references
Aiken 2014
- Aiken ARA, Creinin MD, Kaunitz AM, Nelson AL, Trussell J. Global fee prohibits postpartum provision of the most effective reversible contraceptives. Contraception 2014;90(5):466‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alam 2014
- Alam K, Snover A, Sultana N. Safety and feasibility of intrauterine device insertion following post‐placental delivery. Rawal Medical Journal 2014;39(2):186‐9. [Google Scholar]
Allen 2009
- Allen RH, Goldberg AB, Grimes DA. Expanding access to intrauterine contraception. American Journal of Obstetrics and Gynecology 2009;201(5):456.e1‐5. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bergin 2012
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two‐visit IUD insertion protocols inhibit placement. Contraception 2012;86(6):694‐7. [DOI] [PubMed] [Google Scholar]
Bryant 2013
- Bryant AG, Kamanga G, Stuart GS, Haddad LB, Meguid T, Mhango C. Immediate postpartum versus 6‐week postpartum intrauterine device insertion: a feasibility study of a randomized controlled trial. African Journal of Reproductive Health 2013;17(2):72‐9. [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. Update to CDC's U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. Morbidity and Mortality Weekly Report (MMWR) 2011;60(26):878‐83. [PubMed] [Google Scholar]
Celen 2011
- Celen Ş, Sucak A, Yildiz Y, Danişman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception 2011;84(3):240‐3. [DOI] [PubMed] [Google Scholar]
Conde‐Agudelo 2000
- Conde‐Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. British Medical Journal 2000;321:1255‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Echeverry 1973
- Echeverry G. Family planning in the immediate postpartum period. Studies in Family Planning 1973;4(2):33‐5. [PubMed] [Google Scholar]
Glazer 2011
- Glazer AB, Wolf A, Gorby N. Postpartum contraception: needs vs. reality. Contraception 2011;83(3):238‐41. [DOI] [PubMed] [Google Scholar]
Gueye 2013
- Gueye M, Gaye YFO, Diouf AA, Mbaye M, Niang MM, Gueye SMK, et al. [Trancesarean intra‐uterine device. Pilot study performed at Dakar teaching hospital] [Dispositif intra‐utérin mis en place en cours de césarienne. Étude pilote réalisée au centre hospitalier universitaire de Dakar]. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 2013;42(6):585‐90. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd.
Kapp 2009
- Kapp N, Curtis KM. Intrauterine device insertion during the postpartum period: a systematic review. Contraception 2009;80(4):327‐36. [DOI] [PubMed] [Google Scholar]
Levi 2012
- Levi E, Cantillo E, Ades V, Banks E, Murthy A. Immediate postplacental IUD insertion at cesarean delivery: a prospective cohort study. Contraception 2012;86(2):102‐5. [DOI] [PubMed] [Google Scholar]
Lopez 2015
- Lopez LM, Grey TW, Stuebe A, Chen M, Truitt ST, Gallo MF. Combined hormonal versus nonhormonal versus progestin‐only contraception in lactation. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD003988.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2014
- Mishra S. Evaluation of safety, efficacy, and expulsion of post‐placental and intra‐cesarean insertion of intrauterine contraceptive devices (PPIUCD). Journal of Obstetrics and Gynaecology of India 2014;64(5):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; Vol. 340:c332. [DOI] [PMC free article] [PubMed]
Shukla 2012
- Shukla M, Qureshi S, Chandrawati. Post‐placental intrauterine device insertion ‐ A five year experience at a tertiary care centre in north India. Indian Journal of Medical Research 2012;136(3):432‐5. [PMC free article] [PubMed] [Google Scholar]
Sonalkar 2014
- Sonalkar S, Kapp N. Intrauterine device insertion in the postpartum period: a systematic review. European Journal of Contraception and Reproductive Health Care 2014;14 Nov([Epub ahead of print]):1‐15. [DOI] [PubMed] [Google Scholar]
Stuart 2012
- Stuart GS, Bryant AG, O'Neill E, Doherty IA. Feasibility of postpartum placement of the levonorgestrel intrauterine system more than 6 h after vaginal birth. Contraception 2012;85(4):359‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teal 2014a
- Teal SB. Postpartum contraception: optimizing interpregnancy intervals. Contraception 2014;89(6):487‐8. [DOI] [PubMed] [Google Scholar]
Thiery 1985
- Thiery M, Kets H, Pas H. Immediate postplacental IUD insertion: the expulsion problem. Contraception 1985;31(4):331‐49. [DOI] [PubMed] [Google Scholar]
Trussell 2011
- Trussell J. Contraceptive failure in the United States. Contraception 2011;83(5):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2011
- United Nations, Department of Economic and Social Affairs. World Contraceptive Use ‐ 2011. http://www.un.org/esa/population/publications/contraceptive2011/contraceptive2011.htm (accessed 21 Feb 2013).
WHO 2005
- World Health Organization. Report of a WHO Technical Consultation on Birth Spacing; 2005 Jun 13‐15; Geneva. http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf (accessed 26 August 2014).
WHO 2009
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. Fourth Edition, 2009. http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/. Geneva: World Health Organization, (accessed 23 July 2014):16.
References to other published versions of this review
Grimes 2002
- Grimes D, Schulz K, Vliet H, Stanwood N. Immediate post‐partum insertion of intrauterine devices. Human Reproduction 2002;17:549‐54. [DOI] [PubMed] [Google Scholar]


