Abstract
During a search by computer-aided inspection of two-dimensional (2D) protein gels for ςB-dependent general stress proteins exhibiting atypical induction profiles, a protein initially called Hst23 was identified as a product of the yvyD gene of Bacillus subtilis. In addition to the typical ςB-dependent, stress- and starvation-inducible pattern, yvyD is also induced in response to amino acid depletion. By primer extension of RNA isolated from the wild-type strain and appropriate mutants carrying mutations in the sigB and/or spo0H gene, two promoters were mapped upstream of the yvyD gene. The ςB-dependent promoter drives expression of yvyD under stress conditions and after glucose starvation, whereas a ςH-dependent promoter is responsible for yvyD transcription following amino acid limitation. Analysis of Northern blots revealed that yvyD is transcribed monocistronically and confirmed the conclusions drawn from the primer extension experiments. The analysis of the protein synthesis pattern in amino acid-starved wild-type and relA mutant cells showed that the YvyD protein is not synthesized in the relA mutant background. It was concluded that the stringent response plays a role in the activation of ςH. The yvyD gene product is homologous to a protein which might modify the activity of ς54 in gram-negative bacteria. The expression of a ςL-dependent (ςL is the equivalent of ς54 in B. subtilis) levD-lacZ fusion is upregulated twofold in a yvyD mutant. This indicates that the yvyD gene product, being a member of both the ςB and ςH regulons, might negatively regulate the activity of the ςL regulon. We conclude that (i) systematic, computer-aided analysis of 2D protein gels is appropriate for the identification of genes regulated by multiple transcription factors and that (ii) YvyD might form a junction between the ςB and ςH regulons on one side and the ςL regulon on the other.
The highly sensitive two-dimensional (2D) protein gel electrophoresis technique combined with the computer-aided evaluation of 2D gels is a very powerful tool for the analysis of the global control of gene expression (1, 51, 59). The transcription of the majority of bacterial genes is organized in regulons that are controlled by global regulators such as repressors, activators, or alternative sigma factors. We used the 2D gel electrophoresis methodology to describe the heat stress stimulon of Bacillus subtilis. This heat stress stimulon could be dissected into regulons by searching for proteins that follow the same induction pattern and by analyzing mutants in global regulatory genes (for a review, see reference 20). Increasing attention will be paid to this proteomic approach (59), bearing in mind that a lot of still-unknown regulons were discovered by the sequencing of the B. subtilis genome and should be analyzed in the near future (28).
The largest regulon in the heat stress stimulon is the ςB-dependent general stress regulon of B. subtilis, which presumably contains more than 100 genes (4, 5, 20). The function of this regulon was totally unknown until 1994. By the identification of several ςB-dependent general stress protein genes we and others have obtained evidence that some of these proteins may provide an unspecific, multiple, and prospective general stress resistance to a nongrowing, starving, or stressed B. subtilis cell which is no longer able to grow and divide (for a review, see reference 21).
ςB-dependent stress genes are strongly induced by heat, salt, acid, or ethanol stress as well as by energy depletion (17, 20). The proteins and/or genes belonging to the ςB regulon follow this typical expression pattern, which can be visualized by a computer-aided evaluation of 2D gels (4). However, we also found general stress proteins which were characterized by a slightly modified induction pattern. In addition to the characteristic ςB induction pattern the protein YvyD (formerly Hst23), identified by N-terminal sequencing (4), showed a strong induction by amino acid starvation (4, 57). In this paper, we describe this atypical induction profile of yvyD. We identified—besides the ςB-dependent promoter—a second promoter which is recognized by EςH and which is responsible for the induction of yvyD by amino acid starvation. ςH is used for the transcription of many genes expressed during the transition from exponential growth to the stationary phase (6, 24, 38, 39, 42, 50, 60–63). Such a dual control of a general stress gene by ςB and ςH was already described by Varón et al. (52) for the csb40 operon.
These results show that the 2D protein gel electrophoresis technique is also a useful approach for defining a network of interacting regulons or modulons. We suggest that yvyD (and presumably other genes or operons such as csb40) may form a junction in a global regulatory network between the ςB regulon, the ςH regulon, and most likely the stringent response also.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5α was routinely grown in Luria-Bertani medium and used as the host for cloning experiments (44). B. subtilis strains were cultivated with vigorous agitation at 37°C in a synthetic medium described previously (48). For heat shock and osmotic or ethanol stress experiments, exponentially growing cells of B. subtilis were shifted from 37 to 48°C or were exposed to either 4% (wt/vol) NaCl or 4% (vol/vol) ethanol. Deprivation of glucose, amino acids, or nitrogen was achieved by cultivating bacteria in the synthetic medium with growth-limiting amounts of glucose (0.05%, wt/vol), amino acids (62.4 μM lysine, 62.4 μM tryptophan), or (NH4)2SO4 (1 mM). To generate an artificial amino acid starvation (2, 19), dl-norvaline was added at an optical density at 500 nm (OD500) of 0.5 to a final concentration of 0.05% (wt/vol). B. subtilis BKD11 and BKD12 were cultivated in the synthetic medium with 0.2% (wt/vol) glucose (repressing conditions) or with 0.2% (wt/vol) fructose (inducing conditions) (30).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| E. coli DH5α | F− F80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA69 | 44 |
| B. subtilis | ||
| IS58 (BR16) | trpC2 lys-3 | 45, 49 |
| IS56 (BR17) | trpC2 lys-3 relA | 45, 49 |
| BEK38 | trpC2 lys-3 sigB::ΔHindIII-EcoRV::km | 26a |
| BKD1 | trpC2 lys-3 yvyD::km | pKD11→IS58a |
| BKD2 | trpC2 lys-3 Δspo0H::cat | BH41→IS58 |
| BKD3 | trpC2 lys-3 sigB::ΔHindIII-EcoRV::km Δspo0H::cat | BH41→BEK38 |
| BKD11 | trpC2 lys-3 amyE::PΔB(levD-lacZ) cat | QB5081→IS58 |
| BKD12 | trpC2 lys-3 yvyD::km amyE::PΔB(levD-lacZ) cat | pKD11→BKD11 |
| QB5081 | trpC2 amyE::PΔB(levD-lacZ) cat | 30 |
| BH41 | trpC2 pheA1 Δspo0H::cat | 18 |
| Plasmids | ||
| pBluescript II KS(+) | Cloning vector | Stratagene |
| pKD1 | 1.1-kbp fragment of B. subtilis DNA containing yvyD cloned into pBluescript II KS(+) | This work |
| pKD2 | Internal 0.4-kbp fragment of yvyD cloned into pBluescript II KS(+) | This work |
| pKD11 | pKD1 containing a 1,490-bp Kmr cassette from plasmid pGD780 within the BglII site of yvyD | This work |
| pGD780 | Kmr cassette delivery plasmid | 16 |
An arrow indicates the construction of the strain by transformation.
Construction of B. subtilis mutant strains.
B. subtilis BKD2, BKD3, and BKD11 were constructed by transformation of chromosomal DNA from various B. subtilis strains into the wild-type strain IS58 or the isogenic sigB mutant BEK38. B. subtilis BKD1 and BKD12 were constructed by transformation of the wild-type IS58 or BKD11 with the nonreplicative plasmid pKD11. Correct integration was proved by Southern blotting.
Primer extension and Northern (RNA) blot analysis.
Total RNA of B. subtilis BGH1, BKD2, BKD3, IS58 (BR16), and IS56 (BR17) was isolated from exponentially growing or stressed cells by the acid phenol method described by Majumdar et al. (29) with some modifications (54). The 5′ end of the yvyD mRNA was identified by primer extension as described previously (58). The oligonucleotide 5′-CTTCACATCAGCATCCACGC-3′ labelled with [γ-32P]ATP at the 5′ end was used as the primer. Northern blot analysis was performed as described previously (58) with a digoxigenin-labelled RNA probe which was synthesized in vitro with T7 RNA polymerase and the linearized plasmid pKD2 as a template.
Plasmid constructions.
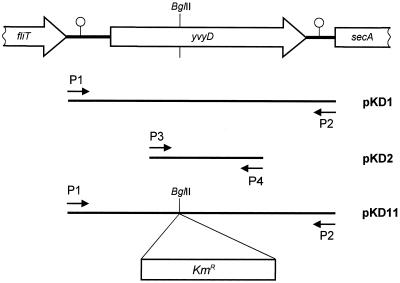
The primers P1 (5′-TTGACCAAATTTTTGCGGAG-3′) and P2 (5′-TCATCACACGCCTATTTTAG-3′) were used for the construction of the plasmid pKD1 (see Fig. 1). The resulting PCR product, after amplification of chromosomal DNA of strain IS58, was cloned into pBluescript II KS(+) linearized with EcoRV. pKD1 contains the entire yvyD gene. The plasmid pKD2 harboring an internal fragment of yvyD was constructed in a similar way. The primers P3 (5′-AGTCTAAGGTTGAGGTTACG-3′) and P4 (5′-GGTACACGACATTTGTAAGG-3′) were used for the amplification of chromosomal DNA of strain IS58, and the resulting fragment was cloned into pBluescript II KS(+) linearized with EcoRV. The plasmid pKD11 was constructed by cloning a 1,490-bp Kmr cassette from plasmid pGD780 (16) within the BglII site of the yvyD gene in the plasmid pKD1.
FIG. 1.
Schematic representation of the yvyD region in the chromosome of B. subtilis. The yvyD gene is located between the fli operon and the secA gene. A rho-independent terminator is located upstream and downstream of yvyD (7). The locations of the primer pair P1-P2, used for construction of plasmid pKD1, and the primer pair P3-P4, used for the construction of plasmid pKD2, are indicated. The BglII site within yvyD was used for the construction of the yvyD mutant by the insertion of a kanamycin resistance cassette in plasmid pKD1, resulting in plasmid pKD11.
β-Galactosidase assays.
The assay for β-galactosidase activity was performed as described previously (53).
2D polyacrylamide gel electrophoresis.
Labelling of cells, 2D polyacrylamide gel electrophoresis, and protein identification on 2D gels were performed as described previously (4, 14).
Computer methods.
Alignments were performed with the Genetics Computer Group package at default settings. Databases used were those of the EMBL and GenBank. Figure 2 was generated by the program BOXSHADE.
FIG. 2.
Multiple, partial alignment (approximately 100 amino acids at the N terminus) of YvyD with proteins functioning as ς54 modulating factors from gram-negative bacteria. Bs, B. subtilis; Ac, Acinetobacter calcoaceticus (orf2) (10); Ae, Alcaligenes eutrophus (Ralstonia eutropha) (orf130) (55); Av, Azotobacter vinelandii (second open reading frame) (32); Bj, Bradyrhizobium japonicum (orf203) (27); Ec, E. coli (orfII) (25); Kp, K. pneumoniae (orf95) (33); Pp, Pseudomonas putida (orf102) (26); St, Salmonella typhimurium (36); Tf, Thiobacillus ferrooxidans (orf3) (3); C, consensus sequence.
General methods.
Plasmid isolation, restriction enzyme analysis, transformation of E. coli, ligation of DNA fragments, and filling in of 3′ termini with Klenow fragments of DNA polymerase I were performed according to standard protocols (44). Chromosomal DNA from B. subtilis was isolated with a genomic DNA purification kit (Promega). Transformation of B. subtilis was carried out according to the method described by Hoch (22).
RESULTS
Identification of Hst23 as the product of the yvyD gene.
YvyD (Hst23) has been described as a general stress protein of B. subtilis (4). In 2D protein gels, YvyD is characterized by the typical ςB-dependent stress and starvation induction pattern; however, in contrast to most products of the ςB-dependent general stress genes YvyD is also induced by amino acid starvation (57).
The sequence encoding the N terminus of YvyD shows 100% identity to orf189, which is located between the fli operon and the secA gene in B. subtilis (7) (Fig. 1). A computer-aided search for similarities with other proteins showed an identity of 30 to 40% between this open reading frame encoding a 189-amino-acid polypeptide and ς54 modulating factors of various gram-negative bacteria (33) (Fig. 2).
During the B. subtilis genome sequencing project, orf189 was renamed yvyD (28). In this paper, we refer to the gene encoding Hst23 (Orf189) as yvyD.
Mapping of the yvyD promoters and Northern blots.
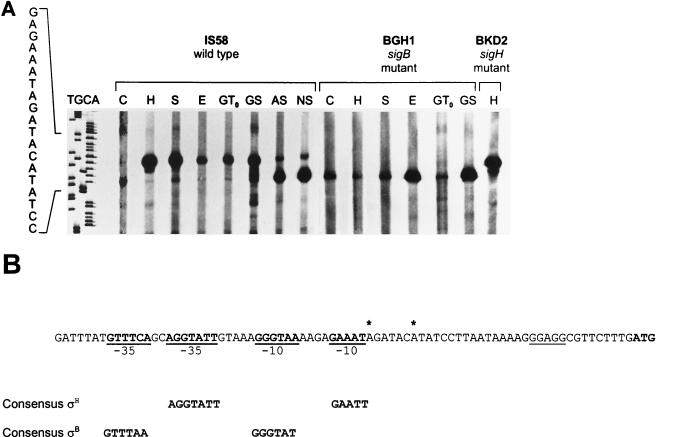
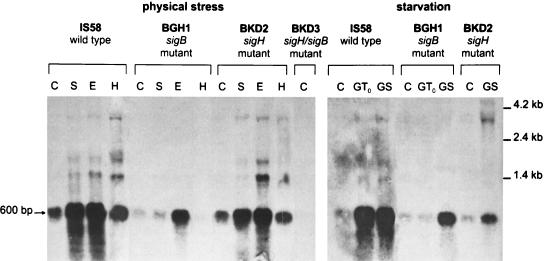
The transcriptional regulation of yvyD was analyzed by primer extension (Fig. 3) and Northern blotting (Fig. 4). By the primer extension technique two transcriptional start sites, separated by 5 nucleotides, were found. The potential −10 and −35 regions of the upstream promoter are similar to those of known ςB-dependent genes. The downstream promoter revealed high similarities to promoters recognized by RNA polymerase containing ςH. In exponentially growing cells of the wild-type strain IS58, transcription is mainly initiated at the ςH-dependent promoter (Fig. 3). In a sigH sigB double mutant there is no transcription of yvyD at all, supporting the hypothesis that only ςB and ςH are involved in transcriptional regulation (Fig. 4). Northern blot analyses showed that yvyD is transcribed monocistronically. A 600-bp signal was detected as the main transcript (Fig. 4). Both sigma factors contribute to the expression pattern. In a ςH mutant the transcription of yvyD depends solely on EςB, showing the typical induction profile for ςB-dependent genes: yvyD is strongly induced by heat, salt, or ethanol stress as well as by glucose starvation (Fig. 3 and 4). In a ςB mutant, however, no heat stress induction occurred at the ςH-dependent promoter, but an induction by glucose starvation (Fig. 3 and 4) and another by amino acid starvation (data not shown) did. However, the transcription at the ςH-dependent promoter appeared to show a delayed induction in response to starvation. Surprisingly, we also found a ςH-dependent induction by ethanol treatment. Concerning the transcriptional regulation of yvyD, we note that the induction by physical stress and glucose starvation depends on ςB and that the induction by amino acid starvation depends solely on ςH (Fig. 3). When both sigma factors are activated (by ethanol stress and glucose starvation) EςB most probably is involved to a larger extent in the transcription of yvyD than EςH. However, the possibility that there really is a competition between both sigma factors for the transcription of yvyD needs further investigation.
FIG. 3.
Determination of the transcriptional start sites of yvyD. (A) Primer extension analysis of RNA. The B. subtilis wild-type strain IS58, the sigB mutant strain BGH1, and the sigH mutant strain BKD2 were exposed at an OD500 of 0.5 to various stresses as described in Materials and Methods. For RNA isolation, bacteria were harvested before exposure (C, control) and 6 min after exposure to the different stressors (H, heat [48°C]; S, salt [4% NaCl]; E, ethanol [4% ethanol]). In cases of nutrient starvation bacteria were harvested at transient phase (glucose depletion [GT0]) or 30 min after entry into stationary phase (deprived of glucose [GS], amino acids [AS], or nitrogen [NS]). A total of 5 μg of each RNA preparation was used for each primer extension reaction. Lanes T, G, C, and A show the sequencing ladder obtained with the same primer as that used for primer extension. (B) DNA sequence of the yvyD promoter region. Potential start sites are indicated by asterisks. The ςB- and ςH-dependent promoters are printed in bold. The consensus sequence for ςB-dependent promoters is taken from the work of Hecker et al. (20), and the consensus sequence for ςH-dependent promoters is derived from the work of Haldenwang (17). The ribosomal binding site is underlined, and the translational start codon is shown in bold.
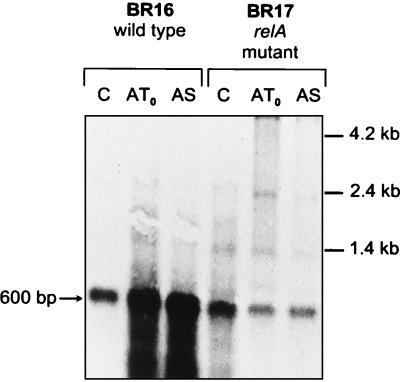
FIG. 4.
Northern blot analysis of stress-inducible yvyD transcription. Total RNA was isolated from B. subtilis IS58, BGH1, BKD2, and BKD3 after exposure to various stress conditions as described in the legend to Fig. 3. A total of 5 μg of RNA was applied in each lane. The locations of RNA molecular size markers and the size of the yvyD transcript are marked. C, control; S, salt; E, ethanol; H, heat; GT0, glucose depletion at transient phase; GS, glucose depletion at stationary phase.
YvyD induction by amino acid starvation in the relA mutant.
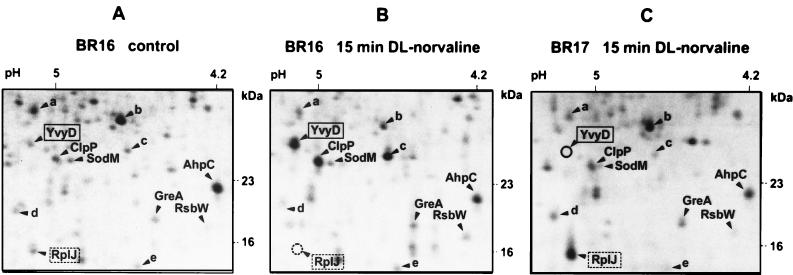
The synthesis of YvyD was strongly stimulated by norvaline treatment, which triggers a stringent response via leucine and isoleucine limitation (2, 19). In accordance with the findings of Wendrich and Marahiel (57), this induction did not occur in a relA mutant (Fig. 5). Figure 5 also demonstrates that the ribosomal protein RplJ is subjected to the characteristic stringent response, i.e., the continued synthesis in the relA mutant versus downregulated synthesis in the wild type. Transcriptional studies by Northern blotting provided additional evidence that yvyD is strongly induced by amino acid depletion at the ςH-dependent promoter only in the wild type and not in the relA mutant (Fig. 6).
FIG. 5.
Sections of 2D protein gels in the YvyD region. Strains BR16 (wild type) and BR17 (relA) were grown to an OD500 of 0.5. For panels B and C an artificial depletion of amino acids was generated by the addition of dl-norvaline to a final concentration of 0.05%. Labelling of cells with [35S]methionine (5 μCi/ml) was performed after 15 min of incubation with dl-norvaline for 3 min. (A) Control (strain BR16, exponentially growing cells, labelling at an OD500 of 0.5, and no dl-norvaline addition); (B) strain BR16 after dl-norvaline treatment; (C) strain BR17 after dl-norvaline treatment. The locations of the proteins ClpP, SodM, GreA, AhpC, and RsbW and of the unidentified proteins a, b, c, d, and e are included as references. Note in panel C that the YvyD protein is no longer induced in the relA mutant and that the protein RplJ is not subjected to the stringent response.
FIG. 6.
Northern blot analysis of stress induction of yvyD transcription in a relA mutant. Total RNA was isolated from B. subtilis BR16 and BR17 before (C, control) and after depletion of amino acids (AT0, transient phase; AS, 30 min after entry into stationary phase). A total of 2.5 μg of RNA was applied in each lane. The locations of RNA molecular size markers and the size of the yvyD transcript are marked. Note that induction of yvyD transcription does not occur in the relA mutant strain.
Analysis of ςL-dependent gene expression in a yvyD mutant.
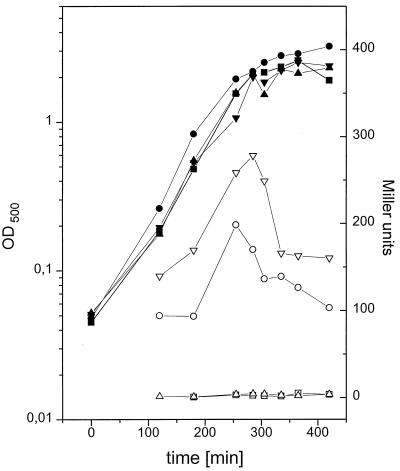
ςL in B. subtilis is the equivalent of ς54 in gram-negative bacteria (9). Because it had been shown that ς54-dependent transcription was elevated by 25% in a strain of Klebsiella pneumoniae carrying a mutation in the gene homologous to yvyD (orf95) (33), we examined the transcription of the ςL-dependent lev operon in B. subtilis. The lev operon is induced by fructose and repressed by glucose in the medium (30).
The β-galactosidase activity originating from a levD-lacZ fusion was measured in the wild-type strain BKD11 and in the yvyD mutant strain BKD12. In the presence of fructose the expression of levD-lacZ was elevated twofold in the yvyD mutant relative to that in the wild type (Fig. 7). The relative amount of the sigL transcript is not increased in the yvyD mutant relative to that in the wild type as revealed by slot blot analysis (data not shown). These results indicate that YvyD might influence the activity of ςL in B. subtilis.
FIG. 7.
β-Galactosidase synthesis originating from a levD-lacZ fusion in the wild type and the yvyD mutant. Strains BKD11 (levD-lacZ) and BKD12 (levD-lacZ yvyD) were grown in minimal medium containing either 0.2% glucose (repressing conditions) (BKD11 [■] and BKD12 [▴]) or 0.2% fructose (inducing conditions) (BKD11 [•] and BKD12 [▾]) as the carbon source. At the times indicated, samples were removed and assayed for β-galactosidase activity (corresponding open symbols).
DISCUSSION
By a careful and comprehensive computer-aided inspection and matching of various 2D gels loaded with radioactively labelled proteins from growing, starved, or stressed B. subtilis cells, it is possible to proceed from a 2D protein index to a more global analysis and description of the gene expression network (1). The proteomic research approach (59) relying mainly on the highly sensitive 2D protein gel electrophoresis technique is a useful approach not only for the definition of stimulons and regulons (20, 51) but, as shown in this study, also for the discovery and preliminary analysis of genes controlled by more than one regulatory circuit.
ςB-dependent general stress genes show an expression pattern induced by heat, salt, acid, or ethanol stress on the one hand and by glucose, oxygen, or phosphate starvation on the other. During a search for general stress proteins exhibiting atypical induction profiles we found YvyD, which shows the typical ςB-dependent induction pattern. However, the strong induction of YvyD caused by amino acid starvation did not fit with the ςB-dependent induction profile. In this paper it is shown that in addition to the ςB promoter, yvyD contains a second, ςH-dependent promoter responsible for this atypical induction profile. Our results indicate that a presumably small subset of ςB-dependent genes are also controlled by ςH, extending the inducing environmental stimuli to amino acid or nitrogen starvation. The first member of this ςB-ςH modulon was described by Varón et al. (52), who found the csb40 operon to be under this dual control. The second open reading frame of the csb40 operon (orf2) showed a significant resemblance to desiccation proteins occurring in dried leaves of plants. The description of a new member of this stationary-phase or general stress modulon seems to support the conclusion of Varón et al. (52) that ςH may be more broadly involved in stress response than previously suggested. Very recently, Gaidenko and Price (13) found ςH to be involved in the stress resistance of B. subtilis. However, the number of genes whose expression is controlled by both sigma factors seems to be rather low, because from about 30 to 40 general stress proteins whose expression profiles were analyzed by a computer-aided evaluation, only Hst23 showed this atypical induction pattern (4).
The ςH regulon and the expression of the spo0H gene have been intensively investigated. ςH has been described as a sigma factor responsible for the transcription of several genes expressed at the transient phase at the start of sporulation and competence development (for reviews, see references 15 and 23). Several genes under ςH control, which are expressed early in the transition period, also possess a ςA-dependent promoter; some genes of the ςH regulon are not directly involved in the sporulation pathway, e.g., citG, rpoD, and the ureABC operon (6, 39, 50, 60). Early-sporulation genes, spo0A, spo0F, kinA, spoVG, and spoVS, are transcribed by EςH (11, 38, 42, 43, 63). The spoIIA operon, encoding the prespore- and forespore-specific sigma factor ςF, is transcribed exclusively by EςH (61, 62). The expression of spo0H itself increases as the culture enters the late logarithmic stage of growth (56). An acidification of the internal pH negatively influences spo0H expression (8). The decreasing level of the transition state regulator AbrB, whose synthesis is repressed by phosphorylated Spo0A, seems to be responsible for the transient derepression of spo0H (11, 35, 46, 47, 56, 63).
We noticed some interesting details about the expression of yvyD. Our transcriptional data appeared to show that in response to glucose starvation the ςB-dependent promoter is activated earlier than the ςH-dependent one. Furthermore, the ςH-dependent induction of yvyD by ethanol in the sigB mutant background is a result which deserves future attention. The most promising result, however, is that in a relA mutant yvyD is no longer induced in response to amino acid starvation. The relA-dependent induction of yvyD occurs at the ςH-dependent promoter because ςB-dependent genes are not induced after ppGpp accumulation (31). An intriguing explanation for this result could be that ςH requires the stringent response for its activity regardless of the mechanism of this proposed activation. It is tempting to speculate that ppGpp is somehow involved in the derepression of spo0H. However, several reports indicate that spo0H expression is regulated at different levels of gene expression, including even the posttranslational level (12, 18, 41, 56). Recently, it has been found that YvyD is accumulated in clpP and clpX mutants (14). This observation can be explained by the recent finding that ςH is a substrate for Clp proteases (34). Further studies are necessary to elucidate the still-putative relationship between SigH activity and the stringent response.
The function of YvyD is still unknown. The protein shows 30 to 40% identity to ς54 modulating factors of gram-negative bacteria; the yvyD gene is transcribed monocistronically. In contrast to B. subtilis, the genes encoding the ς54 modulating factors are organized in operons coding for ς54-like factors (3, 10, 25–27, 32, 33, 36, 55) and for proteins highly similar to components of the phosphotransferase system (37) in gram-negative bacteria. Reizer et al. (40) proposed that the phosphotransferase system catalyzed protein phosphorylation functions in the regulation of ς54-dependent gene expression and supposed a link between carbon and nitrogen metabolic pathways. The ς54 modulating proteins do not seem to act as repressors but may inhibit the activity of the sigma factor via a still-unknown mechanism (33). In a yvyD mutant of B. subtilis, transcription of the ςL-dependent operon levD seems to be elevated, indicating that YvyD may also exert a negative effect on ςL activity. Despite the fact that we do not yet know the physiological role of YvyD, the outstanding location of yvyD in the gene regulation network linking ςB and ςH on the one side with ςL on the other might promise a very important function of this protein in the genetic network of the transition phase which needs further investigation.
ACKNOWLEDGMENTS
We thank M. Débarbouillé for the generous gift of strain QB5081, D. Zeigler of the Bacillus Genetic Stock Center for plasmid pGD780, T. Msadek for strain BH41, and E. Krüger for strain BEK38. We are grateful to C. Scharf for help with computer work and to P. J. Piggot and two anonymous referees for critical and helpful comments on the manuscript.
This work was supported by grants from the DFG, the BMBF, and Fonds der Chemischen Industrie to M.H.
REFERENCES
- 1.Antelmann H, Bernhardt J, Schmid R, Mach H, Völker U, Hecker M. First steps from a two-dimensional protein index to a response-regulation map for Bacillus subtilis. Electrophoresis. 1997;18:1451–1463. doi: 10.1002/elps.1150180820. [DOI] [PubMed] [Google Scholar]
- 2.Belitskii B R, Shakulov R S. Guanosine polyphosphate concentration and stable RNA synthesis in Bacillus subtilis following suppression of protein synthesis. Mol Biol (Moscow) 1980;14:1342–1353. . (In Russian.) [PubMed] [Google Scholar]
- 3.Berger D K, Woods D R, Rawlings D E. Complementation of Escherichia coli ς54 (NtrA)-dependent formate hydrogenlyase activity by a cloned Thiobacillus ferrooxidans ntrA gene. J Bacteriol. 1990;172:4399–4406. doi: 10.1128/jb.172.8.4399-4406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional gel electrophoresis study. Microbiology. 1997;143:999–1013. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 5.Boylan S A, Redfield A R, Price C W. Transcription factor ςB of Bacillus subtilis controls a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter H L, III, Wang L-F, Doi R H, Moran C P., Jr rpoD operon promoter used by ςH-RNA polymerase in Bacillus subtilis. J Bacteriol. 1988;170:1617–1621. doi: 10.1128/jb.170.4.1617-1621.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Helmann J D. The Bacillus subtilis ςD-dependent operon encoding the flagellar proteins FliD, FliS, and FliT. J Bacteriol. 1994;176:3093–3101. doi: 10.1128/jb.176.11.3093-3101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosby W M, Zuber P. Regulation of Bacillus subtilis ςH (Spo0H) and AbrB in response to changes in external pH. J Bacteriol. 1997;179:6778–6787. doi: 10.1128/jb.179.21.6778-6787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Débarbouillé M, Martin-Verstraete I, Kunst F, Rapoport G. The Bacillus subtilis sigL gene encodes an equivalent of ς54 from gram negative bacteria. Proc Natl Acad Sci USA. 1991;88:9092–9096. doi: 10.1073/pnas.88.20.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrt S, Ornston L N, Hillen W. RpoN (ς54) is required for conversion of phenol to catechol in Acinetobacter calcoaceticus. J Bacteriol. 1994;176:3493–3499. doi: 10.1128/jb.176.12.3493-3499.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisby D, Zuber P. Analysis of the upstream activating sequence and site of carbon and nitrogen source repression in the promoter of an early-induced sporulation gene of Bacillus subtilis. J Bacteriol. 1991;173:7557–7564. doi: 10.1128/jb.173.23.7557-7564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisby D, Zuber P. Mutations in pts cause catabolite-resistant sporulation and altered regulation of spo0H in Bacillus subtilis. J Bacteriol. 1994;176:2587–2595. doi: 10.1128/jb.176.9.2587-2595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaidenko T A, Price C W. General stress transcription factor ςB and sporulation transcription factor ςH each contribute to survival of Bacillus subtilis under extreme growth conditions. J Bacteriol. 1998;180:3730–3733. doi: 10.1128/jb.180.14.3730-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerth U, Krüger E, Derré I, Msadek T, Hecker M. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol Microbiol. 1998;28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 15.Grossman A D. Genetic networks controlling the initiation of sporulation and development of competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 16.Guérout-Fleury A M, Shazand K, Frandsen N, Stragier P. Antibiotic resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 17.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy J, Weir J, Smith I, Losick R. Post-transcriptional control of a sporulation regulatory gene encoding transcription factor sigmaH in Bacillus subtilis. Mol Microbiol. 1991;5:477–487. doi: 10.1111/j.1365-2958.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 19.Hecker M, Richter A, Schroeter A, Wölfel L, Mach F. Synthesis of heat shock proteins following amino acid or oxygen limitation in Bacillus subtilis relA+ and relA strains. Z Naturforsch Teil C. 1987;42:941–947. . (In German.) [PubMed] [Google Scholar]
- 20.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 21.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoch J A. Genetic analysis in Bacillus subtilis. Methods Enzymol. 1991;204:305–320. doi: 10.1016/0076-6879(91)04015-g. [DOI] [PubMed] [Google Scholar]
- 23.Hoch J A. Control of cellular development in sporulating bacteria by the phosphorelay two-component signal transduction system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 129–144. [Google Scholar]
- 24.Jaacks K J, Healy J, Losick R, Grossman A D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones D H, Franklin F C, Thomas C M. Molecular analysis of the operon which encodes the RNA polymerase sigma factor ς54 of Escherichia coli. Microbiology. 1994;140:1035–1043. doi: 10.1099/13500872-140-5-1035. [DOI] [PubMed] [Google Scholar]
- 26.Köhler T, Alvarez J F, Harayama S. Regulation of the rpoN, orf102 and orf154 genes in Pseudomonas putida. FEMS Microbiol Lett. 1994;115:177–184. doi: 10.1111/j.1574-6968.1994.tb06634.x. [DOI] [PubMed] [Google Scholar]
- 26a.Krüger, E. Unpublished data.
- 27.Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer H-M. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the ς54 gene (rpoN) J Bacteriol. 1991;173:1125–1138. doi: 10.1128/jb.173.3.1125-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 29.Majumdar D, Avissar Y J, Wyche J H. Simultaneous and rapid isolation of bacterial and eucaryotic DNA and RNA. BioTechniques. 1991;11:94–96. [PubMed] [Google Scholar]
- 30.Martin-Verstraete I, Débarbouillé M, Klier A, Rapoport G. Mutagenesis of the Bacillus subtilis “−12, −24” promoter of the levanase operon and evidence for the existence of an upstream activating sequence. J Mol Biol. 1992;226:85–99. doi: 10.1016/0022-2836(92)90126-5. [DOI] [PubMed] [Google Scholar]
- 31.Maul B, Völker U, Riethdorf S, Engelmann S, Hecker M. ςB dependent regulation of gsiB in response to multiple stimuli in Bacillus subtilis. Mol Gen Genet. 1995;248:114–120. doi: 10.1007/BF02456620. [DOI] [PubMed] [Google Scholar]
- 32.Merrick M, Gibbins J, Toukdarian A. The nucleotide sequence of the sigma factor gene ntrA (rpoN) of Azotobacter vinelandii: analysis of conserved sequences in NtrA proteins. Mol Gen Genet. 1987;210:323–330. doi: 10.1007/BF00325701. [DOI] [PubMed] [Google Scholar]
- 33.Merrick M J, Coppard J R. Mutations in genes downstream of the rpoN gene (encoding ς54) of Klebsiella pneumoniae affect expression from ς54 dependent promoters. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 34.Nanamiya H, Ohashi Y, Asai K, Moriya S, Ogasawara N, Fujita M, Sadaie Y, Kawamura F. ClpC regulates the fate of a sporulation initiation sigma factor, ςH protein, in Bacillus subtilis at elevated temperatures. Mol Microbiol. 1998;29:505–513. doi: 10.1046/j.1365-2958.1998.00943.x. [DOI] [PubMed] [Google Scholar]
- 35.Perego M, Wu J-J, Spiegelman G B, Hoch J A. Mutational dissociation of the positive and negative regulatory properties of the Spo0A sporulation transcription factor of Bacillus subtilis. Gene. 1991;100:207–212. doi: 10.1016/0378-1119(91)90368-l. [DOI] [PubMed] [Google Scholar]
- 36.Popham D, Keener J, Kustu S. Purification of the alternative ς factor, ς54, from Salmonella typhimurium and characterization of ς54-holoenzyme. J Biol Chem. 1991;266:19510–19518. [PubMed] [Google Scholar]
- 37.Powell B S, Court D L, Inada T, Nakamura Y, Michotey V, Cui X, Reizer A, Saier M H, Jr, Reizer J. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. J Biol Chem. 1995;270:4822–4839. doi: 10.1074/jbc.270.9.4822. [DOI] [PubMed] [Google Scholar]
- 38.Predich M, Nair G, Smith I. Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by the RNA polymerase containing ςH. J Bacteriol. 1992;174:2771–2778. doi: 10.1128/jb.174.9.2771-2778.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price V A, Feavers I M, Moir A. Role of sigma H in expression of the fumarase gene (citG) in vegetative cells of Bacillus subtilis 168. J Bacteriol. 1989;171:5933–5939. doi: 10.1128/jb.171.11.5933-5939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reizer J, Reizer A, Saier M H, Jr, Jacobson G R. A proposed link between nitrogen and carbon metabolism involving protein phosphorylation in bacteria. Protein Sci. 1992;1:722–726. doi: 10.1002/pro.5560010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resnekov O. Presented at the Eleventh International Spores Conference, Marine Biological Laboratories, Woods Hole, Mass. 1992. Post-transcriptional control of the gene for ςH by the sporulation gene spoVG. [Google Scholar]
- 42.Resnekov O, Driks A, Losick R. Identification and characterization of sporulation gene spoVS from Bacillus subtilis. J Bacteriol. 1995;177:5628–5635. doi: 10.1128/jb.177.19.5628-5635.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson J B, Gocht M, Marahiel M A, Zuber P. AbrB, a regulator of gene expression in Bacillus, interacts with the transcription initiation regions of a sporulation gene and an antibiotic biosynthesis gene. Proc Natl Acad Sci USA. 1989;86:8457–8461. doi: 10.1073/pnas.86.21.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Smith I, Paress P, Cabane K, Dubnau E. Genetics and physiology of the rel system of Bacillus subtilis. Mol Gen Genet. 1980;178:271–279. doi: 10.1007/BF00270472. [DOI] [PubMed] [Google Scholar]
- 46.Strauch M A, Webb V, Spiegelman G, Hoch J A. The Spo0A protein is a repressor of the abrB gene. Proc Natl Acad Sci USA. 1990;87:1801–1805. doi: 10.1073/pnas.87.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strauch M A, Hoch J A. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol Microbiol. 1993;6:337–342. doi: 10.1111/j.1365-2958.1993.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 48.Stülke J, Hanschke R, Hecker M. Temporal activation of β-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J Gen Microbiol. 1993;139:2041–2045. doi: 10.1099/00221287-139-9-2041. [DOI] [PubMed] [Google Scholar]
- 49.Swanton M, Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972;46:583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]
- 50.Tatti K M, Carter III H L, Moir A, Moran C P., Jr Sigma H-directed transcription of citG in Bacillus subtilis. J Bacteriol. 1989;171:5928–5932. doi: 10.1128/jb.171.11.5928-5932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Bogelen R A, Abshire K Z, Moldover B, Olson E R, Neidhardt F C. Escherichia coli proteome analysis using the gene-protein database. Electrophoresis. 1997;18:1243–1251. doi: 10.1002/elps.1150180805. [DOI] [PubMed] [Google Scholar]
- 52.Varón D, Brody M S, Price C W. Bacillus subtilis operon under the dual control of the general stress transcription factor ςB and the sporulation transcription factor ςH. Mol Microbiol. 1996;20:339–350. doi: 10.1111/j.1365-2958.1996.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 53.Voelker U, Dufour A, Haldenwang W G. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of ςB. J Bacteriol. 1995;177:114–122. doi: 10.1128/jb.177.1.114-122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 55.Warrelmann J, Eitinger M, Schwartz E, Römermann D, Friedrich B. Nucleotide sequence of the rpoN (hno) gene cluster of Alcaligenes eutrophus: evidence for a conserved gene cluster. Arch Microbiol. 1992;158:107–114. doi: 10.1007/BF00245213. [DOI] [PubMed] [Google Scholar]
- 56.Weir J, Predich M, Dubnau E, Nair G, Smith I. Regulation of spo0H, a gene coding for the Bacillus subtilis ςH factor. J Bacteriol. 1991;173:521–529. doi: 10.1128/jb.173.2.521-529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wendrich T M, Marahiel M A. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol Microbiol. 1997;26:65–79. doi: 10.1046/j.1365-2958.1997.5511919.x. [DOI] [PubMed] [Google Scholar]
- 58.Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkins M R, Williams K L, Appel R D, Hochstrasser D F, editors. Proteome research: new frontiers in functional genomics. Berlin, Germany: Springer-Verlag; 1997. [Google Scholar]
- 60.Wray L V, Jr, Ferson A E, Fisher S H. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J Bacteriol. 1997;179:5494–5501. doi: 10.1128/jb.179.17.5494-5501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J-J, Gaukler Howard M, Piggot P J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989;171:692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J-J, Piggot P J, Tatti K M, Moran C P., Jr Transcription of the Bacillus subtilis spoIIA locus. Gene. 1991;101:113–116. doi: 10.1016/0378-1119(91)90231-y. [DOI] [PubMed] [Google Scholar]
- 63.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]