Abstract
Objective:
Stem cells (SCs) can improve the functional defects of brain injury. Rodents use their whiskers to get tactile information from their surroundings. The aim of this study was to investigate whether the transplantation of SCs into the lesioned barrel cortex can help neuronal function in the contralateral cortex.
Materials and Methods:
Sixteen male Wistar rats (200-230 g) were used in this experimental study. We induced a mechanical lesion in the right barrel cortex area of rats by removing this area by a 3 mm skin punch. Four groups containing one intact group of rats: group 1: control, and three lesion groups, group 2: lesion+un-differentiated dental pulp SCs (U-DPSCs), group 3: lesion+differentiated dental pulp SCs (D-DPSCs), and group 4: cell medium (vehicle) that were injected in the lesion area. Three weeks after transplantation of SCs or cell medium, the rats’ responses of left barrel cortical neurons to controlled deflections of right whiskers were recorded by using the extracellular single-unit recordings technique.
Results:
The results showed that the neural spontaneous activity and response magnitude of intact barrel cortex neurons in the lesion group decreased significantly (P<0.05) compared to the control group while ON and OFF responses were improved in the D-DPSCs (P<0.001) group compared to the vehicle group three weeks after transplantation.
Conclusion:
Transplantation of dental pulp mesenchymal SCs significantly improved the neural responses of the left barrel cortex that was depressed in the vehicle group.
Keywords: Brain Injury, Electrophysiology, Rats, Somatosensory Cortex, Stem Cells
Introduction
The discrete clustering of its layer IV neurons, collectively known as barrels distinguishes the barrel cortex (1). Each layer IV barrel receives precise sensory information from a single whisker via the thalamus. Extracellular field potential recordings have been used to study this precise structure-function relationship in brain slices (2).
The sensory info integration among the cerebral hemispheres is important for numerous perceptual tasks which need bi-lateral coordination. The right and left somatic sensory cortex function is narrowly linked through the corpus callosum (3). The sensory info integration through the corpus callosum is yet a mystery at the cellular level, however, some new articles have started to discover the interhemispheric cellular dynamics in the somatosensory system (4). Still, how the number of changes of real-time activity in one cortical area is mirrored in that of its contralateral counterpoint is yet an unsolved question (5). The subcortical pathways from the whiskers on the left and right side of the face are kept quite separate from the periphery to the cortex causing a cortex functional lateralization (6).
Nevertheless, when barrel neurons reply best to contralateral whiskers stimulations, they are also affected by the whiskers on the ipsilateral side of the face as well. Ipsilateral whiskers stimulation induces both local field potential and spikes in the layers of IV and V neurons in the barrel cortex field (7). Cortical neurons’ responses to the ipsilateral whiskers are mediated by callosal links since blocking activity in one hemisphere removes all replies in the hemisphere ipsilateral to the stimulated whiskers (8). This may be why, in anesthetized rats, the contralateral intact neuronal activity to the damaged barrel cortex is reduced (9). Also, cerebral cortex lesions generate several complex responses to damage of the brain. Well-reported hypo-excitability impacts caused by cortical lesions consist of extensive hypo-metabolism (10), decreases in blood flow (11), reductions in glucose metabolism (12) and low amplitude of electroencephalogram (EEG) activity (13) in the contralateral along with ipsilateral cortex after damage of the brain. Some of the overall metabolic impacts are observed through extensive areas, while other deficits seem to be limited to areas in the brain connected by their neural links to the damaged areas (14).
To reach functional recovery, injured neuronal network repair and links in the contralateral hemisphere area are needed. A perfect illustration of these therapeutic influences in stem cell (SC) therapy though, has been problematic and infrequently stated before (15). Dental pulp SCs are non-invasive and can be easily isolated from the pulp of adult and postnatal elongated teeth, they can be a suitable option for cell therapy of central nervous system injuries.
SC transplantation could increase axon density and axonal sprouting in the lesion area (16, 17). Several studies highlight the idea that neurotrophic factors released from SC grafts have an important role in the valuable influence of SC transplantation therapy (18-20).
The current research aimed to calculate the effect of SCs therapy on the ON and OFF neuronal response of intact contralateral cortical barrels by the single unit recording technique in rats with a lesioned barrel cortex on the opposite side.
Materials and Methods
Animals
The Kerman Neuroscience Research Center’s Research Ethics Committee (IR.KMU.REC.1399.510) gave its approval to the experimental study. Eighteen male adult Wistar rats (200-230 g) were used in this study. The animals had clean cages and were kept in an animal house that was temperature-controlled, well-ventilated, and had free access to a regular diet and water.
Isolation and characterization of DPSC
Isolation and characterization methods were described in our previous study (19). Briefly, the pulp of lower and upper jaw teeth was extracted heterogeneously from 2 Wistar rats (200-300 g). Their pulp tissues were crumbed and then added into a falcon tube containing collagenase I (Serva). Cell cultivation was performed.
As some of these cells exhibited an oval or fibroblastlike shape before neural differentiation, a third passage was used for immunophenotypic analysis to trigger differentiation. Flow cytometry was used for analyzing the immunophenotypic characterization of cells. After neural differentiation, the cell morphology revealed neuronallike cells with large, rounded cell bodies and neuron-like processes. SCs were differentiated (19).
Extracellular single-unit recording
Surgery and groups
Sixteen rats were randomly divided into four experimental groups (n=4). After the rats were anesthetized with Ketamine/xylazine (80/10 mg/ kg), the head of the rat was fixed in the stereotaxic frame (Stoelting, USA). The right parietal skull was exposed posteriorly (1 to 4 mm) and lateral (4 to 7 mm) to the position of the bregma and was removed by a dental drill. The barrel cortex was cut by a 3-mm skin punch, and then the lesion area was washed with normal saline at 37°C. The skin was stitched and the rats were returned to their cage. See this protocol in our previous study (19).
In this study there was 4 groups. i. Control group was without any surgery or injection, ii. A total of 1×106 undifferentiated dental pulp stem cells (lesion+UDPSC group) in 80 μl of DMEM, iii. A total of 1×106 differentiation dental pulp stem cells (lesion+D-DPSC group) in 80 μl of DMEM, and iv. Lesion+vehicle group, the rats received 80 μl of DMEM which was injected directly into the lesion site three days after lesion induction.
Then, the neural activities of the left intact cortical barrels to the right whiskers deflection of rats were recorded by the single-unit recording approach. The number of neurons in each group was as follows: control (n=16 neurons), lesion+vehicle (n=27 neurons), lesion+U-DPSC (n=18 neurons), and lesion+D-DPSC (n=30 neurons).
Each rat was intraperitoneally anesthetized with urethane (1.2 mg/kg), and then the rat’s was fixed head in a stereotaxic frame. A dental drill was used to expose and remove the left parietal cortex. Using a servo-controlled heating pad, the body temperature was set to 37°C. Injecting 10% of the initial dose of urethane was used to control the spontaneous movements of whiskers and irregular breathing when changing anesthesia depth. A tungsten microelectrode (1-3 MÙ, FHC) was perpendicularly inserted into the posterior medial field of the barrel cortex by a micro driver (WPI, USA). All units were recorded from depths 600 to 1000 mm of the cortex. The bandpass of the amplifier was 0.3-10 KHz, a preamplifier amplified the signals. The obtained data were saved on a computer (Science beam, Iran). Neuron electrical activities were considered single units’ activity by a signal-to-noise ratios of at least 3:1. An offline sorter of window discriminator was then used to isolate each neuron (8, 21, 22).
Mechanical whisker deflection
A miniature speaker connecting to a glass micropipette (with an inner diameter of 0.68 mm and an outer diameter of 1.2 mm) was used to deflect the whiskers of the right mystacial pad in each rat by a controlled-mechanical whisker deflection.
Then whiskers were cut by 10 mm from the surface of the face. After determining a whisker of D2, the whisker was placed in glass tubes connected to the speaker. The whisker was moved randomly 50 times (23).
A graduated microscope was used to calibrate the amplitude of down-upward (500 ìm) deflections, hold phase (200 ms) and rise time (5 ms). The ON neural response magnitudes (moving the whiskers down from the resting position) and the OFF neural response magnitudes (moving back to the resting position) were recorded. The recorded bin size was adjusted to 1000 ms. By counting the spikes/bin, the unit activity was summed and illustrated as peri-stimulus time histograms (PSTHs) (24, 25).
Statistical analysis
Testing the normality of data distribution was performed using the Kolmogorov-Smirnov test.
All statistical analyzes were performed using SPSS software (version 22, IBM, USA). A one-way ANOVA analysis followed by Bonferroni post hoc test was applied. Data are expressed as mean ± SEM. A P<0.05 was considered statistically significant.
Results
Spontaneous activity
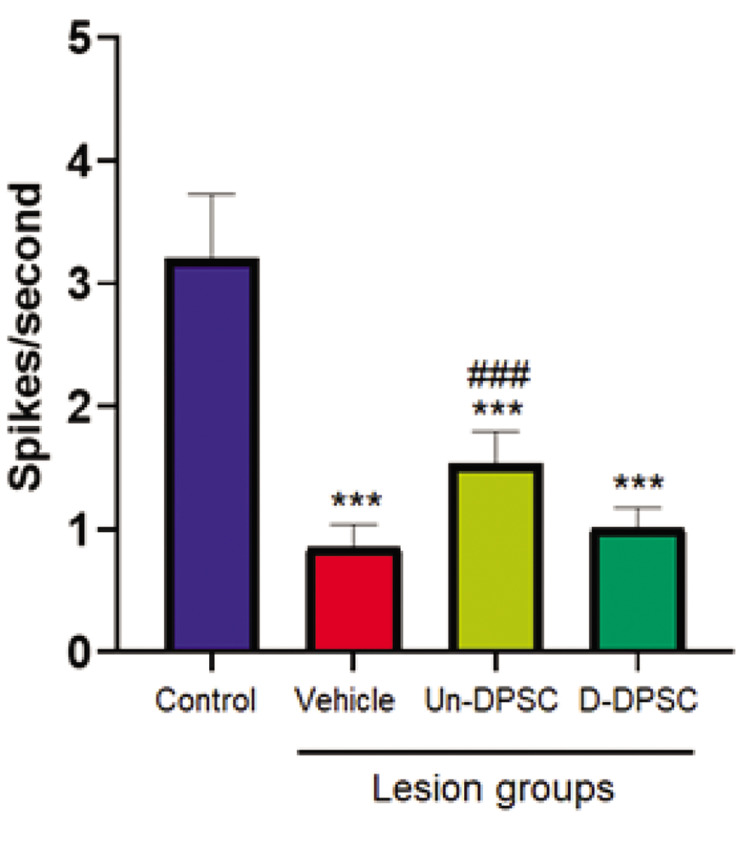
Spontaneous activity of neurons to the D2 whisker deflection (0-300 ms) is shown in different groups (Fig .1). ANOVA revealed a significant main effect [F (3, 87) =10.151, P=0.0006] for the spontaneous activity variable. The results showed that a lesion in the right barrel cortex leads to a significant reduction in spontaneous activity in the right intact barrel cortex of the lesion+vehicle group compared to the control group.
Fig.1.
The mean of spontaneous activity of neurons. The spontaneous activity of the left intact cortex of the lesion+Un-DPSC, lesion+D-DPSC, and lesion+vehicle groups were compared to that of the control group. The sign of (*) shows a significant statistical difference between the lesion group and the control group. The sign of (#) shows a significant statistical difference in each group compared with the lesion+vehicle group. Data are expressed as mean ± SEM. Un-DPSCs; Un-differentiated dental pulp stem cells, D-DPSCs; Lesion+differentiated dental pulp stem cells, ***; P<0.001, and ###; P<0.001.
The analysis also reported a significant increase in spontaneous activity in the lesion+Un-DPSC compared to the vehicle groups. Results showed that SC therapy cannot restore spontaneous activity to the control level (P<0.001, Fig .1).
A cumulative peri-stimulus time histogram (PSTH) was used to measure and compare neuronal responses in the barrel cortex in the control (n=16 neurons), lesion+vehicle (n=27 neurons), lesion+U-DPSC (n=18 neurons), and Lesion+D-DPSC (n=30 neurons) groups. Figure 2 shows a sample response of cumulative PSTH neurons to PW (D2) deflection.
Response magnitudes
The mean evoked response magnitude of barrel neurons in layer IV of the left hemisphere was analyzed following stimulation on whisker D2.
Fig.2.
A peri-stimulus time histogram (bin=1 second) showing responses to the whisker of D2 in the control, lesion+vehicle, lesion+Un-DPSC, and lesion+D-DPSC groups. Un-DPSC; Un-differentiated dental pulp stem cell, and D-DPSC; Lesion+differentiated dental pulp stem cell.
ON responses
Analysis of data by One-way ANOVA revealed that there was a main significant effect on the average of the ON response magnitudes of barrel cortical neurons [F (3, 87) =10.151, P=0.000]. Post hoc analysis revealed that the mean of response magnitude in the intact barrel neurons reduced significantly in the lesion+vehicle group (P<0.001) and lesion+Un-DPSC (P<0.01) compared to the control group. The mean of response magnitude revealed a significant increase in the lesion+D-DPSC group compared to the lesion+vehicle group (P<0.01). The mean of response magnitude of the lesion+Un-DPSC group in comparison with the lesion+vehicle group, did not show any significant change (Fig .3).
Fig.3.
ON responses. ON response magnitudes of the D2 barrel neurons to the whisker deflection in different groups are shown. Data are expressed as mean ± SEM. Un-DPSC; Undifferentiated dental pulp stem cell, D-DPSC; Lesion+differentiated dental pulp stem cell, **; P<0.01, ***; P<0.001, compared to control group, and ##; P<0.01, compared to lesion+vehicle group.
OFF responses
There was a significant effect on the OFF-response magnitudes of barrel cortical neurons [F (3, 87)=8.409, P=0.001]. The mean of OFF response magnitude at the time of stimulation did not show any significant change between the control and the lesion+vehicle groups. When the D2 whisker was deflected, there was a significant increase in the response magnitude of lesion+D-DPSC compared with lesion+vehicle groups (P<0.001). The mean magnitude of the OFF response was not a significant change in the lesion+Un-DPSC compared to the lesion+vehicle group (Fig .4).
Fig.4.
OFF Responses. Magnitudes of the OFF response of the D2 barrel neurons to the whisker deflection in different groups are shown. Data are expressed as mean ± SEM. Un-DPSC; Un-differentiated dental pulp stem cell, D-DPSC; Lesion+differentiated dental pulp stem cell, and ###; P<0.001 compared to lesion+vehicle group.
Latency
Latency ON
There was a main effect on the latency of neuronal responses [F (3, 87)=10.328, P=0.001]. Results revealed that latency in the lesion+vehicle group increased significantly compared to the control group (P<0.01). There was a significant reduction between lesion+Un-DPSC and lesion+D-DPSC in comparison to the lesion+vehicle group (<0.01, Fig .5).
Fig.5.
Response latency ON. Response latency of the D2 barrel neurons to the whisker deflection. Data are expressed as mean ± SEM. Un-DPSC; Un-differentiated dental pulp stem cell, D-DPSC; Lesion+differentiated dental pulp stem cell, **; P<0.01, as compared to the control group, and ##; P<0.01, as compared to lesion+vehicle group.
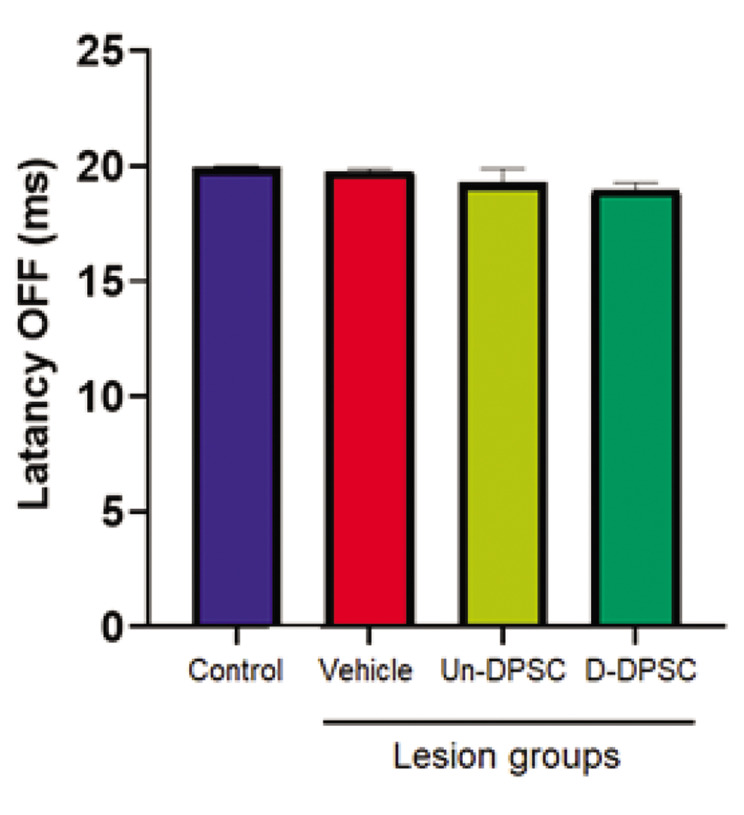
Latency OFF
There was not a main effect on the latency OFF of neuronal responses [F (3, 87)=1.886, P=0.138]. There was no significant difference between the control and lesion, and also between the lesion and SCs groups (Fig .6).
Fig.6.
Response latency OFF. Response latency of the D2 barrel neurons to the whisker deflection. Data are expressed as mean ± SEM. Un-DPSC; Undifferentiated dental pulp stem cell, D-DPSC; Lesion+differentiated dental pulp stem cell.
Discussion
The present study revealed that the spontaneous activity, latency, and features of response magnitude in the barrel cortex opposing the damaged barrel cortex declined compared to the control group’s neural activity, three weeks after the unilateral focal lesion in the right barrel cortex. Lesions in the contralateral barrel cortex could change the response patterns of the neural responses of the intact homotopic cortex, which might also influence the animals’ behavior (19). This response can improve in the groups that were injected with SCs in the lesioned area compared to the vehicle group three weeks after injection.
Reduced activity in spontaneous and evoked responses after 3 weeks in the lesion group, as well as disorders in the experience-dependent plasticity in the intact barrel cortex neurons following a lesion in the homotopic region in the opposing cerebral hemisphere, have been reported (14) which is consistent with the present study.
Reduced input from the lesioned hemisphere into the intact barrel cortex through the corpus callosum inputs is a possible mechanism for changes due to the lesion (5). According to a chronic study a barrel field cortex, sub-pial aspiration lesion could meaningfully degrade both evoked activity and background in the barrel field cortex of the contralateral (14).
Still, several types of change, including reactive events, reduced growth factors, and release of neurotransmitters from the lesioned area and also eliminating the activity of ongoing interhemispheric signals originating from the lesioned area can be produced by a cortical lesion. These events are considered the possible modulators for firing features in neurons on the contralateral side (5). Changes in the dendritic morphology and growth factor performance might influence the function and structure of the contralateral barrel cortex of the lesion area (26). Also, the reduced cortical excitability mentioned in this study is probably the result of reduced excitatory neurotransmitter levels in the contralateral cortex (27) which can be investigated in further studies.
Besides, one could argue that thalamocortical projections play a role in improving animal performance (28) as a potential supporter for performance recovery following bilateral or unilateral SI and SII cortex (29).
We observed neural responses improved in both U-DPSC and D-DPSC groups, this improvement was significant in the D-DPSCs group compared to the vehicle group. However, a significant proportion of grafted SCs migrated to the contralateral side of the brain, whether this was lesioned or intact, showing that SCs are attracted by and interact with both reorganization and degeneration regions (30). Behavioral recovery after brain damage can be improved by SC transplantation in animal models but there is no understanding of the functional integration of SC-derived neurons into injured brain circuitry (20).
Because a large part of this area is eliminated by our mechanical lesion, therefore rearrangement of these neuronal barrel clusters by SC injection is far from expected. However, SC transplantation can induce events to enhance performance at the molecular level in the ipsilateral cortex. The ability of DPSCs to provide trophic factors in the peri-lesioned area was proved in our previous study (19).
Regeneration of damaged neuronal axons (31), reduction of apoptosis (32), glial scar (33), and inflammation (34), the release of neuroprotective factors, and the ability to differentiate various cell types, for example, oligodendrocytes, functional neurons, and astrocytes (35) have been considered as feasible mechanisms for functional improvement. Sc therapy may reopen the SII and the contralateral barrel cortex plasticity as well as the plasticity of the remaining barrel cortex that escaped the lesion (19). Still, we do not know the exact mechanism of how the neurons connected to the thalamus and the barrel contralateral cortex. Further investigations are needed to find the exact mechanism.
Transplanted human embryonic SCs in rodents, send axonal projections to extensive brain areas, including the contralateral cortex (19). Following a stroke in rats, axonal projections from intracortical transplanted SC-derived (36) cortical neurons extend to both contralateral and ipsilateral hemispheres. Whether an activity in the grafted neurons influences sensorimotor function as well as these efferent connections become incorporated into stroke-injured host neural circuitry remains unclear (20). In our previous study, it was shown that transplantation of DPSCs into a lesioned somatosensory (barrel) cortex can facilitate the recovery of function at week 2-4 compared to the vehicle group and affected a range of neuronal markers such as nestin, NeuN, Olig 2, BDNF, neuroligin1 and GFAP in the barrel cortex post-lesion (19). After cortical stroke, transcallosal connections are important and involved in behavioral recovery and spontaneous interhemispheric structural rearrangement. Transcallosal connections of transplanted neurons are likely to participate in this reorganization (37). The results of several recent studies in other experimental models confirm this idea which is in line with our findings, that regeneration of the cortical neural circuitry is probable by cell transplantation in the damaged adult brain (20).
The current study had limitations for recording from the lesioned- barrel cortex and there is a lack of information about how SCs injection can consequently influence neural function.
Conclusion
Injection of D-DPSCs or U-DPSCs into the lesioned barrel cortex area can improve the homologous opposite cortical neural responses. Although the injection of SCs is not expected to reorganize the barrel, the changes in the contralateral barrel cortex could be observed after lesion and SC therapy in neural connections with the area of brain damage.
Acknowledgments
The present study was supported by a research grant from the Council for Development Stem Cells Sciences and Technologies (Grant No.: 82710/11) and by funds from Kerman Neuroscience Research Center (Grant No.: KNRC/EC/95-63). The authors declared no potential conflicts with respect to the research, authorship, and/or publication of this article.
Author’s Contributions
M.R.A., V.Sh.; Conceptualization and Supervision. M.S., M.R.A., V.Sh.; Methodology. M.S., A.D.; Investigation. V.SH.; Funding acquisition. M.S., M.R.A., A.D.; Writing- Reviewing and Editing. All authors read and approved the final manuscript.
References
- 1.Crandall SR, Patrick SL, Cruikshank SJ, Connors BW. Infrabarrels are layer 6 circuit modules in the barrel cortex that link long-range inputs and outputs. Cell Rep. 2017;21(11):3065–3078. doi: 10.1016/j.celrep.2017.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldmeyer D, Brecht M, Helmchen F, Petersen CC, Poulet JF, Staiger JF, et al. Barrel cortex function. Prog Neurobiol. 2013;103:3–27. doi: 10.1016/j.pneurobio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Chance SA, Crow TJ. Distinctively human: cerebral lateralisation and language in Homo sapiens. J Anthropol Sci. 2007;85:83–100. [Google Scholar]
- 4.Ku RY, Torii M. New molecular players in the development of callosal projections. Cells. 2020;10(1):29–29. doi: 10.3390/cells10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Rema V, Ebner FF. Chronic suppression of activity in barrel field cortex downregulates sensory responses in contralateral barrel field cortex. J Neurophysiol. 2005;94(5):3342–3356. doi: 10.1152/jn.00357.2005. [DOI] [PubMed] [Google Scholar]
- 6.Tsytsarev V, Arakawa H, Zhao S, Chédotal A, Erzurumlu RS. Behavioral consequences of a bifacial map in the mouse somatosensory cortex. J Neurosci. 2017;37(30):7209–7218. doi: 10.1523/JNEUROSCI.0598-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheibani V, Shamsizadeh A, Afarinesh MR, Rezvani ME. Neonatal capsaicin treatment modulates experience-dependent plasticity in the rat barrel cortex. J Comp Neurol. 2010;518(17):3427–3438. doi: 10.1002/cne.22384. [DOI] [PubMed] [Google Scholar]
- 8.Shuler MG, Krupa DJ, Nicolelis MA. Bilateral integration of whisker information in the primary somatosensory cortex of rats. J Neurosci. 2001;21(14):5251–5261. doi: 10.1523/JNEUROSCI.21-14-05251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H, Wang L, Rea WW, Brynildsen JK, Jaime S, Zuo Y, et al. Lowbut not high-frequency LFP correlates with spontaneous BOLD fluctuations in rat whisker barrel cortex. Cereb Cortex. 2016;26(2):683–694. doi: 10.1093/cercor/bhu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9(6):740–747. doi: 10.1016/s0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 11.Baron JC. Testing cerebral function: will it help the understanding or diagnosis of central nervous system disease? Ciba Found Symp. 1991;163:250–261. [PubMed] [Google Scholar]
- 12.Cappa SF, Perani D, Grassi F, Bressi S, Alberoni M, Franceschi M, et al. A PET follow-up study of recovery after stroke in acute aphasics. Brain Lang. 1997;56(1):55–67. doi: 10.1006/brln.1997.1737. [DOI] [PubMed] [Google Scholar]
- 13.Juhász C, Kamondi A, Szirmai I. Spectral EEG analysis following hemispheric stroke: evidences of transhemispheric diaschisis. Acta Neurol Scand. 1997;96(6):397–400. doi: 10.1111/j.1600-0404.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 14.Rema V, Ebner FF. Lesions of mature barrel field cortex interfere with sensory processing and plasticity in connected areas of the contralateral hemisphere. J Neurosci. 2003;23(32):10378–10387. doi: 10.1523/JNEUROSCI.23-32-10378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song M, Mohamad O, Gu X, Wei L, Yu SP. Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant. 2013;22(11):2001–2015. doi: 10.3727/096368912X657909. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Sundström E. Stem cell therapies for central nervous system trauma: the 4 Ws-what, when, where, and why. Stem Cells Transl Med. 2022;11(1):14–25. doi: 10.1093/stcltm/szab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang QM, Deng KQ, Zhang M, Wu XC, Yang RL, Fu SP, et al. Multiple strategies enhance the efficacy of MSCs transplantation for spinal cord injury. Biomed Pharmacother. 2023;157:114011–114011. doi: 10.1016/j.biopha.2022.114011. [DOI] [PubMed] [Google Scholar]
- 18.Bao X, Wei J, Feng M, Lu S, Li G, Dou W, et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103–113. doi: 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 19.Sabzalizadeh M, Afarinesh MR, Esmaeili-Mahani S, Farsinejad A, Derakhshani A, Arabzadeh E, et al. Transplantation of rat dental pulp stem cells facilities post-lesion recovery in the somatosensory whisker cortex of male Wistar rats. Brain Res Bull. 2021;173:150–161. doi: 10.1016/j.brainresbull.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Palma-Tortosa S, Tornero D, Grønning Hansen M, Monni E, Hajy M, Kartsivadze S, et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci USA. 2020;117(16):9094–9100. doi: 10.1073/pnas.2000690117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabzalizadeh M, Mollashahi M, Afarinesh MR, Mafi F, Joushy S, Sheibani V. Sex difference in cognitive behavioral alterations and barrel cortex neuronal responses in rats exposed prenatally to valproic acid under continuous environmental enrichment. Int J Dev Neurosci. 2022;82(6):513–527. doi: 10.1002/jdn.10206. [DOI] [PubMed] [Google Scholar]
- 22.Sabzalizadeh M, Afarinesh MR, Esmaeili-Mahani S, Sheibani V. Focal unilateral mechanical lesion in barrel cortex impairs rat’s abilities to discriminate textures. Somatosens Mot Res. 2021;38(1):1–10. doi: 10.1080/08990220.2020.1828055. [DOI] [PubMed] [Google Scholar]
- 23.Shafiei F, Afarinesh MR, Golshan F, Haghpanah T, Sabzalizadeh M, Zangiabadi I, et al. Comparison of pre-pulse inhibition, tactile discrimination learning and barrel cortical neural response in adult male rats following chronic exposure to morphine, methadone and buprenorphine. Physiol Behav. 2019;212:112694–112694. doi: 10.1016/j.physbeh.2019.112694. [DOI] [PubMed] [Google Scholar]
- 24.Afarinesh MR, Sheibani V, Arabzadeh S, Shamsizadeh A. Effect of chronic morphine exposure on response properties of rat barrel cortex neurons. Addict Biol. 2008;13(1):31–39. doi: 10.1111/j.1369-1600.2007.00087.x. [DOI] [PubMed] [Google Scholar]
- 25.Afarinesh MR, Shafiei F, Sabzalizadeh M, Haghpanah T, Taheri M, Parsania S, et al. Effect of mild and chronic neonatal hypothyroidism on sensory information processing in a rodent model: a behavioral and electrophysiological study. Brain Res Bull. 2020;155:29–36. doi: 10.1016/j.brainresbull.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Madinier A, Quattromani MJ, Sjölund C, Ruscher K, Wieloch T. Enriched housing enhances recovery of limb placement ability and reduces aggrecan-containing perineuronal nets in the rat somatosensory cortex after experimental stroke. PLoS One. 2014;9(3):e93121–e93121. doi: 10.1371/journal.pone.0093121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews RJ. Transhemispheric diaschisis.A review and comment. Stroke. 1991;22(7):943–949. doi: 10.1161/01.str.22.7.943. [DOI] [PubMed] [Google Scholar]
- 28.Tamè L, Braun C, Holmes NP, Farnè A, Pavani F. Bilateral representations of touch in the primary somatosensory cortex. Cogn Neuropsychol. 2016;33(1-2):48–66. doi: 10.1080/02643294.2016.1159547. [DOI] [PubMed] [Google Scholar]
- 29.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Giedzinski E, Angulo MC, Lui T, Lu C, Park AL, et al. Functional equivalence of stem cell and stem cell-derived extracellular vesicle transplantation to repair the irradiated brain. Stem Cells Transl Med. 2020;9(1):93–105. doi: 10.1002/sctm.18-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zhou Y, Li H, Wang R, Yang D, Li B, et al. Transplanted dental pulp stem cells migrate to injured area and express neural markers in a rat model of cerebral ischemia. Cell Physiol Biochem. 2018;45(1):258–266. doi: 10.1159/000486772. [DOI] [PubMed] [Google Scholar]
- 32.Hosseini SM, Farahmandnia M, Razi Z, Delavari S, Shakibajahromi B, Sarvestani FS, et al. Combination cell therapy with mesenchymal stem cells and neural stem cells for brain stroke in rats. Int J Stem Cells. 2015;8(1):99–105. doi: 10.15283/ijsc.2015.8.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peruzzaro ST, Andrews MMM, Al-Gharaibeh A, Pupiec O, Resk M, Story D, et al. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury. J Neuroinflammation. 2019;16(1):2–2. doi: 10.1186/s12974-018-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu W, Feng Z, Xu J, Jiang Z, Feng M. Brain-derived neurotrophic factor modified human umbilical cord mesenchymal stem cellsderived cholinergic-like neurons improve spatial learning and memory ability in Alzheimer’s disease rats. Brain Res. 2019;1710:61–73. doi: 10.1016/j.brainres.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Nudi ET, Jacqmain J, Dubbs K, Geeck K, Salois G, Searles MA, et al. Combining enriched environment, progesterone, and embryonic neural stem cell therapy improves recovery after brain injury. J Neurotrauma. 2015;32(14):1117–1129. doi: 10.1089/neu.2014.3618. [DOI] [PubMed] [Google Scholar]
- 36.Somaa FA, Wang TY, Niclis JC, Bruggeman KF, Kauhausen JA, Guo H, et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017;20(8):1964–1977. doi: 10.1016/j.celrep.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 37.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22(14):6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]