Abstract
Objective:
Non-small cell lung cancer (NSCLC) stands as a prominent contributor to cancer-related fatalities on a global scale, necessitating the search for novel therapeutic agents. SP-8356, a derivative of (1S)-(–)-verbenone, has shown promise as an anticancer agent in preclinical studies. However, specific mechanisms underlying its effects in NSCLC remain to be elucidated. The aim of this research was to explore the in vitro anti-NSCLC effects of SP-8356, elucidate its mechanisms of action, and assess its efficacy in inhibiting tumor formation in a murine model.
Materials and Methods:
n this experimental study, NSCLC cell lines were treated with various concentrations of SP- 8356. Cell viability and proliferation were assessed using MTT and colony formation assays, respectively. Cell cycle distribution was analyzed by flow cytometry, and apoptosis was evaluated by determining apoptotic protein expression. Western blot analysis was conducted to assess protein expression levels of the both p53 and MDM2. Additionally, we evaluated efficacy of the SP-8356 in inhibiting tumor formation of the nude mouse model.
Results:
SP-8356 demonstrated a concentration-dependent inhibition of cell proliferation in the NSCLC cell lines. Flow cytometric analysis showed that SP-8356 led to cell cycle arrest at the G2/M phase, indicating its potential influence on regulating the cell cycle. SP-8356 treatment was associated with the downregulation of CDK1 and Cyclin B1. Additionally, SP-8356 significantly enhanced apoptosis in NSCLC cells. SP-8356 treatment was associated with the downregulation of Bcl-2, while Bax expression was upregulated. Mechanistically, SP-8356 led to accumulation of the p53 protein levels within the NSCLC cells. This accumulation was mediated through inhibition of its negative regulator, MDM2. Using a nude mouse model demonstrated that SP-8356 effectively inhibited tumor formation in vivo.
Conclusion:
Our findings shed light on the molecular mechanisms underlying anticancer activity of SP-8356 and highlight its potential as a promising therapeutic candidate for NSCLC treatment.
Keywords: Apoptosis, Non-Small Cell Lung Cancer, p53, Proliferation, SP-8356
Introduction
Lung cancer is a leading cause of tumor-related deaths in both men and women (1). Standard treatments like surgery, chemotherapy, and radiotherapy have revolutionized lung cancer treatment (2). Pulmonary neoplasms are broadly divided into non-small cell lung cancer (NSCLC) and small cell cancers; the former represents 80% of all lung cancer cases (3). NSCLC is associated with aberrant protein expression, resulting in extensive proliferation and malignancy (4). A great need has emerged to look for the molecular mechanism related to the NSCLC pathogenesis to improve disease outcomes.
p53 is a tumor suppressor protein, frequently mutated or inactivated in many types of cancer, including NSCLC (5). In NSCLC, p53 mutations are found in approximately 50% of cases, while the majority of mutations occurring in the DNA binding domain of the protein (6). These mutations can result in the loss of p53 function, leading to accumulation of the DNA damage and promotion of tumor growth and metastasis. In addition to mutations, other mechanisms can also contribute to the inactivation of p53 in NSCLC, such as increased expression of its negative regulator MDM2, which targets p53 for degradation, or alterations in other proteins that regulate p53 activity (7). Loss of p53 function in NSCLC has been associated with a poorer prognosis as well as resistance to chemotherapy and radiation therapy. Therefore, understanding the mechanisms that regulate p53 in NSCLC and developing strategies to restore its function may have important implications for the treatment of this disease.
Natural compounds and their derivatives have become a significant area of interest in drug discovery, due to their diverse pharmacological properties and potential therapeutic applications (8). Among these compounds, (1S)-(–)-verbenone, a naturally occurring monoterpene found in essential oils of various plant species, has been shown to cause promising bioactivities (9). As a result, researchers have synthesized and investigated derivatives of (1S)-(–)-verbenone to enhance its properties and develop novel therapeutic agents (10).
One such derivative is SP-8356, which has garnered attention for its potential pharmacological effects in various areas, including cancer research (11). The unique chemical structure and biological activities of SP-8356 have led to investigating its potential, as an anticancer agent. Preclinical studies have shown encouraging results, indicating that SP-8356 may exhibit significant anticancer properties by targeting specific molecular pathways involved in tumorigenesis (12). The present study was formulated with the aim of exploring role of SP-8356 with respect to the p53 protein in NSCLC cells.
Materials and Methods
Cell culture
In this experimental study, human NSCLC cells (NCI-H460 and A549) and normal human fetal lung fibroblast cell line (MRC-5) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in appropriate growth media supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, USA) and 1% penicillinstreptomycin (Thermo Scientific, USA). The cells were cultured in a humidified incubator at 37°C with 5% CO2.
Chemicals and reagents
SP-8356, a derivative of (1S)-(–)-verbenone, was synthesized using the previously established methods (13). Stock solutions of SP-8356 were prepared in dimethyl sulfoxide (DMSO) at appropriate concentrations and stored at -20°C.
Cell viability assay (MTT assay)
To assess cell proliferation, NSCLC cells were seeded into 96-well plates at a density of 5000 cells per well and allowed to adhere overnight. The cells were then treated with various concentrations of SP-8356 (5 ìM, 10 ìM, 15 ìM and 20 ìM) or vehicle control (DMSO) for 24 hours. Following the specified length of treatment duration, the culture medium was substituted with fresh media containing 0.5 mg/ml of MTT (3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide) and then incubated for an additional 4 hours at 37°C. DMSO was used to dissolve formazan crystals, and the absorbance was determined at 570 nm using a microplate reader.
Colony formation assay
NSCLC cells were plated in 6-well dishes at a sparse density of 200 cells per well and permitted to attach overnight. Subsequently, the cells were exposed to a range of SP-8356 concentrations (5 ìM, 10 ìM, 15 ìM and 20 ìM) or vehicle control (DMSO) for 24 hours. Following the treatment, the existing culture medium was substituted with fresh complete growth media, and the cells were incubated for 14 days to facilitate formation of colonies. The colonies were fixed using methanol and then stained with crystal violet. Count of colonies was conducted manually.
Cell cycle analysis
To explore impact of SP-8356 on the cell cycle, NSCLC cells were exposed to SP-8356 (15 ìM) for 24 hours. Following the treatment, the cells were collected, rinsed with phosphate-buffered saline (PBS), fixed in ice-cold 70% ethanol, and preserved at -20°C. Before analysis, the fixed cells were washed with PBS, treated with 50 ìl RNase A (from 100 ìg/ml stock solution, Sigma-Aldrich, USA), and stained with 200 ìl propidium iodide (PI; from 50 ìg/ml stock solution, Sigma-Aldrich, USA). A FACS Calibur flow cytometer (BD Biosciences, USA) was used to carry out flow cytometric analysis to ascertain distribution of the cell cycle. BD CellQuest Pro-Software was used for analyzing the results.
Western blotting
To examine the protein expression levels, NSCLC cells were treated with treated with various concentrations of SP-8356 (5 ìM, 10 ìM, 15 ìM and 20 ìM) or vehicle control (DMSO) for 24 hours. Following treatment, the cells were lysed using RIPA buffer supplemented with 1X protease (Sigma-Aldrich, USA) and phosphatase inhibitors (prepared from 100X protease/phosphatase inhibitor cocktail; Sigma-Aldrich, USA). Total protein concentration was quantified utilizing a protein assay kit (Pierce™ BCA Protein Assay Kit; Thermo Scientific, USA). Equivalent quantities of protein were segregated through 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked and probed with primary antibodies against anti-CDK1 (ab133327, abcam, USA, 1:1000 dilution), anti-Cyclin B1 (ab32053, abcam, USA; 1:800 dilution), anti-Bax (ab216494, abcam, USA; 1:1000 dilution), anti-Bcl-2 (ab59348, abcam, USA; 1:1000 dilution), anti-p53 (ab131442, abcam, USA; 1:1000 dilution), anti-MDM2 (ab16895, abcam, USA; 1:1000 dilution), and anti-GAPDH (ab8245, abcam, USA; 1:1000 dilution). After rinsing with TBST buffer, the membranes were exposed to peroxidase-conjugated anti-rabbit secondary antibodies (diluted at 1:5000; Hangzhou Multi Sciences [Lianke] Biotech Co., China) at room temperature. Protein signals were detected using an enhanced chemiluminescence (ECL) technique.
Experimental animals
Fifteen male athymic BALB/c nude mice, aged 5-6 weeks, were accommodated in regular cages maintained at 25°C with a 12-hours light and 12-hours dark cycle. All animal procedures were performed following the protocol approved by People’s Hospital of Chongqing Liang jiang New Area (Chongqing, China, PHCL/1231.101). The mice were provided with a standard pellet diet and ad libitum access to water. They were randomly divided into three groups, each containing five animals:
Group I: control mice were received an intravenous injection of isotonic saline (25 ml/kg body weight) along with subcutaneous injection of 2×106 A549 cells on one side of the posterior flank.
Group II: nude mice were subcutaneously injected with 2×106 A549 cells on one side of the posterior flank, along with an oral low-dose treatment of 10 μM/kg body weight of SP-8356.
Group III: mice were subcutaneously injected with 2×106 A549 cells on one side of the posterior flank, along with an oral high-dose treatment of 10 mg/kg body weight of SP-8356.
At the conclusion of the experimental period on day 19, the mice were anesthetized with ketamine (30 mg/ kg body weight) and then euthanized. The tumor tissue was excised, washed with saline, and dried, and its weight was measured. Tumor homogenates (10%) were prepared using a 0.1 M Tris HCl buffer (pH=7.4) and a homogenizer.
To evaluate impact of SP-8356 on tumor cell growth, dimension and weight of the tumors were measured in the mice carrying tumors. Tumor size was calculated using the formula (L×S2)×0.5, where L represented the greatest diameter and S represented the shortest diameter of the tumor. Weight of the tumors was determined by weighing the moist tumor tissue.
Statistical analysis
Data from triplicate or quadruplicate experiments were expressed as mean ± standard deviation (SD). Statistical significance was determined using appropriate statistical tests, such as Student’s t test or one-way analysis of variance (ANOVA), followed by post hoc comparisons. P<0.05 were regarded as statistically significant.
Results
SP-8356 significantly curbed NSCLC cell viability and proliferation
The MTT cell viability assay revealed a notable dosedependent reduction in cell viability upon treatment with increasing concentrations of SP-8356. The IC50 values for A549 and NCI-H460 NSCLC cells were found to be 15 ìM (Fig .1A).
Fig.1.
SP-8356 suppresses cell viability and proliferation in NSCLC cells. A. MTT cell viability assay illustrates a decline in cell viability that is directly proportional to the rising concentrations of SP-8356, indicating a dose-dependent effect. Treatment with SP-8356 significantly decrease cell viability in NCI-H460 and A549 NSCLC cells compared to MRC-5 cells (control fibroblasts). B. Colony formation assay confirms the inhibitory effect of SP-8356 on cell proliferation in NSCLC cells. SP-8356 treatment significantly decrease number of colonies formed by NSCLC cells compared to the control group. The concentration-dependent response highlights the ability of SP-8356 to impede the clonogenic potential of NSCLC cells. The experiments were conducted in triplicate and replicated three times. *; P<0.05 and NSCLC; Non-small cell lung cancer.
Furthermore, the inhibitory effect of SP-8356 on cell proliferation was confirmed through a colony formation assay. Treatment with SP-8356 resulted in a significant decrease in the number of colonies formed by NSCLC cells compared to the control group. The concentrationdependent response demonstrated the ability of SP-8356 to impede the clonogenic potential of NSCLC cells (Fig .1B).
These results collectively highlight the robust inhibitory effects of SP-8356 on the both NSCLC cell viability and proliferation. The findings suggested that SP- 8356 was held as a promising therapeutic candidate for combating NSCLC by targeting cell growth and survival pathways. Further investigations are warranted to explore its mechanism of action and potential translational applications for NSCLC treatment.
SP-8356 regulated G2/M transition
SP-8356 treatment significantly altered the cell cycle progression in NSCLC cells. Flow cytometric analysis demonstrated a rise in the proportion of cells arrested at the G2/M phase, following the SP-8356 treatment in the A549 cells compared to control (Fig .2A, B) and similar results were obtained in NCI-H460 NSCLC cells (Fig .2C, D). Conversely, a significant reduction in the G1 and S phase populations was observed in response to SP-8356 treatment. These findings suggested that SP-8356 triggered G2/M cell cycle arrest in NSCLC cells, implying its potential role in regulating the G2/M transition. These findings collectively suggested that SP- 8356 was involved in the regulation of G2/M cell cycle transition in NSCLC cells.
Fig.2.
SP-8356 regulates G2/M transition in NSCLC cells. Flow cytometric analysis of cell cycle distribution in NSCLC cells treated with SP-8356. A. Untreated NCI-H460 cells, B. NCI-H460 cells treated with SP-8356, C. Untreated A549 cells, D. A549 cells treated with SP-8356. SP-8356 treatment resulted in a notable increase in the percentage of cells arrested in the G2/M phase, as shown by the shift in the cell population towards G2/M in NCI-H460 and A549, compared to respective controls. Conversely, there was a notable decrease in the G1 and S phase populations in response to SP-8356 treatment. NSCLC; Non-small cell lung cancer.
SP-8356 regulated cell cycle and apoptotic associated protein levels
Western blot analysis was performed to evaluate effects of SP-8356 on cell cycle protein levels in NSCLC cells. Treatment with SP-8356 resulted in notable changes in the expression levels of proteins associated with cell cycle regulation. More specifically, there was a decrease in the expression of CDK1, cyclin B1, and Bcl-2, coupled with an increase in Bax expression observed in the both NCI-H460 (Fig .3A) and A549 (Fig .3B) cell lines. Figure 3C shows densitmety analysis of Figure 3A while as Figure 3D shows densitmety analysis of Figure 3B.
Fig.3.
SP-8356 modulates protein levels associated with the cell cycle and apoptosis in NSCLC cells. A. Western blot analysis of cell cycle and apoptoticassociated protein levels in NCI-H460 cells treated with SP-8356. B. Western blot analysis of cell cycle and apoptotic-associated protein levels in A549 cells treated with SP-8356. Treatment with SP-8356 resulted in notable changes in the expression levels of cell cycle and apoptosis proteins. C. Quantitative analysis of western blot “A” using densitometry. D. Quantitative analysis of western blot “B” using densitometry. The experiments were conducted in triplicate and replicated three times. *; P<0.05, **; P<0.001, and NSCLC; Non-small cell lung cancer.
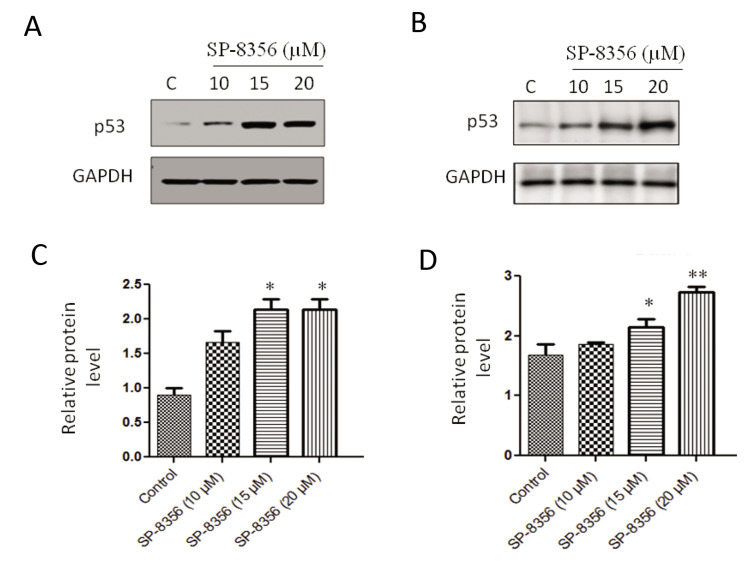
SP-8356 upregulated p53 levels
Treatment with SP-8356 led to a significant upregulation of p53 protein levels in NSCLC cells. Western blot analysis unveiled a significant elevation in p53 expression following SP-8356 treatment in comparison with the control group. Upregulation of p53 was observed in the both A549 and NCI-H460 NSCLC cell lines, indicating a consistent effect of SP-8356 on p53 levels in different cellular contexts (Fig .4).
Fig.4.
SP-8356 upregulates p53 levels in NSCLC cells. A. Expression analysis of p53 protein levels in NCI-H460 cells treated with SP-8356. B. Expression analysis of p53 protein levels in A549 cells treated with SP-8356. C. Quantitative analysis of western blot “A” using densitometry. D. Quantitative analysis of western blot “B” using densitometry. The experiments were conducted in triplicate and replicated three times. *; P<0.05, **; P<0.001, and NSCLC; Non-small cell lung cancer.
SP-8356 acted on p53 through MDM2
To understand the mechanism by which SP-8356 upregulated p53 levels, we investigated its impact on MDM2, a key negative regulator of p53. Western blot analysis revealed that treatment with SP-8356 led to a significant reduction in MDM2 protein expression in NSCLC cells. This downregulation of MDM2 was observed in the both A549 and NCI-H460 NSCLC cell lines (Fig .5).
Fig.5.
SP-8356 acts on p53 through MDM2. A. Expression analysis of MDM2 protein levels in NCI-H460 cells treated with SP-8356. B. Expression analysis of MDM2 protein levels in A549 cells treated with SP-8356. C. Quantitative analysis of western blot “A” using densitometry. D. Quantitative analysis of western blot “B” using densitometry. The experiments were conducted in triplicate and replicated three times. *; P<0.05 and **; P<0.001.
These findings indicated that SP-8356 acted on p53 through MDM2, inhibiting function of MDM2 and thereby preventing p53 degradation. As a consequence, p53 protein level was increased, leading to its activation and subsequent induction of downstream cellular responses, including cell cycle arrest and apoptosis.
Influence of SP-8356 on tumor size and weight
Impact of SP-8356 on tumor growth inhibition was evaluated by weighing and measuring tumor size. The results presented in Figure 6A, B clearly demonstrated a notable reduction in tumor size of the both low-dose and high-dose SP-8356-treated groups, compared to the control group.
Furthermore, the high-dose SP-8356-treated group exhibited more significant decrease in tumor size than the low-dose-treated group. Similarly, when examining tumor weight (Fig .1B), both of the low-dose and highdose SP-8356-treated groups displayed the marked reductions compared to the control group.
Moreover, the high-dose SP-8356-treated group demonstrated a significant decrease in tumor weight compared to the low-dose-treated group. These findings suggested that SP-8356 administration effectively inhibited tumor growth, with higher doses leading to more pronounced effects on the both tumor size and weight.
Fig.6.
Effect of SP-8356 on tumor volume and tumor weight. A. The graph depicts the impact of SP-8356 treatment on tumor volume. Both of the low-dose (10 ìM) and high-dose (20 ìM) SP-8356-treated groups (groups II and III, respectively) show a significant decrease in tumor volume compared to the control group (group I). B. The bar chart illustrates effect of SP-8356 on tumor weight. In the both of low-dose and high-dose SP- 8356-treated groups (groups II and III, respectively), tumor weight is markedly decreased compared to the control group (group I). *; P<0.01, when compared to the group I, while as #; P<0.05, when compared to the group II.
Discussion
NSCLC stands as one of the most prevalent and formidable malignancies globally, accounting for a substantial proportion of cancer-related deaths (14). Despite the significant advancements in therapeutic strategies, management of NSCLC remains challenging, necessitating exploration of novel and effective therapeutic agents (15, 16). In this context, SP-8356, a derivative of (1S)-(–)-verbenone, has emerged as a promising candidate for potential anti-cancer activity in NSCLC cells. The present study investigated anticancer properties of the SP-8356 in NSCLC cells. Our results demonstrated that SP-8356 significantly suppressed cell viability and proliferation in NSCLC cells in a dose-dependent manner, as evidenced by the MTT cell viability assay and colony formation assay, without affecting MRC-5 cells. MRC-5 cells are a type of normal human lung fibroblast cells, often used in research as a representative of noncancerous, healthy cells. SP-8356 appeared to selectively affect NSCLC cancer cells, specifically without impacting MRC-5 cells. This selectivity could be due to several factors, such as differences in the genetic makeup or signaling pathways between cancer cells and normal cells (17, 18). These findings suggested that SP-8356 had a potent inhibitory effect on NSCLC cell growth, making it a promising candidate for NSCLC treatment.
One of the mechanisms underlying anticancer effects of SP-8356 involves its regulation of the cell cycle progression (10). Flow cytometric analysis revealed that SP-8356 induced G2/M cell cycle arrest in NSCLC cells. G2/M arrest is a critical checkpoint in the cell cycle, where cells are subjected to thorough DNA damage repair before entering mitosis. Ability of SP-8356 to induce G2/M arrest in NSCLC cells may disrupt the uncontrolled cell cycle progression, leading to reduced cell proliferation and increased susceptibility to apoptosis.
Apoptosis is a key process in controlling cellular homeostasis and eliminating damaged or abnormal cells (19). In this study, SP-8356 was found to enhance apoptosis in NSCLC cells, as demonstrated by Annexin V staining and Western blot analysis of apoptotic-associated protein levels. Increase in the pro-apoptotic proteins, like Bax, and decrease in the anti-apoptotic proteins, such as Bcl-2, suggested activation of the intrinsic apoptotic pathway. These findings suggested that SP-8356 can trigger apoptosis in NSCLC cells, contributing to its anticancer effects. Taken together, these results suggested that SP-8356 played role in modulating the G2/M cell cycle transition and induced apoptosis in NSCLC cells. The observed alterations in apoptotic-associated protein levels further supported potential of SP-8356, as a candidate therapeutic agent for inducing cell cycle arrest and promoting apoptosis in NSCLC. Additional research is warranted to uncover the underlying molecular mechanisms and to investigate its therapeutic potential for treating NSCLC.
Upregulation of p53 protein levels was a prominent observation in our study, following the SP-8356 treatment. P53 is a crucial tumor suppressor protein that plays a central role in regulating cell cycle progression, DNA repair, and apoptosis in response to cellular stress (20, 21). The increased levels of p53 in NSCLC cells treated with SP-8356 indicated the compound impacts of p53 expression. Further investigation of the underlying mechanism revealed that SP-8356 acted on p53 through MDM2, a negative regulator of p53. SP-8356 interfered with the interaction between p53 and MDM2, preventing p53 ubiquitination and subsequent degradation. As a result, p53 level was accumulated within the cells, triggering downstream cellular responses, like cell cycle arrest and apoptosis. The results suggested that SP-8356 was capable to upregulate p53. The increased p53 levels may contribute to the observed anti-cancer effects of SP-8356 in NSCLC cells. Exploring the specific molecular mechanisms through which SP-8356 upregulate p53 expression will yield valuable insights into its potential as a therapeutic agent for NSCLC treatment.
Modulation of the p53-MDM2 axis represented a critical mechanism through which SP-8356 exerted its anticancer effects in NSCLC cells. Disruption of this regulatory pathway enhanced stability and function of p53, promoting its tumorsuppressive activities. The ability of SP-8356 to upregulate p53 and inhibit its ubiquitination highlighted its potential as a targeted therapeutic agent for p53-associated malignancies, including NSCLC. Modulation of the p53-MDM2 axis by SP-8356 highlighted a crucial mechanism underlying its anticancer effects in NSCLC cells. Disruption of this regulatory pathway may represent a promising therapeutic strategy for targeting p53 and promoting tumor suppression. Further investigations into the specific interactions between SP-8356, MDM2, and p53 will provide deeper insights into the molecular mechanisms mediating the compound’s impact on p53 regulation and its potential as a novel therapeutic agent for NSCLC treatment.
In conclusion, our study demonstrated that SP-8356, a (1S)-(–)-verbenone derivative, exhibiting potent anticancer properties in NSCLC cells. The compound significantly suppressed cell viability and proliferation, induced G2/M cell cycle arrest, and enhanced apoptosis. Furthermore, SP-8356 upregulated p53 levels by inhibiting its ubiquitination through disruption of the p53-MDM2 interaction. These findings provided valuable insights into the molecular mechanisms that underlie SP-8356 anticancer effects and highlight its potential as a promising therapeutic candidate for NSCLC treatment. Future studies, including in vivo investigations and translational research, are warranted to further validate the therapeutic potential of SP-8356 and to advance its development as a targeted therapy for NSCLC patients.
Conclusion
SP-8356, a (1S)-(–)-verbenone derivative, demonstrated potent anticancer effects in NSCLC cells. The compound substantially inhibited cell proliferation, triggered G2/M cell cycle arrest, and fostered apoptosis. Its upregulation of p53 levels via MDM2 inhibition contributed to its anticancer activity. SP-8356 held promise as a targeted therapeutic agent for NSCLC treatment, warranting further investigations for its clinical development.
Acknowledgments
There is no financial support and conflict of interest in this study.
Author’s Contributions
L.Y.; Conceptualization, Methodology, and Investigation. L.H.; Original draft preparation, Statistical analysis, Confirmed the authenticity of the raw data, and Supervision. All authors read and approved the final manuscript.
References
- 1.Sung H, Ferlay J, Siegel RL, Javersanne M, Soerjomataram I, Jemal A, et al. global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wirsdörfer F, de Leve S, Jendrossek V. Combining radiotherapy and immunotherapy in lung cancer: can we expect limitations due to altered normal tissue toxicity? Int J Mol Sci. 2018;20(1):24–24. doi: 10.3390/ijms20010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-smallcell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon N, Goller K, Wang F, Soibam B, Gagea M, Jain AK, et al. Aberrant expression of embryonic mesendoderm factor MESP1 promotes tumorigenesis. EBioMedicine. 2019;50:55–66. doi: 10.1016/j.ebiom.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baslan T, Morris JP 4th, Zhao Z, Reyes J, Ho YJ, Tsanov KM, et al. Ordered and deterministic cancer genome evolution after p53 loss. Nature. 2022;608(7924):795–802. doi: 10.1038/s41586-022-05082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh MM, Scheffler M, Merkelbach-Bruse S, Scheel AH, Ulmer B, Wolf J, et al. Comprehensive analysis of TP53 and KEAP1 mutations and their impact on survival in localized- and advanced-stage NSCLC. J Thorac Oncol. 2022;17(1):76–88. doi: 10.1016/j.jtho.2021.08.764. [DOI] [PubMed] [Google Scholar]
- 7.Ni L, Xu J, Zhao F, Dai X, Tao J, Pan J, et al. MiR-221-3p-mediated downregulation of MDM2 reverses the paclitaxel resistance of nonsmall cell lung cancer in vitro and in vivo. Eur J Pharmacol. 2021;899:174054–174054. doi: 10.1016/j.ejphar.2021.174054. [DOI] [PubMed] [Google Scholar]
- 8.Atanasov AG, Zotchev SB, Dirsch VM. International Natural Product Sciences Taskforce.Supuran CT.Natural products in drug discovery.advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perricone M, Arace E, Corbo MR, Sinigaglia M, Bevilacqua A. Bioactivity of essential oils: a review on their interaction with food components. Front Microbiol. 2015;6:76–76. doi: 10.3389/fmicb.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mander S, Kim DH, Thi Nguyen H, Yong HJ, Pahk K, Kim EY, et al. SP-8356, a (1S)-(-)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-κB signaling. Sci Rep. 2019;9(1):6595–6595. doi: 10.1038/s41598-019-41224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui W, Yang D, Chen X, Yu H. SP-8356 (a verbenone derivative) inhibits proliferation, suppresses cell migration and invasion and decreases tumor growth of osteosarcoma: role of PGC-1α/TFAM and AMPK-activation. Cell J. 2023;25(5):291–299. doi: 10.22074/CELLJ.2023.557404.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DH, Yong HJ, Mander S, Nguyen HT, Nguyen LP, Park HK, et al. SP-8356, a (1S)-(-)-verbenone derivative, inhibits the growth and motility of liver cancer cells by regulating NF-κB and ERK signaling. Biomol Ther (Seoul) 2021;29(3):331–341. doi: 10.4062/biomolther.2020.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju C, Song S, Hwang S, Kim C, Kim M, Gu J, et al. Discovery of novel (1S)-(-)-verbenone derivatives with anti-oxidant and antiischemic effects. Bioorg Med Chem Lett. 2013;23(19):5421–5425. doi: 10.1016/j.bmcl.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai RL, Chen NF, Li LY, Cui JW. A brand new era of cancer immunotherapy: breakthroughs and challenges. Chin Med J (Engl) 2021;134(11):1267–1275. doi: 10.1097/CM9.0000000000001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pucci C, Martinelli C, Ciofani G. Innovative approaches for cancer treatment: current perspectives and new challenges. Ecancermedicalscience. 2019;13:961–961. doi: 10.3332/ecancer.2019.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blagosklonny MV. Selective protection of normal cells from chemotherapy, while killing drug-resistant cancer cells. Oncotarget. 2023;14:193–206. doi: 10.18632/oncotarget.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7(3):339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toufektchan E, Toledo F. The guardian of the genome revisited: p53 downregulates genes required for telomere maintenance, DNA repair, and centromere structure. Cancers (Basel) 2018;10(5):135–135. doi: 10.3390/cancers10050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marvalim C, Datta A, Lee SC. Role of p53 in breast cancer progression: an insight into p53 targeted therapy. Theranostics. 2023;13(4):1421–1442. doi: 10.7150/thno.81847. [DOI] [PMC free article] [PubMed] [Google Scholar]