Abstract
Following the process of vasculogenesis during development, angiogenesis generates new vascular structures through a variety of different mechanisms or modes. These different modes of angiogenesis involve, for example, increasing microvasculature density by sprouting of endothelial cells, splitting of vessels to increase vascular surface area by intussusceptive angiogenesis, fusion of capillaries to increase blood flow by coalescent angiogenesis, and the recruitment of non-endothelial cells by vasculogenic mimicry. The recent reporting on coalescent angiogenesis as a new mode of vessel formation warrants a brief overview of angiogenesis mechanisms to provide a more complete picture. The journal Angiogenesis is devoted to the delineation of the different modes and mechanisms that collectively dictate blood vessel formation, inhibition and function in health and disease.
The modes of angiogenesis
During embryonic development, blood vessels are formed by vasculogenesis. This process involves (haem)angioblasts which are endothelial precursor cells that aggregate into the de novo assembly of endothelial cell-lined tubes. Further development of the vasculature is dependent on the process of sprouting angiogenesis. In 2022 Angiogenesis published the description of a new mode of angiogenesis, which is based on fusion or coalescence of capillaries to increase blood flow where needed [1]. Although a similar mechanism may have been reported before in the context of avian embryonic development [2], with this publication coalescent angiogenesis has been recognized as a bona fide mechanism of blood vessel formation. It remains elusive whether coalescent angiogenesis plays a role after embryonic development or whether it occurs during pathological angiogenesis. Since this new form of angiogenesis is now open for discussion and further investigation, we suggest it is useful here to provide an updated and modern overview of the different modes of angiogenesis. In a recent report, we reviewed the different modes of angiogenesis in detail [3], but a more complete overview is now presented herein (Figure 1).
Figure 1.
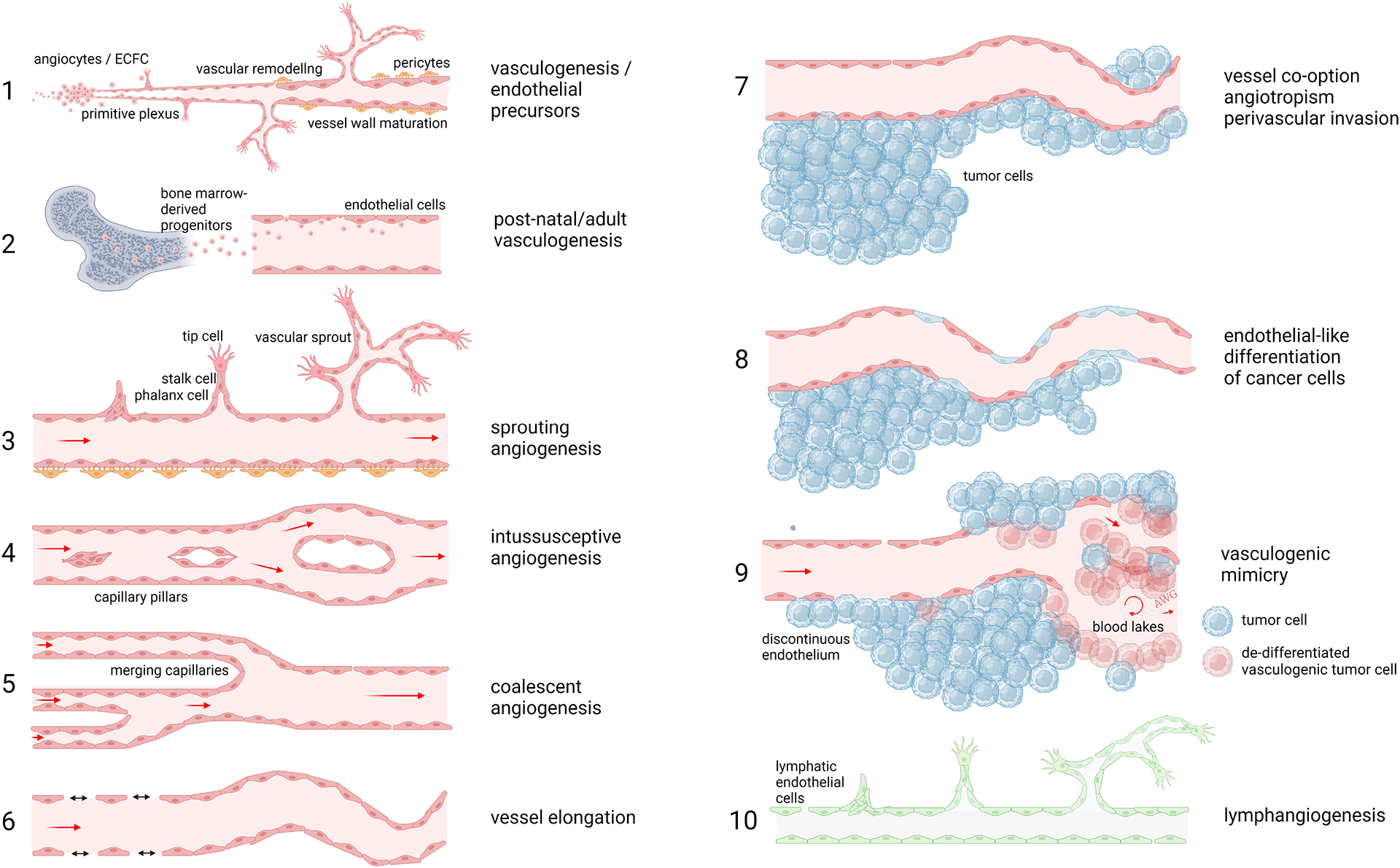
The 10 different modes of angiogenesis.
1. Vasculogenesis. 2. Post-natal angiogenesis. 3. Sprouting angiogenesis. 4. Intussusceptive angiogenesis. 5. Coalescent angiogenesis. 6. Vessel elongation. 7. Vessel cooption. 8. Endothelial-like differentiation of cancer cells. 9. Vasculogenic mimicry. 10. Lymphangiogenesis.
Interestingly, a process resembling vasculogenesis can also occur after embryonic development, even during adult life. Such post-natal vasculogenesis occurs when circulating endothelial colony forming (ECFCs) [4] cells are recruited to tumors or sites of ischemia [5]. These ECFCs may directly incorporate into the vasculature or promote angiogenesis via release of paracrine factors. The major and most well-known mode of angiogenesis is sprouting angiogenesis. In this process, endothelial cells – in response to angiogenic growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor – undergo all cellular changes necessary to generate a new functional blood vessel including the production of proteases to degrade the extracellular matrix, differentiation and migration of tip cells, proliferation, lumen formation, attraction of pericytes, deposition of extracellular matrix, and maturation [6]. This type of angiogenesis is considered the fastest way to vascularize growing tissue. Another mechanism is intussusceptive angiogenesis. This is considered the opposite of coalescent angiogenesis, with endothelial cells forming trans-vascular pillars between opposing vessels walls, thus splitting the vessel into two separate blood vessels [7]. This process is not restricted to capillaries but can also occur in smaller arteries and veins [8]. Vessel co-option (also known as angiotropism or perivascular invasion) is the process observed in tumors where outgrowth of the cancerous tissue occurs via existing blood vessels, without the need for vascular proliferation [9]. Vessel co-option and intussusceptive angiogenesis are often referred to as non-angiogenic processes, because they do not directly involve vascular proliferation. However, although both processes might indeed, at least initially, lack the mitogenic activity in endothelial cells, they both involve phenotypic and functional changes in the vasculature. These changes are the result of exposure to the same angiogenic growth factors that stimulate sprouting angiogenesis [10]. Also, these processes eventually result in enhanced numbers of microvessels or improved circulation, leading to increased oxygenation and nutrition of the tissue (it could therefore be considered whether the term ‘non-angiogenic growth’ is a misnomer).
In vasculogenic mimicry tumor cells masquerade as endothelial-like cells, by dedifferentiation into a mesenchymal-like program and/or acquiring an endothelial phenotype. By using this process tumor cells can also functionally behave as endothelial cells, by expressing typical endothelial cell markers (e.g. VE-cadherein or PECAM1) and factors that impair the coagulation of blood [11]. Vasculogenic mimicry presents a challenge for antiangiogenic therapy, because it is suggested that such therapy can be overcome by relying on or switching towards vasculogenic mimicry for maintaining blood circulation. A similar situation holds true for intussusceptive angiogenesis and vessel co-option, where counteracting vascular proliferation is considered less effective when vascular proliferation is absent.
The lymphatic system, which functions to promote the recirculation of interstitial fluid back into the blood circulation, is made up of and regulated by lymphatic endothelial cells with a different phenotype and function compared to blood endothelial cells. The lymphatic system is assembled during embryonic development and can be adapted during pathogenic conditions by neoformation through lymphangiogenesis (e.g. in cancer or lymphatic malformations). Although many of the processes mentioned above can contribute to lymphangiogenesis, lymphatic endothelial cell sprouting is the most well-known mechanism [12].This process is propelled by growth factors VEGF-C and -D signaling via VEGF-R3. While lymphangiogenesis can contribute to cancer metastasis formation [13], it is recently becoming apparent that it can positively contribute to anti-tumor immunity and immunotherapy [14]. In addition, tertiary lymphoid structures and the lymph node-associated high endothelial venules play important roles in immune surveillance and anti-cancer immunity [15].
Angiogenesis in review
If there is one thing that the SARS-CoV-2 pandemic has taught us, it would be that COVID-19 is a vascular pathology [16, 17]. Angiogenesis continues to receive many excellent submissions on this subject, describing predictive vascular markers [18–22], mechanisms of infection trough ACE2 [23] and specific damage to the vasculature [24–28]. A large body of research has been performed to characterize the diversity, heterogeneity, and plasticity of endothelial cells. Novel techniques such as spatial single cell sequencing and proteomics allows for identification of new endothelial cell subtypes [29–31]. In 2021 Angiogenesis published a dedicated special issue on endothelial heterogeneity and plasticity [32–38]. In the series of educational reviews, Angiogenesis published a review by Corvera et al. on angiogenesis in obesity and diabetes [39]. This was complemented by a report on the mechanisms of coronary endothelial dysfunction in diabetes [40].
Angiogenesis’ future
In 2022 Angiogenesis has continued to maintain its strength by recruiting and publishing high-impact papers and prolonging a strong impact factor of 9.800. We as editors will continue, as initiated before [41, 42], maintaining the rigorous, fair and fast peer-review process of submitted manuscripts. We also continue to invite strong educational reviews in the fields of angiogenesis and vascular biology and we will aim to release additional special issues. Of course, maintaining Angiogenesis as the primary journal in the field of angiogenesis and vascular biology is not possible without the trust and support of our authors, the editorial board members and the large group of outside peer-reviewers. We are grateful to all that have contributed and we highly encourage researchers to submit their exciting research to Angiogenesis and communicate new ideas for invited reviews and special issues to further improve the journal.
References
- 1.Nitzsche B, Rong WW, Goede A, Hoffmann B, Scarpa F, Kuebler WM, Secomb TW, Pries AR: Coalescent angiogenesis-evidence for a novel concept of vascular network maturation. Angiogenesis 2021, 25(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pezzella F, Kerbel RS: On coalescent angiogenesis and the remarkable flexibility of blood vessels. Angiogenesis 2022, 25(1):1–3. [DOI] [PubMed] [Google Scholar]
- 3.Dudley AC, Griffioen AW: Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis 2023:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Y, Banno K, Gil CH, Myslinski J, Hato T, Shelley WC, Gao H, Xuei X, Liu Y, Basile D et al. : Origin, prospective identification, and function of circulating endothelial colony forming cells in mouse and man. JCI Insight 2023, 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dight J, Zhao J, Styke C, Khosrotehrani K, Patel J: Resident vascular endothelial progenitor definition and function: the age of reckoning. Angiogenesis 2022, 25(1):15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillen F, Griffioen AW: Tumor vascularization; sprouting angiogenesis and beyond. Cancer Met Rev 2007, 26(3–4):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djonov V, Schmid M, Tschanz SA, Burri PH: Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res 2000, 86(3):286–292. [DOI] [PubMed] [Google Scholar]
- 8.Djonov VG, Kurz H, Burri PH: Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev Dyn 2002, 224(4):391–402. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang S, Dudley AC: Models and molecular mechanisms of blood vessel co-option by cancer cells. Angiogenesis 2020, 23(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Bianchi F, Ferguson M, Cesario A, Margaritora S, Granone P, Goldstraw P, Tetlow M, Ratcliffe C, Nicholson AG et al. : Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene 2005, 24(7):1212–1219. [DOI] [PubMed] [Google Scholar]
- 11.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ: Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999, 155(3):739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li A, Zhu L, Lei N, Wan J, Duan X, Liu S, Cheng Y, Wang M, Gu Z, Zhang H et al. : S100A4-dependent glycolysis promotes lymphatic vessel sprouting in tumor. Angiogenesis 2022, 26(1):19. [DOI] [PubMed] [Google Scholar]
- 13.Chen XJ, Wei WF, Wang ZC, Wang N, Guo CH, Zhou CF, Liang LJ, Wu S, Liang L, Wang W: A novel lymphatic pattern promotes metastasis of cervical cancer in a hypoxic tumour-associated macrophage-dependent manner. Angiogenesis 2021, 24(3):549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, Da Costa E, Hauert S, Rincon-Restrepo M, Tremblay C et al. : Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med 2017, 9(407). [DOI] [PubMed] [Google Scholar]
- 15.Blanchard L, Girard JP: High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 2021, 24(4):719–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J, Jonigk D, Chocron R, Pier GB, Gendron N et al. : COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis 2021, 24(4):755–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann M, Mentzer SJ, Jonigk D: Pulmonary Vascular Pathology in Covid-19. Reply. N Engl J Med 2020, 383(9):888–889. [DOI] [PubMed] [Google Scholar]
- 18.Smadja DM, Guerin CL, Chocron R, Yatim N, Boussier J, Gendron N, Khider L, Hadjadj J, Goudot G, Debuc B et al. : Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020, 23(4):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippe A, Chocron R, Gendron N, Bory O, Beauvais A, Peron N, Khider L, Guerin CL, Goudot G, Levasseur F et al. : Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021, 24(3):505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippe A, Gendron N, Bory O, Beauvais A, Mirault T, Planquette B, Sanchez O, Diehl JL, Chocron R, Smadja DM: Von Willebrand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: insight from VWF/ADAMTS13 ratio imbalance. Angiogenesis 2021, 24(3):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhogal P, Paul G, Collins G, Jaffer O: Letter in response to: circulating von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021, 24(3):413–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovas A, Buscher K, Osiaevi I, Drost CC, Sackarnd J, Tepasse PR, Fobker M, Kuhn J, Braune S, Gobel U et al. : Microvascular and proteomic signatures overlap in COVID-19 and bacterial sepsis: the MICROCODE study. Angiogenesis 2022, 25(4):503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klouda T, Hao Y, Kim H, Kim J, Olejnik J, Hume AJ, Ayyappan S, Hong X, Melero-Martin J, Fang Y et al. : Interferon-alpha or -beta facilitates SARS-CoV-2 pulmonary vascular infection by inducing ACE2. Angiogenesis 2022, 25(2):225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osiaevi I, Schulze A, Evers G, Harmening K, Vink H, Kumpers P, Mohr M, Rovas A: Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2022, 26(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riera-Mestre A, Iriarte A, Moreno M, Del Castillo R, Lopez-Wolf D: Angiogenesis, hereditary hemorrhagic telangiectasia and COVID-19. Angiogenesis 2021, 24(1):13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werlein C, Ackermann M, Stark H, Shah HR, Tzankov A, Haslbauer JD, von Stillfried S, Bulow RD, El-Armouche A, Kuenzel S et al. : Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis 2023, 26(2):233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry BM, de Oliveira MHS, Cheruiyot I, Benoit JL, Cooper DS, Lippi G, Le Cras TD, Benoit SW: Circulating level of Angiopoietin-2 is associated with acute kidney injury in coronavirus disease 2019 (COVID-19). Angiogenesis 2021, 24(3):403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, Kuhn J, Braune S, Gobel U, Tholking G et al. : Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis 2021, 24(1):145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasut A, Becker LM, Cuypers A, Carmeliet P: Endothelial cell plasticity at the single-cell level. Angiogenesis 2021, 24(2):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch PS, Lee KH, Goerdt S, Augustin HG: Angiodiversity and organotypic functions of sinusoidal endothelial cells. Angiogenesis 2021, 24(2):289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marziano C, Genet G, Hirschi KK: Vascular endothelial cell specification in health and disease. Angiogenesis 2021, 24(2):213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margadant C: Endothelial heterogeneity and plasticity. Angiogenesis 2021, 24(2):197–198. [DOI] [PubMed] [Google Scholar]
- 33.Jafree DJ, Long DA, Scambler PJ, Ruhrberg C: Mechanisms and cell lineages in lymphatic vascular development. Angiogenesis 2021, 24(2):271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauri C, van Impel A, Mackay EW, Schulte-Merker S: The adaptor protein Grb2b is an essential modulator for lympho-venous sprout formation in the zebrafish trunk. Angiogenesis 2021, 24(2):345–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruter DL, Liu Z, Ngo KM, X S, Marvin A, Buglak DB, Kidder EJ, Bautch VL: SMAD6 transduces endothelial cell flow responses required for blood vessel homeostasis. Angiogenesis 2021, 24(2):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canu G, Ruhrberg C: First blood: the endothelial origins of hematopoietic progenitors. Angiogenesis 2021, 24(2):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong X, Oh N, Wang K, Neumeyer J, Lee CN, Lin RZ, Piekarski B, Emani S, Greene AK, Friehs I et al. : Human endothelial colony-forming cells provide trophic support for pluripotent stem cell-derived cardiomyocytes via distinctively high expression of neuregulin-1. Angiogenesis 2021, 24(2):327–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X, Wang JJ, Wang J, Abboud HE, Chen Y, Zhang SX: Endothelium-specific deletion of Nox4 delays retinal vascular development and mitigates pathological angiogenesis. Angiogenesis 2021, 24(2):363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corvera S, Solivan-Rivera J, Yang Loureiro Z: Angiogenesis in adipose tissue and obesity. Angiogenesis 2022, 25(4):439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun D, Wang J, Toan S, Muid D, Li R, Chang X, Zhou H: Molecular mechanisms of coronary microvascular endothelial dysfunction in diabetes mellitus: focus on mitochondrial quality surveillance. Angiogenesis 2022, 25(3):307–329. [DOI] [PubMed] [Google Scholar]
- 41.Griffioen AW, Dudley AC: Angiogenesis: a year in review. Angiogenesis 2021, 24(2):195–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffioen AW, Dudley AC: The rising impact of angiogenesis research. Angiogenesis 2022, 25(4):435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]


