Abstract
Endometriosis, a common gynecological disease, causes chronic pelvic pain and infertility in women of reproductive age. Due to the limited efficacy of current therapies, a critical need exists to develop new treatments for endometriosis. Inflammatory dysfunction, instigated by abnormal macrophage (MΦ) function, contributes to disease development and progression. However, the fundamental role of the heterogeneous population of peritoneal MΦ and their potential druggable functions is uncertain. Here we report that GATA6-expressing large peritoneal MΦ (LPM) were increased in the peritoneal cavity following lesion induction. This was associated with increased cytokine and chemokine secretion in the peritoneal fluid (PF), as well as MΦ infiltration, vascularization and innervation in endometriosis-like lesions (ELL). Niclosamide, an FDA-approved anti-helminthic drug, was effective in reducing LPM number, but not small peritoneal MΦ (SPM), in the PF. Niclosamide also inhibits aberrant inflammation in the PF, ELL, pelvic organs (uterus and vagina) and dorsal root ganglion (DRG), as well as MΦ infiltration, vascularization and innervation in the ELL. PF from ELL mice stimulated DRG outgrowth in vitro, whereas the PF from niclosamide-treated ELL mice lacked the strong stimulatory nerve growth response. These results suggest LPM induce aberrant inflammation in endometriosis promoting lesion progression and establishment of the inflammatory environment that sensitizes peripheral nociceptors in the lesions and other pelvic organs, leading to increased hyperalgesia. Our findings provide the rationale for targeting LPM and their functions with niclosamide and its efficacy in endometriosis as a new non-hormonal therapy to reduce aberrant inflammation which may ultimately diminish associated pain.
Keywords: endometriosis, inflammation, macrophages, mice, niclosamide
1 |. INTRODUCTION
Endometriosis is known as a chronic and incurable inflammatory gynecological disorder estimated to affect approximately 10% of reproductive age women.1 It is associated with debilitating chronic pelvic pain, infertility or both, which substantially reduces the quality of life in women.2–4 Because endometriosis is an estrogen-dependent disorder, current treatments focus on suppression and/or inhibition of local or systemic estrogen production and function. Oral contraceptive pills and GnRH agonists are preferentially used for the first-or second-line medical treatments of endometriosis-associated pain in order to inhibit estrogen production and function.4,5 Laparoscopic surgery to remove lesions can provide some pain relief. However, hormonal treatments as well as laparoscopic surgery are often of limited efficacy with high recurrence rates, frequent side effects, additional costs, and potential morbidity.6 The recurrence rate of surgical excision of lesions is over 50% after five years.7–9 GnRH agonist therapy induces temporary menopause with unwanted side effects10 and over 50% of women exhibit recurrence of symptoms within two years.11,12 Progesterone resistance is a major complication for progestin therapy, leading to the escalation of estrogen activity.13 Thus, a critical need exists to develop more effective therapies for endometriosis that target biologically important mechanisms that underpin the pathophysiology of the disease.
Aberrant inflammatory and immune dysfunctions are believed to play major roles in the pathophysiology of endometriosis.6,14–17 Especially, macrophages (MΦ) are considered to be key players18: abundant MΦ are present in ectopic lesions19 where they undergo alternative activation to promote disease progression by enhancing cell proliferation, vascularization and innervation of lesions.20–22 These processes likely contribute to pelvic pain associated with endometriosis.20,23,24 MΦ populations are also elevated in peritoneal fluid (PF),25 and contribute to the establishment of an inflammatory environment in the pelvic cavity by secreting cytokines and chemokines, which encourage lesion growth and progression.20,26 However, no therapeutic strategy addressing inflammatory and immune dysfunctions has been developed in endometriosis.
We have recently determined that a small molecule, niclosamide, could be a potential new effective therapy for endometriosis.27–29 Our recent findings have demonstrated that niclosamide reduces the growth and progression of endometriosis-l ike lesions (ELL) and inhibits inflammatory signaling such as STAT3 and NFκB in the ELL in a mouse model of induced endometriosis.27 RNA-sequencing analysis has shown that transcripts associated with inflammatory responses were reduced by niclosamide.27 Furthermore, niclosamide inhibits MΦ-dependent cell viability and cytokine/chemokine secretion in an endometriotic cell line (12Z) and primary endometriotic stromal cells,28,29 indicating that niclosamide could be an inhibitor of lesion progression by blocking inflammatory pathways. However, our previous study27 focused on targeting only ELL with niclosamide, and the inflammatory milieu was not considered. While red or deep-infiltrated lesions possess active proliferative function, it is hard to classify endometriosis progression based on the size and/or pathology of the lesions. Furthermore, endometriosis-associated symptoms are not always correlated with the current classification (rASRM/rAFS) based on lesion size, location and/or extent of lesion infiltration into tissues and the presence of adhesions.30 Thus, inflammatory factors and immune systems surrounding the endometriosis environment in the pelvic cavity might cause severe symptoms observed in endometriosis patients, and those need to be considered and could be targeted. Therefore, the present study complements and expands findings from our initial study to examine the efficacy of niclosamide on peritoneal inflammation and immune cells established by lesion induction. Because it is expected that aberrant inflammation can be critical to induce endometriosis-associated pain-related hypersensitivity, not only lesion but other organs (uterus and vagina) as sources of peripheral hyperalgesia, as well as dorsal root ganglion (DRG) were assessed.
In our prior study,27 small uterine fragments were sutured to the peritoneal wall of mice to mimic the lesions.31–33 Although this model was sufficient to study the efficacy of niclosamide on lesion growth (size and weight), the suturing of entire uterine pieces with both endometrium and myometrium does not mimic retrograde menstruation as the source of endometriotic lesions in human. Furthermore, it is now thought that “suturing” not only induces an artificial inflammatory response, but also could mask local inflammation in the pelvic cavity established by induction of the lesions. Additionally, endometriotic lesions are heterogeneous and show multiple manifestations. Therefore, the present study was performed using an alternative established mouse model.21,34,35 In the current model, a menses-l ike event was induced in donor mice,36 and then mouse menstrual-like endometrium was introduced via injection into the peritoneal cavity of recipient mice to mirror spontaneous attachment of endometrial tissue during retrograde menstruation. This model exhibits similarities to human peritoneal lesions with respect to ESR expression, inflammation, vascularization, MΦ infiltration and innervation.21,34,35 Furthermore, the induction of ELL results in significantly higher levels of hyperalgesia-associated behaviors.37
The present results highlight the importance of large peritoneal MΦ (LPM) in creating an aberrant inflammatory environment in endometriosis, which could be critical for endometriosis-associated pain hypersensitivity. The results also indicate the efficacy of niclosamide in targeting LPM and their related functions in endometriosis as a unique non-hormonal therapy.
2 |. MATERIALS AND METHODS
2.1 |. Animals
All animal experiments were performed at Southern Illinois University according to the NIH guidelines for the care and use of laboratory animals (protocol # 16–0 38). B6. Cg-Tg(Csf1r-EGFP)1Hume (MacGreen, Jax #18549) and C57BL/6 (Jax #5304) mice were obtained from the Jackson Laboratory. The genotypes of MacGreen mice were determined by PCR analysis of tail genomic DNA as previously described.38
2.2 |. Mouse model of endometriosis
An experimental mouse model of endometriosis was established adopting procedures described previously.21,34,35,37 Briefly, a “menses-like” event was induced in ovariectomized E2-and P4-primed donor mice following an established protocol.36 Then, mouse menses-like endometrium, scraped from myometrium and cut into fragments (1–2 mm per side), were introduced as the source of syngeneic mouse endometrium (donor) via injection (in 0.2 mL PBS) into the peritoneal cavity of ovariectomized E2-primed mice (recipient) under anesthesia via inhaled isoflurane. To track origin, infiltration and localization of MΦ, we used Csf1r-eGFP mice (MacGreen),38,39 where monocytes and MΦ are GFP-labeled, as donors or recipients. When MacGreen mice were used as donors, C57BL/6 mice were used as recipients or vice vasa.
2.3 |. Study design
Three weeks after the induction of ELL or Sham, niclosamide was orally dosed at 0 or 200 mg/kg/day for a total of 3 weeks (Figure 1A), and tissues and PF were collected at 6 weeks for further characterization. We have previously reported that oral treatment of niclosamide at doses of 100 and 200 mg/kg/day is effective in inhibiting ELL growth.27 Specifically, a dose of 200 mg/kg/day was more effective, reducing the size of ELL by targeting inflammatory mechanisms such as STAT3 and NFκB signaling.27–29 The FDA-approved oral dosage of niclosamide is 2000 mg taken daily for an anthelmintic purpose in adult. Using a standard conversion factor for mice (=12.3) for deriving a human equivalent dose (HED) according to the FDA guidance, the estimated equivalent mouse dose is 410 mg/kg/day. Thus, our dose of 200 mg/kg/day in mice is approximately half that approved by FDA. Indeed, all mice treated with niclosamide (~200 mg/kg/day) for 3 weeks appeared healthy with no obvious adverse effects including weight loss.27 Furthermore, niclosamide did not disrupt normal ovarian and uterine functions.27 Therefore, we chose this dose of niclosamide to further understand the efficacy of niclosamide on inflammatory dysfunction in the peritoneal environment in endometriosis. For oral administration, niclosamide was mixed in gelatin (Knox) with artificial flavors (Sweetener, Splenda, and Berry Pomegranate, MiO). Note: Mice were generally housed 4 per cage, but during treatment, mice were placed individually in a cage devoid of bedding, and provided the gelatin mix. After a few days of training, more than 95% of mice eat their complete dosage of niclosamide (~150 mm3 gelatin) within 30 minutes. The mice which did not completely eat gelatin were eliminated from the studies. Mice were euthanized at 6 weeks post-induction, and PF was recovered by lavage (4 mL × 2 of ice-cold PBS with 3% FBS); ELL, uterus, vagina, and lumbosacral (L5-S 1) DRG were collected for further analysis.
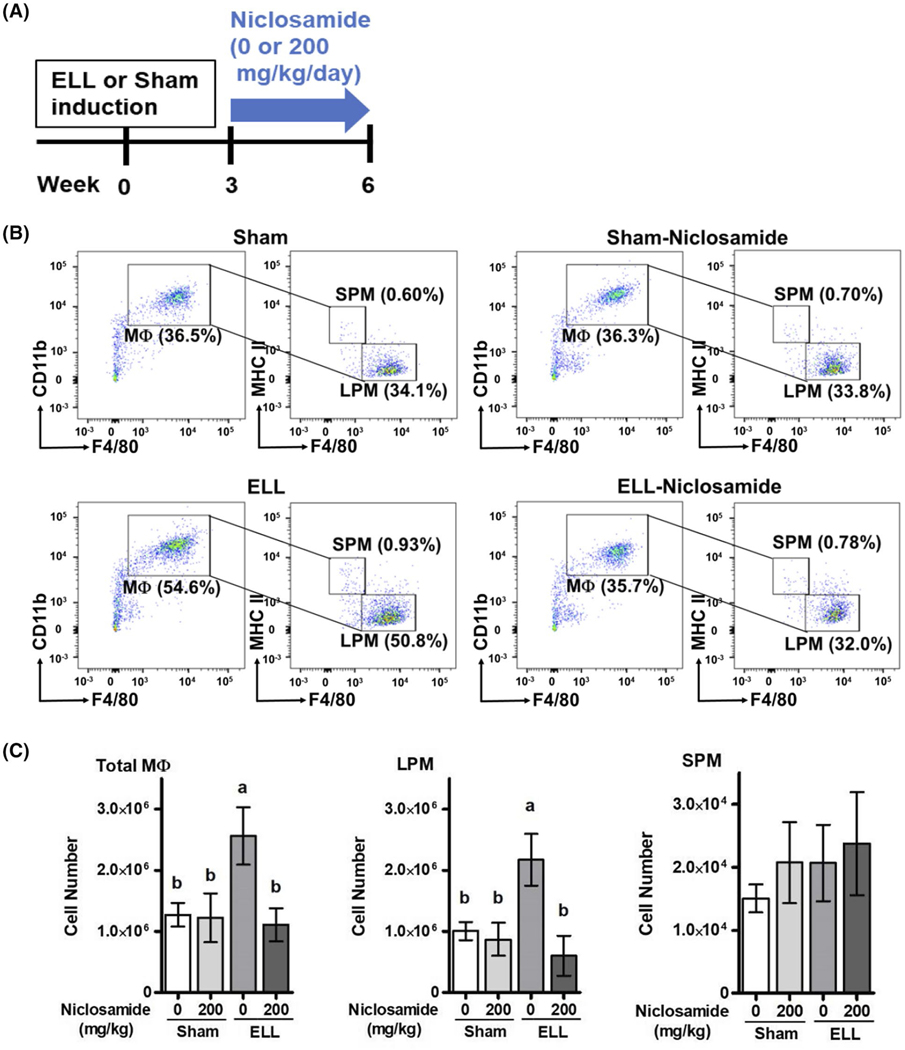
FIGURE 1.
Niclosamide inhibits ELL-induced peritoneal MΦ. A, Experimental study design as described in Materials and Methods. B, Flow cytometry analysis for MΦ subsets in peritoneal exudate cells of Sham, Sham-N, ELL or ELL-N mice. CD19−CD11b + F4/80+ MΦ (left panel) were further gated with F4/80 and MHC II for SPM and LPM (right panel). Percentages of MΦ, LPM and SPM were calculated from a total of peritoneal exudate cells. Representative flow plots are shown. C, Total MΦ, LPM and SPM numbers in the peritoneal lavages were calculated with Absolute Counting Beads (n = 6 per group), and are shown as mean ± SEM (a vs b, P < .05). Legend: ELL, endometriosis-like lesions; MΦ, macrophages; LPM, large peritoneal MΦ; SPM, small peritoneal MΦ; NS, not significant
2.4 |. Flow cytometry
Peritoneal lavages were spun down to collect peritoneal exudate cells that were used to identify immune cell profiles by flow cytometry. Red blood cells were lysed and cell suspensions were pre-incubated with FcBlock anti-C D16/CD32 (Thermo Fisher) for 20 minutes and then stained with fluorochrome-conjugated monoclonal antibodies (Supporting Information Table S1) for 1 hour. Cells were stained with propidium iodide (BD Bioscience), and then analyzed with the BD LSRII Flow Cytometer using BD FACSDiva software (BD Bioscience). For ARG1 and GATA6, cells were fixed and permeabilized using Intracellular Fixation & Permeabilization Buffer Set or Transcription Factor Staining Buffer Set (eBioscience). Compensation was performed on the BD LSRII Flow Cytometer at the beginning of each analysis. Data were analyzed with FlowJo v10. Absolute numbers of cells in each sample were calculated using CountBright Absolute Counting Beads (Thermo Fisher).
2.5 |. Quantitative real-time PCR analysis
Total RNA was isolated from tissues or cells, and cDNA was synthesized from 1 μg of total RNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher). Relative gene expression was determined by SYBR green (BD Bioscience) incorporation using a Bio-Rad CFX as described previously.40 Primer sequences were determined using NCBI’s design tools and are provided in Supporting Information Table S2.
2.6 |. Protein array and IQELISA
Harvested PF were analyzed using the Cytokine Array kits (ARY022B, R&D Systems) according to the manufacturer’s instructions. Quantitative analysis of spot density was performed using Image J [imagej.nih.gov, 41]. ANGPT2, BDNF, CXCL12, FGF15, IL1A, IL17A, PTX3, SERPINE1, and SPP1 were further quantified by IQELISA kits (Supporting Information Table S1, RayBiotech) according to the manufacturer’s instructions.
2.7 |. Immunofluorescence
Immunostaining of CD68, CGRP, GFP, pan (mixture) cytokeratin, LYVE-1, Neurofilament and PGP9.5 was performed with cross-sections (5 μm) of paraffin-embedded tissues or fixed cells using specific primary antibodies (Supporting Information Table S1) and AlexaFluor 488 and 568-conjugated F(ab’) secondary antibody (Molecular Probe). Cell-specific CD68, GFP, LYVE-1 and PGP9.5 positive cell number were counted in the area of 0.0475 or 0.7326 mm2 (3 different areas from each section and 4 different animals), and quantitatively analyzed. The relative intensity of CGRP in the DRG was quantified by Image J.
2.8 |. DRG culture
DRG culture was performed following an established protocol23 with minor modification. DRG isolated from rat embryo day 18 was purchased from BrainBits. DRG was plated onto poly-D-lysine coated 24-well plate and cultured with growth media (GM; NbActiv4, BrainBits) for 2 days. Then, DRG was cultured with GM or GM supplemented with PF from mice with/without ELL, or similarly prepared PF following niclosamide treatment, using a mix of 50% GM and 50% PBS or PF for 4 days, and then stained with neurofilament antibody. Three methods for quantification of DRG outgrowth were used. The body size was established by measuring the diameter of the DRG. The radius of filaments was measured from the body to the last positive cell in 4 different directions, and the average was used for each sample. Filaments, each no more than 20 μm away from the DRG body, were counted in the size of 25 μm2 at four different areas.
2.9 |. Statistical analysis
Data were subjected to one-w ay ANOVA, and Tukey multiple-comparison post-test was used to identify differences between individual means using Prism software (Ver. 5.0, GraphPad). All data met the necessary criteria for ANOVA analysis including equal variance as determined by Bartlett’s test. QPCR data in the ELL was analyzed by two-tailed Student’s t test comparing between 0 and 200 mg/kg niclosamide treatment. F-test was used to determine whether two groups possessed equal variances. All experimental data are presented as mean with standard error of the mean (SEM). Unless otherwise indicated, a P value less than .05 was considered to be statistically significant.
3 |. RESULTS
3.1 |. Niclosamide restores aberrant peritoneal MΦ distribution induced by ELL
To examine the efficacy of niclosamide on endometriosis-associated inflammation, menses-like tissues were inoculated into the peritoneal cavity of recipient mice. ELL morphology at 6 weeks after the implantation (3 weeks after niclosamide treatment) is shown in Supporting Information Figure S1A. ELL were identified on the peritoneal wall, uterus, gut, intestine, mesentery and/or within adipose tissues with natural, white/yellow/brown or red colorings. ELL number was reduced by niclosamide treatment (Supporting Information Figure S1B). Histologic examination showed that ELL contain hemorrhage, glandular epithelium and stroma (Supporting Information Figure S1C). Not all the inoculated tissues adhered to the peritoneum, with some floating tissues detected at harvest, consistent with the original report.35
When MacGreen donor endometrium was injected into C57BL/6 wild type recipients, GFP+ macrophages (also CD68+) were identified in the ELL (Supporting Information Figure S2A), demonstrating that endometrial MΦ are incorporated into lesions as previously described.35 We next focused on peritoneal MΦ using combinations of MacGreen and C57BL/6 mice for donor and recipient or vice versa to determine whether endometrial MΦ present in the “refluxed” menses-like endometrium contribute peritoneal MΦ. After the collection of PF (approximately 7 mL of peritoneal lavage was collected from each mouse), 200 μL of peritoneal lavage was mixed with 3 mL RPMI1640 media, and then peritoneal exudate cells were allowed to adhere to cell culture plates for 2 hours. When peritoneal exudate cells recovered from MacGreen recipient mice were plated, many GFP+cells adhered to the plastic culture plate (Supporting Information Figure S2B). Conversely, when the donor was MacGreen, no adherent GFP+ cells were observed (Supporting Information Figure S2C). Thus, endometrial tissue MΦ do not contribute to peritoneal MΦ pools in mice with induced endometriosis. We also realized that niclosamide treatment tended to reduce GFP+ cells attached to culture plates (Supporting Information Figure S2B). Therefore, peritoneal MΦ targeted by niclosamide were further assessed using the flow cytometer.
We next characterized peritoneal MΦ (CD19− CD11b+ F4/80+) into the two physically, functionally and developmentally different peritoneal MΦ subsets42: LPM and small peritoneal MΦ (SPM, Figure 1B). Left panels show CD19−CD11b+ F4/80+ MΦ, and then the MΦ were further gated with F4/80 and MHC II for LPM and SPM (right panels). Percentages of MΦ, LPM and SPM were calculated from a total of peritoneal exudate cells. In the Sham control, within the exudate cells, approximately 30%−4 0% were MΦ that consisted of a large population of LPM (F4/80hi MHCIIlo) and a small population of SPM (F4/80lo MHCIIhi). These ratios in the peritoneal exudate cells are consistent with a previous study.42 We also observed an increased ratio of both peritoneal MΦ and LPM in the exudates from ELL mice (Figure 1B). The total numbers of MΦ, LPM and SPM in the peritoneal lavages were thus calculated with Absolute Counting Beads (n = 6 per group, Figure 1C). The cell number of total MΦ and LPM, but not SPM, was significantly increased in mice with ELL. This increase in total MΦ and LPM populations in mice with ELL was reduced by niclosamide treatment. Interestingly, this inhibition by niclosamide was not observed in Sham-niclosamide (N) mice, suggesting that MΦ that are not disease-m odified may be unaffected by niclosamide, but that aberrantly induced MΦ are somehow subject to inhibition. In addition, we did not observe significant changes in CD19b+ (ie, B-cells, Supporting Information Figure S3A), Ly6C+ or Ly6G+ (ie, monocytes or neutrophils, Supporting Information Figure S3B), and CD8+ or CD4+ (ie, Killer or Helper T-cells, Supporting Information Figure S3C) in the PF by ELL induction or niclosamide treatment.
3.2 |. Niclosamide targets LPM
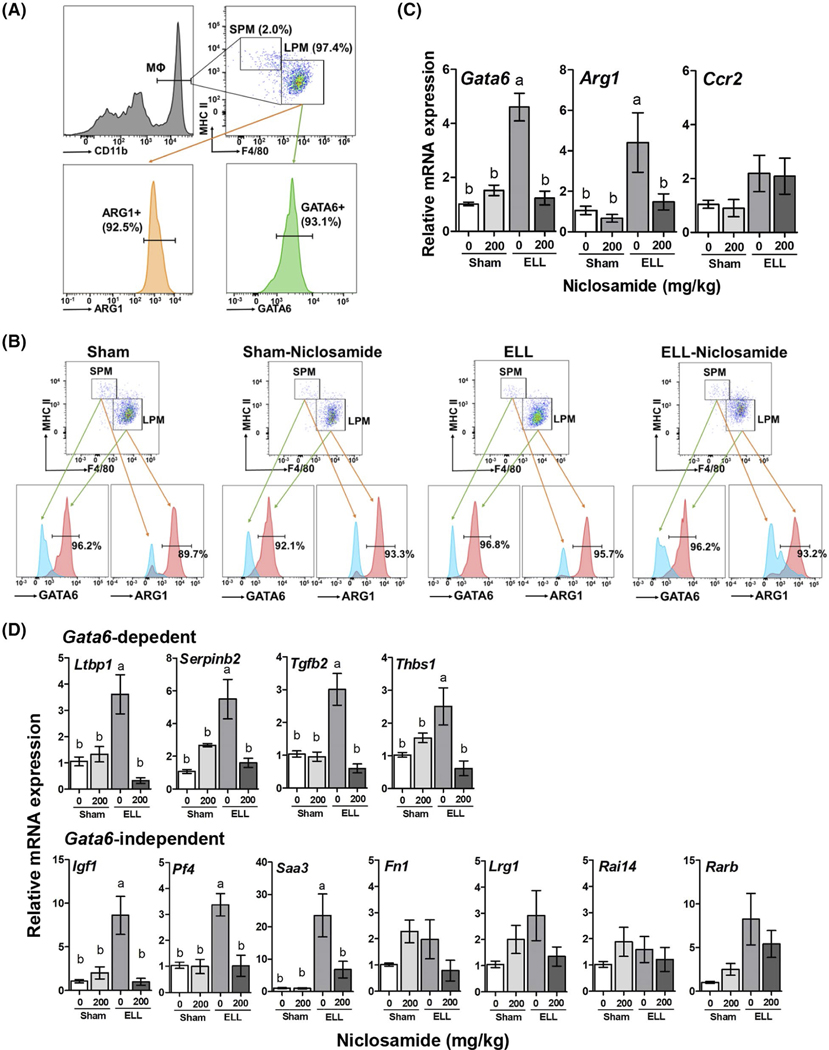
Our results showed that over 90% of LPM express GATA6, a selective marker of F4/80hi LPM42,43 and ARG1, a marker of alternatively activated or pro-repair MΦ44,45 (Figure 2A). In contrast, pro-inflammatory SPM did not express GATA6 and ARG1 (Figure 2B). The ratio of GATA6 and ARG1 was not altered in mice with ELL induction or niclosamide treatment. This is in line with their prototypical phenotypes under steady-state conditions and indicates that ELL does not induce a shift in the ratio of LPM and SPM and is in line with previous findings from the Greaves’ group.46 Relative Gata6 and Arg1 mRNA expression in peritoneal exudate cells was also increased in ELL mice, and niclosamide inhibited this increase (n = 5 per group, Figure 2C), whereas Ccr2, which is highly expressed in SPM and critical in monocyte recruitment,47 was not altered by ELL induction or niclosamide treatment (n = 5 per group, Figure 2C). GATA6 controls LPM specific genes to regulate proliferation, survival and metabolism of LPM.43,48,49 Gata6-dependent and Gata6-independent genes were thus examined (Figure 2D). The Gata6-dependent genes (Ltbp1, Serpinb2, Tgfb2 and Thbs1), and some Gata6-independent genes (Igf1, Pf4 and Saa3)43 exhibited increased mRNA expression in ELL mice, and were subsequently downregulated in ELL-N mice. However, several peritoneal MΦ specific, but Gata6-independent genes (Fn1, Lrg1, Rai14 and Rarb), were not affected by ELL or niclosamide, indicating that gene regulation by niclosamide is not globally affected.
FIGURE 2.
Characterization of peritoneal MΦ. A, Flow cytometer analysis for LPM and SPM subsets (% were calculated from CD11b+ cells, top left panel). LPM were further stained with ARG1 or GATA6 (% were calculated from LPM). B, LPM and SPM subsets further gated with ARG1 or GATA6 in the groups of Sham, Sham-Niclosamide, ELL and ELL-Niclosamide mice. C, mRNA expression of Gata6, Arg1 and Ccr (n = 5 per group). D, Gata6-dependent and -independent genes in the peritoneal exudate cells (n = 5 per group). The relative gene expression is shown as mean ± SEM (a vs b, P < .05)
3.3 |. Niclosamide inhibits peritoneal cytokines and chemokines
We next screened for inflammatory cytokines and chemokines secreted in the PF using an antibody array kit containing 105 specific antibodies. The relative intensity of a subset of 14 of these cytokines and chemokines selected from this protein array (Supporting Information Figure S4) are shown in Figure 3A (n = 4~5 per group). ANGPT2, BDNF, CD40LG, CXCL12, CXCL15 (IL-8), FGF15, IFNG, IL1A, IL17A, KLKB1, PTX3, SERPINE1, SPP1 and TFRC were increased in the PF by ELL and/or inhibited by niclosamide. On the other hand, those secreted factors were not affected in Sham-N mice except CXCL12. To further quantify the protein concentrations, ANGPT2, BDNF, CXCL12, FGF15, IL1A, IL17A, PTX3, SERPINE1 and SPP1 in the PF were analyzed by IQELISA (n = 10~12 per group, Figure 3B). The peritoneal protein concentrations of ANGPT2, BDNF, FGF15, IL17A and SERPINE1 showed patterns that correlated precisely to those in the protein array with respect to being increased by ELL and subsequently inhibited niclosamide. IL1A concentration was not affected by ELL induction but reduced by niclosamide treatment. This correlation was not universal as CXCL12 and SPP1 did not exhibit significantly different levels among any groups and did not match the results that were observed in the protein array. The detection of PTX3 was below the minimum sensitivity of the IQELISA kit and thus could not be verified.
FIGURE 3.
Niclosamide inhibits the secretion of peritoneal cytokines and chemokines. A, Antibody array was used to identify cytokine and chemokine profiles in the PF induced by ELL and targeted by niclosamide. Quantification of representative proteins (14 targets from 105 examined) are shown (n = 4–5 per group). The relative intensity of protein is shown as mean ± SEM (a vs b vs c, P < .05). B, IQELISA kits were further used to quantified protein concentrations in the PF. (n = 10~12 per group). The concentration of protein is shown as mean ± SEM (a vs b, P < .05)
3.4 |. Niclosamide inhibits MΦ infiltration, vascularization, innervation and inflammation in ELL
We next addressed the effects of niclosamide on the pathophysiology of ELL owing to the contribution of MΦ on endometriosis lesion progression.20,21,23,27,50 MΦ are critical for development, vascularization and innervation of the lesions.18 Thus, we examined MΦ, endothelial cells (LYVE-1 ) and neural cells (PGP9.5) in the ELL (n = 4 per group, Figure 4A). Co-staining of GFP and CD68 exhibited that many GFP+ (green) cells were co-localized with CD68+ (red) in the ELL using C57BL/6 mice as donors and MacGreen mice as recipients, suggesting that peritoneal MΦ or monocytes are infiltrated into the ELL. Niclosamide mainly reduced GFP+ CD68+ cells in the ELL, whereas there were still either GFP+ or CD68+ cells being detected. Abundant LYVE-1 + endothelial cells and PGP9.5+ neural cells in the ELL were decreased by niclosamide treatment (Figure 4A). Furthermore, niclosamide inhibited mRNA expression of cytokines/chemokines and related factors (Bdnf, Cxcl12, Ifng, Il17a, Klkb1, Ngf, Nr3c1 (Gr), Ptger2 (Ep2), Ptger4 (Ep4), Ptgs2 (Cox2), Ptx3 and Serpine1) in the ELL (n = 10~12, Figure 4B). The relative expression of candidate genes identified from the secreted PF protein array that were subsequently examined in ELL, which were not affected by niclosamide treatment, is shown in Supporting Information Figure S5.
FIGURE 4.
Niclosamide inhibits MΦ infiltration, vascularization, innervation and inflammation in the ELL. A, Left panel: GFP and CD68 were co-stained to determine MΦ infiltration into the ELL (Donor: C57BL/6, Recipient: MacGreen). Immunostaining of pan (mixture) cytokeratin and LYVE-1 at the middle panel, and pan-cytokeratin and PGP9.5 at the right panel are shown to detect epithelial cells with endothelial cells and epithelial cells with neural cells, respectively, in the ELL. Quantitative results of each staining were performed using 3 areas of each ELL section (n = 4 per group). CD68+/GFP+, LYVE-1+ or PGP9.5+ cells per 0.0475 mm2, 0.7326 mm2 or 0.475 mm2, respectively, are shown as mean ± SEM ( ** or *** vs control vehicle, P < .01 or P < .001, respectively). B, mRNA expressions of inflammatory factors in the ELL (n = 10–12 per group). The relative gene expression is shown as mean ± SEM (*, ** or *** vs control vehicle, P < .05, .01 or P < .001, respectively)
3.5 |. Niclosamide inhibits inflammatory mediators in the uterus, vagina and DRG
Aberrant accumulation of inflammatory factors can stimulate peripheral nerve terminals of nociceptor neurons innervating different tissues in peripheral organs,51 resulting in an increase in the expression of transient receptor potential channels eg TRPA1 and TRPV1. Activation of peripheral nerves is also associated with increased release of neurotransmitters and neuromodulators such as SP, CGRP, and BDNF. BDNF is known to regulate both initiation and maintenance of chronic endometriosis-associated pain52,53 involving neuroangiogenesis21 and innervation in the pelvic organs.51 We thus examined the inflammatory mediators, neurotransmitters and neuromodulators in the uterus, vagina and lumbosacral (L5-S 1) DRG, which are the primary spinal ganglia receiving sensory input from pelvic organs. Induction of ELL increased inflammatory mediators, related enzymes and receptors, neurotransmitters and/or the nociceptor-specific sodium channel NaV1.9 in pelvic organs and DRG, and this was inhibited by niclosamide: specifically, Bdnf, Calca (Cgrp), Ifng, Ptger4 (Ep4) and Ptgs2 (Cox2) in the uterus (Figure 5A); Bdnf, Calca, Ifng, Il17a and Scn11a in the vagina (Figure 5B); Bdnf, Cgrp, Ngf, Ptger4 and Trpv1 in the DRG (Figure 5C). Other inflammatory factors, neurotransmitters and receptor channels that were examined by QPCR that were not affected by ELL induction and/or niclosamide treatment in the uterus, vagina or DRG are shown in Supporting Information Table S3. Immunostaining of CGRP in the DRG showed that the increased intensity of CGRP induced by ELL was inhibited by niclosamide treatment (Figure 5D). These results suggest that ELL induction stimulates endometriosis-associated peripheral inflammatory mediators, and niclosamide is able to inhibit them in pelvic organs and peripheral sensory neurons.
FIGURE 5.
The effect of niclosamide on inflammatory mediators in (A) uterus, (B) vagina and (C) DRG (n = 8–15 per group). The relative gene expression is shown as mean ± SEM (a vs b, P <.05). D, Immunofluorescence CGRP and neurofilament as a positive control of nerve cells in DRG. The relative intensity of CGRP in the DRG was quantified by Image J (n = 4 per group) and is shown as mean ± SEM (a vs b, P < .05)
3.6 |. Niclosamide inhibits DRG outgrowth
It has been reported that PF from endometriosis patients stimulates neurite outgrowth compared with those without endometriosis.23,54 To understand whether niclosamide affects neurite outgrowth, we cultured DRG from rat embryo in the presence of GM or GM supplemented with PF from mice with and without ELL, or similarly prepared PF following niclosamide treatment, using a mix of 50% GM and 50% PBS or PF for 4 days. PF from ELL mice stimulated DRG outgrowth (DRG body size, as well as radius and number of filaments) compared with GM, PBS or PF from Sham mice (Figure 6A,B). The inclusion of PF from ELL-N mice lacked its strong stimulatory nerve growth response. Thus, endometriosis-associated factors in PF can be inflammatory mediators to stimulate/sensitize peripheral sensory neurons, and niclosamide is able to control their sensitization by reducing the inflammatory mediators in the PF.
FIGURE 6.
The effect of niclosamide on DRG outgrowth. A, DRG outgrowth was examined culturing with growth media (GM), a mix of 50% GM and PBS, or PF from: Sham, ELL or ELL-Niclosamide mice for 4 days. DRG were fixed and stained with neurofilament antibody. B, DRG body size, as well as radius and the number of filaments were analyzed (n = 6 per group), and shown as mean ± SEM. a vs b vs c, P < .05
4 |. DISCUSSION
Despite the large number of women who suffer from chronic pelvic pain and infertility from complications associated with endometriosis, current treatments that are available only temporarily relieve the symptoms of the disease. In the present study, our results highlight that LPM and their related factors could be targetable by niclosamide to improve endometriosis-associated hyperalgesia at several points of relevance in the progression and pathology of the disease. The important findings are that niclosamide was able to reduce the number of LPM and LPM-associated gene expression, which were increased by ELL induction, as well as reducing total cytokine/chemokine secretion in the pelvic cavity. In addition, niclosamide reduced MΦ infiltration, vascularization, and innervation of the ELL. Reduction of inflammatory factors, which could serve as mediators to stimulate peripheral neurons, was observed in both ELL and pelvic organs, such as uterus and vagina. Thus, niclosamide shows the potential to suppress sensory stimuli leading to endometriosis-a ssociated hypersensitivity by improving the inflammatory environment established in the pelvic cavity and peripheral organs (Figure 7).
FIGURE 7.

Overall findings and hypothesis in the present study. Created with BioRender.com
Niclosamide has been orally administered for the treatment of intestinal helminthic infections. It was originally evaluated and reported by the WHO and the Food and Agriculture Organization of the United Nations (FAO) in 1988.55 The toxicity kinetics of niclosamide administered orally in rats for 4 weeks elicits no adverse effects up to 2000 mg/kg daily. Similarly, niclosamide treatment in dogs is safe at doses up to 4500–6000 mg/day for 4 weeks. No signs of intoxication have been observed in humans treated at 1000 mg/day. However, nausea and abdominal pain have been reported in 10% of human patients following an oral dosage of 2000 mg/day. Acute toxicity in mice is reported as LD50 = >1500 mg/kg. Based on these reports, the maximum dosage, 200 mg/kg, used for the present and previous27 studies, is considered to be low enough to safely evaluate the efficacy of niclosamide using a mouse model of endometriosis. Indeed, daily administration of niclosamide at a dose of 200 mg/kg for 3 weeks did not show weight loss or abnormal behaviors in the present and previous27 studies, indicating that the dose of niclosamide (200 mg/kg/day) in mice is not pharmacologically high.
Immune dysfunction has been indicated to be a central component in the development and progression of endometriosis by establishing a chronic inflammatory environment.56,57
Endometriotic cell implantations are dependent and sensitive to peritoneal inflammation driven by a variety of immune cells, as well as their secreted products, within the PF.58 Peritoneal MΦ are a large immune cell population in the pelvic cavity and known to be both increased in number and altered in function in endometriosis patients.20,25 In the present study, the total number of peritoneal MΦ (CD19−CD11b+ F4/80+), especially GATA6-expressing LPM (F4/80hi MHCIIlo), were increased in the pelvic cavity at 6 weeks when ELL has been established. This result supports the findings from Foster et al23 demonstrating abundant LPM (F4/80hi Ly6C−) in mice with ELL using the same model. On the other hand, Yuan et al59 have reported fewer numbers of LPM (F4/80hi CD11bhi) and more abundant SPM (F4/80lo CD11blo) in the ELL mice compared to control from 0.25 to 42 days post-induction. However, the definition of LPM and SPM in our study follows the current categorization of peritoneal MΦ due to the differential expressions of F4/80 and MHCII,42 representing LPM should be the majority of peritoneal MΦ in mice. Foster et al23 also demonstrate a similar peritoneal MΦ population to our study. The differences observed in the results from us and Yuan et al could result from a different definition of LPM and SPM, as their SPM population is much larger than those in the currently accepted definition of SPM, as well as a slightly different mouse model of endometriosis used by that group.
Peritoneal inflammation can induce the macrophage disappearance reaction (MDR), by which the reduction of LPM could occur. Bain et al60 have reported that some of Ly6C+ monocytes that entered the pelvic cavity can be precursors of F4/80hi GATA6+. However, we did not observe the signs of MDR, and altered SPM and Ly6Chi monocyte populations in our study. Recently, a study has demonstrated that LPM and monocytes can infiltrate into ELL, that the presence of lesions triggers increased monocyte recruitment and monocyte input into the LPM population. Interestingly, inhibition of monocyte recruitment increases the number of lesions at 2 weeks after the lesion induction, suggesting that newly-recruited monocyte-derived MΦ can have an anti-e ndometriosis role.46 Although some questions remain regarding MΦ ontogeny in endometriosis, it is clear that ELL establish a persistent increase in the population of LPM in the pelvic cavity.
In the present study, niclosamide decreased the aberrant LPM population in the pelvic cavity that was increased by ELL induction. The LPM specific marker, Gata6,that was increased by the presence of ELL was also inhibited by niclosamide. Gata6-dependent genes,43 Tgfb2, Ltbp1 and Thbs1, were also reduced in expression by the niclosamide treatment. The TGFB2 isoform, the latent TGFβ binding protein (LTBP1) and a regulator of latent TGFβ activation (THBS1) are known as extracellular components of TGFβ signaling.61 Increased levels of TGFβ1 in the peritoneal fluids, serum, lesions and peritoneum of women with endometriosis have been observed [summarized in62]. An increase in TGFβ1 has been associated with cell survival, proliferation, attachment, invasion and angiogenesis during lesion development.63–69 Tgfb1 deficiency in the recipient mice suppresses endometriotic lesion development.70 Furthermore, MΦ proliferation and recruitment in the endometriotic lesions are also increased by the activation of TGFβ signaling.70,71 Thus, TGFβ signaling, especially Gata6-dependent factors, could play a major role in increasing the LPM population in the pelvic cavity with the presence of lesions, and niclosamide might target this pathway. On the other hand, our previous results showed that niclosamide targets STAT3 and NFκB activities to inhibit inflammatory factors in the lesions and endometriotic cells.27–29 Aberrant STAT372–76 and/or NFκB77,78 activities are key to the onset of endometriosis. The activation of STAT3 signaling is induced by MΦ in the peritoneal fluids from patients with endometriosis.72 Gata6 is one of the STAT3 target genes,79 and STAT3 can bind to the Gata6 promoter.80 Thus, targeting aberrant STAT3 signaling can also lead to the inhibition of Gata6-dependent factors. Although it still remains unclear whether niclosamide is effective in targeting TGFβ or STAT3 signaling or both, our recent work (unpublished) has suggested that niclosamide can directly interact with RNA-b inding proteins to regulate their downstream target transcripts. Thus, the process of Gata6 translation could also be a target of niclosamide. Nevertheless, the Gata6-d ependent TGFβ and STAT3 signaling mechanisms might have therapeutic potential.
The present study showed that the secretion of cytokines and chemokines was elevated in the PF and reduced by niclosamide. Inflammatory factors such as BDNF,81 CXCL12,82,83 CXCL15 (IL-8),84–87 IFNG88 and IL17A89 have been reported in the PF of women with endometriosis. Most of these factors are known to play substantial roles in MΦ polarization and activation, and enhance the production of additional cytokines. In our model, the presence of ELL consistently increased the levels of ANGPT2, BDNF, FGF15, IL17A and SERPINE1 detected by both protein array and ELISA, whereas CXCL12, CXCL15 and IFNG were only identified by the protein array as stimulated by ELL. ANGPT2, a proangiogenic cytokine, and its receptor, TIE2 positive MΦ infiltrate into the lesion for maintaining the viability of newly formed vessels.50 ANGPT2 has been reported as a downstream mediator of TGFβ signaling in vascular malformations.90 SERPINE1, aka plasminogen activator inhibitor-1 , PAI-1, is highly expressed in deep infiltrating endometriosis, and its expression is associated with severe dysmenorrhea.91 PAI-1 can increase STAT3 activity in MΦ,92 and its expression can be regulated by TGFβ1.93 While FGF15 (known as FGF19 in human) has not been recognized in endometriosis, FGF19-FGFR4 signaling contributes to tumor growth in cancer, enhancing epithelial-m esenchyme transition and activating STAT3 signaling.94 BDNF is known as one of the major regulatory factors of STAT3 signaling,95 and STAT3 activation is critical IL17A-mediated inflammatory response.96 Thus, those factors could be downstream regulators of TGFβ and/or STAT3 signaling and niclosamide’s downstream targets. Additionally, inhibition of Bdnf, Il17a and Serpine1 was also observed in both lesions and PF from the ELL-n iclosamide mice. The infiltrated MΦ could be a source of inflammatory factors in the lesions, as niclosamide also inhibited infiltrated peritoneal MΦ in the ELL mice. However, our previous studies have shown that endometriotic cells secrete abundant inflammatory factors when stimulated by MΦ, suggesting that endometriotic cells also could be a source of cytokines and chemokines.28,29
The present study shows that innervation in the ELL was inhibited by niclosamide. BDNF is a neurotrophic family member and has been recognized as a regulator in the formation and maintenance of chronic pain in various chronic disorders97–99 including endometriosis.52,53 Increased production of BDNF stimulates neurite outgrowth from DRG, resulting in increased neuroangiogenesis.21 Interestingly, increased expression of Bdnf was observed not only in the ELL but also in the uterus, vagina and lumbosacral DRG Ptgs2 (Cox2), prostaglandin receptors (Ep2 or Ep4), transient receptor potential channel (Trpv1), nociceptor-specific sodium channel NaV1.9 (Scn11a), and/or neuromodulator CGRP (Calca) in pelvic organs including DRG were also elevated by ELL induction. Thus, ELL-i nduced inflammatory factors in the PF could be inflammatory mediators to stimulate and sensitize peripheral sensory neurons in the pelvic organs. This result was supported by our finding that PF from ELL mice stimulated DRG outgrowth. Importantly, the signs of sensitization of peripheral neurons observed in the ELL, uterus, vagina and DRG, as well as innervation in the ELL and DRG outgrowth were reduced in the ELL-niclosamide mice. This suggests that the improvement of the inflammatory environment established in the pelvic cavity could be critical to prevent endometriosis-a ssociated hypersensitivity.
The present study shows the efficacy of niclosamide on an aberrant immune system in the endometriotic milieu and its associated consequences using a mouse model of endometriosis. We have assessed immune cell profiles, especially LPM, in the pelvic cavity induced by ELL. We screened inflammatory factors secreted within the pelvic cavity and found aberrant cytokines and chemokines induced by ELL, which would be critical in establishing the inflammatory environment and targeted by niclosamide. We further suggest those factors could be inflammatory mediators for peripheral stimulation of sensory nerve fibers not only in the lesions but also in other pelvic organs (uterus and vagina). Therefore, targeting inflammatory factors associated with LPM might have a greater impact than targeting the heterogeneous lesions. However, it remains unclear how aberrant LPM contribute to endometriosis progression and how niclosamide improves the inflammatory environment. Our on-going study has further indicated that LPM are also heterogeneous and may have uncharacterized populations that are critical to the growth and progression of endometriosis. Thus, understanding LPM functions and further characterization of LPM needs to be examined. Nevertheless, our results suggest that either acute or chronic aberrant inflammation caused by lesion induction can be targetable by niclosamide, and its action may reduce the pathology of the disease.
5 |. SUMMARY
Due to the low efficacy and numerous unwanted side effects of current treatment options for endometriosis, new therapeutic targets and efficient drugs need to be identified. Targeting inflammatory dysfunction in the peritoneal environment to inhibit endometriosis and improving endometriosis-associated symptoms are a new strategy compared to current hormonal treatments and needs further development. Here we present the critical contribution that large peritoneal macrophages play toward the establishment of the chronic inflammatory environment, and that niclosamide is effective in inhibiting aberrant inflammatory dysfunction not only in the lesions but also in the pelvic cavity and peritoneal organs, potentially improving endometriosis-associated hyperalgesia. Thus, this non-h ormonal drug, niclosamide, would have a high impact on the treatment of endometriosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stacey McGee for DRG immunostaining.
Funding information
This work was supported by NIH grant R21HD092739 (to KH)
Abbreviations:
- DRG
dorsal root ganglion
- E2
estradiol-17β
- ELL
endometriosis-like lesions
- FAO
Food and Agriculture Organization
- FBS
fetal bovine serum
- FDA
Food and Drug Administration
- GnRH
gonadotropin-releasing hormone
- HED
human equivalent dose
- LPM
large peritoneal macrophages
- MΦ
macrophages
- P4
progesterone
- PBS
phosphate buffered saline
- PF
peritoneal fluid
- rAFS
revised American Fertility Society
- rASRM
revised American Society for Reproductive Medicine
- SPM
small peritoneal macrophages
- WHO
World Health Organization
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- 1.Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- 2.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- 4.Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev. 2019;40:1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudice LC. Clinical practice. Endometriosis. New England J Med. 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.As-Sanie S, Black R, Giudice LC, et al. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol. 2019;221:86–94. [DOI] [PubMed] [Google Scholar]
- 7.DeCherney AH. Endometriosis: recurrence and retreatment. Clin Ther. 1992;14:766–772; discussion 765. [PubMed] [Google Scholar]
- 8.Evers JL, Dunselman GA, Land JA, Bouckaert PX. Is there a solution for recurrent endometriosis? Br J Clin Pract Suppl. 1991;72:45–50; discussion 51–43. [PubMed] [Google Scholar]
- 9.Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15:441–461. [DOI] [PubMed] [Google Scholar]
- 10.Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst Rev. 2009(no.1):CD005997. [DOI] [PubMed] [Google Scholar]
- 11.Practice Committee of American Society for Reproductive, M. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90:S260–S269. [DOI] [PubMed] [Google Scholar]
- 12.Waller KG, Shaw RW. Gonadotropin-releasing hormone analogues for the treatment of endometriosis: long-term follow-up. Fertil Steril. 1993;59:511–515. [DOI] [PubMed] [Google Scholar]
- 13.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. [DOI] [PubMed] [Google Scholar]
- 15.Symons LK, Miller JE, Kay VR, et al. The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24:748–762. [DOI] [PubMed] [Google Scholar]
- 16.Patel BG, Lenk EE, Lebovic DI, Shu Y, Yu J, Taylor RN. Pathogenesis of endometriosis: interaction between endocrine and inflammatory pathways. Best Pract Res Clin Obstet Gynaecol. 2018;50:50–60. [DOI] [PubMed] [Google Scholar]
- 17.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4:9–33. [DOI] [PubMed] [Google Scholar]
- 18.Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol. 2020;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capobianco A, Rovere-Q uerini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacci M, Capobianco A, Monno A, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greaves E, Temp J, Esnal-Zufiurre A, Mechsner S, Horne AW, Saunders PT. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am J Pathol. 2015;185:2286–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran LV, Tokushige N, Berbic M, Markham R, Fraser IS. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod. 2009;24:835–841. [DOI] [PubMed] [Google Scholar]
- 23.Forster R, Sarginson A, Velichkova A, et al. Macrophage-d erived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019;33:11210–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JA, Faris HM, Schiff I, Anderson DJ. Characterization of leukocyte subpopulations in the peritoneal fluid of women with endometriosis. Fertil Steril. 1988;50:216–222. [PubMed] [Google Scholar]
- 26.Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925–930. [PubMed] [Google Scholar]
- 27.Prather GR, MacLean JA 2nd, Shi M, Boadu DK, Paquet M, Hayashi K. Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model. Biol Reprod. 2016;95:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekulovski N, Whorton AE, Shi M, MacLean JA II, Hayashi K. Endometriotic inflammatory microenvironment induced by macrophages can be targeted by niclosamidedagger. Biol Reprod. 2019;100:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekulovski N, Whorton AE, Tanaka T, et al. Niclosamide suppresses macrophage induced inflammation in endometriosis. Biol Reprod. 2020;102(5):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamson GD. Endometriosis classification: an update. Curr Opin Obstet Gynecol. 2011;23:213–220. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Chen Y, Kuang Y, et al. Multiple beneficial roles of repressor of estrogen receptor activity (REA) in suppressing the progression of endometriosis. Endocrinology. 2016;157:900–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Gong P, Chen Y, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7:271ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Li Q, Katzenellenbogen BS, et al. Estrogen-i nduced CCN1 is critical for establishment of endometriosis-like lesions in mice. Mol Endocrinol. 2014;28:1934–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaves E, Collins F, Esnal-Zufiaurre A, Giakoumelou S, Horne AW, Saunders PT. Estrogen receptor (ER) agonists differentially regulate neuroangiogenesis in peritoneal endometriosis via the repellent factor SLIT3. Endocrinology. 2014;155:4015–4026. [DOI] [PubMed] [Google Scholar]
- 35.Greaves E, Cousins FL, Murray A, et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol. 2014;184:1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HO, Saunders PT. Evidence from a mouse model that epithelial cell migration and mesenchymal-e pithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE. 2014;9:e86378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greaves E, Horne AW, Jerina H, et al. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci Rep. 2017;7:44169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasmono RT, Oceandy D, Pollard JW, et al. A macrophage colony-stimulating factor receptor-g reen fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. [DOI] [PubMed] [Google Scholar]
- 39.Sasmono RT, Williams E. Generation and characterization of MacGreen mice, the Cfs1r-E GFP transgenic mice. Methods Mol Biol. 2012;844:157–176. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi K, Erikson DW, Tilford SA, et al. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 2009;80:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosn EE, Cassado AA, Govoni GR, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okabe Y, Medzhitov R. Tissue-s pecific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Kasmi KC, Qualls JE, Pesce JT, et al. Toll-l ike receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. [DOI] [PubMed] [Google Scholar]
- 46.Hogg C, Panir K, Dhami P, et al. Macrophages inhibit and enhance endometriosis depending on their origin. Proceedings of the National Academy of Sciences. 2021;118(no.6):e2013776118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gautier EL, Ivanov S, Williams JW, et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J Exp Med. 2014;211:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosas M, Davies LC, Giles PJ, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capobianco A, Monno A, Cottone L, et al. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. 2011;179:2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding S, Zhu T, Tian Y, et al. Role of brain-derived neurotrophic factor in endometriosis pain. Reprod Sci. 2017;25(no.7):1045–1057. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi H, Yamada Y, Morioka S, Niiro E, Shigemitsu A, Ito F. Mechanism of pain generation for endometriosis-a ssociated pelvic pain. Arch Gynecol Obstet. 2014;289:13–21. [DOI] [PubMed] [Google Scholar]
- 54.Arnold J, Barcena de Arellano ML, Ruster C, et al. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav Immun. 2012;26:132–141. [DOI] [PubMed] [Google Scholar]
- 55.Organization, W. H., and Geneva. Who specifications and evaluations for public health pesticides niclosamide 2′,5-dichloro-4′-nitros alicylanilide. 2002. [Google Scholar]
- 56.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. [DOI] [PubMed] [Google Scholar]
- 57.Vallve-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 2019;25:564–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo M, Bafligil C, Tapmeier T, et al. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: a characterisation study. BMC Med. 2020;18:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan M, Li D, An M, Li Q, Zhang L, Wang G. Rediscovering peritoneal macrophages in a murine endometriosis model. Hum Reprod. 2017;32:94–102. [DOI] [PubMed] [Google Scholar]
- 60.Bain CC, Hawley CA, Garner H, et al. Long-l ived self-renewing bone marrow-d erived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun. 2016;7:ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood. 2000;96:2022–2036. [PubMed] [Google Scholar]
- 62.Young VJ, Ahmad SF, Duncan WC, Horne AW. The role of TGF-beta in the pathophysiology of peritoneal endometriosis. Hum Reprod Update. 2017;23:548–559. [DOI] [PubMed] [Google Scholar]
- 63.Beliard A, Noel A, Goffin F, Frankenne F, Foidart JM. Adhesion of endometrial cells labeled with 111Indium-t ropolonate to peritoneum: a novel in vitro model to study endometriosis. Fertil Steril. 2003;79(Suppl 1):724–729. [DOI] [PubMed] [Google Scholar]
- 64.Demir AY, Groothuis PG, Nap AW, et al. Menstrual effluent induces epithelial-mesenchymal transitions in mesothelial cells. Hum Reprod. 2004;19:21–29. [DOI] [PubMed] [Google Scholar]
- 65.Johnson MC, Torres M, Alves A, et al. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol. 2005;3:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. Role of TGF-betas in normal human endometrium and endometriosis. Hum Reprod. 2010;25:101–109. [DOI] [PubMed] [Google Scholar]
- 67.Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis. 2006;27:2148–2156. [DOI] [PubMed] [Google Scholar]
- 68.Young VJ, Brown JK, Maybin J, Saunders PT, Duncan WC, Horne AW. Transforming growth factor-b eta induced Warburg-like meta-bolic reprogramming may underpin the development of peritoneal endometriosis. J Clin Endocrinol Metab. 2014;99:3450–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu YG, Tekmal RR, Binkley PA, Nair HB, Schenken RS, Kirma NB. Induction of endometrial epithelial cell invasion and c-f ms expression by transforming growth factor beta. Mol Hum Reprod. 2009;15:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hull ML, Johan MZ, Hodge WL, Robertson SA, Ingman WV. Host-derived TGFB1 deficiency suppresses lesion development in a mouse model of endometriosis. Am J Pathol. 2012;180:880–887. [DOI] [PubMed] [Google Scholar]
- 71.Dou Q, Williams RS, Chegini N. Inhibition of transforming growth factor-beta 1 alters the growth, anchor-dependent cell aggregation and integrin mRNA expression in human promonocytes: implications for endometriosis and peritoneal adhesion formation. Mol Hum Reprod. 1997;3:383–391. [DOI] [PubMed] [Google Scholar]
- 72.Itoh F, Komohara Y, Takaishi K, et al. Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril. 2013;99:1705–1713. [DOI] [PubMed] [Google Scholar]
- 73.Kim BG, Yoo JY, Kim TH, et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Mol Hum Reprod. 2013;19:160–168. [DOI] [PubMed] [Google Scholar]
- 75.Okamoto M, Nasu K, Abe W, et al. Enhanced miR-2 10 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod. 2015;30:632–641. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Chen H, Wang N, et al. Combined 17beta-estradiol with TCDD promotes M2 polarization of macrophages in the endometriotic milieu with aid of the interaction between endometrial stromal cells and macrophages. PLoS ONE. 2015;10:e0125559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-k appaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Ramos R, Rocco J, Rojas C, et al. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil Steril. 2012;97:645–651. [DOI] [PubMed] [Google Scholar]
- 79.Snyder M, Huang XY, Zhang JJ. Identification of novel direct Stat3 target genes for control of growth and differentiation. J Biol Chem. 2008;283:3791–3798. [DOI] [PubMed] [Google Scholar]
- 80.Wu CS, Wei KL, Chou JL, et al. Aberrant JAK/STAT signaling suppresses TFF1 and TFF2 through epigenetic silencing of GATA6 in gastric cancer. Int J Mol Sci. 2016;17(no.9):1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding S, Zhu T, Tian Y, et al. Role of brain-derived neurotrophic factor in endometriosis pain. Reprod Sci. 2018;25:1045–1057. [DOI] [PubMed] [Google Scholar]
- 82.Leconte M, Chouzenoux S, Nicco C, et al. Role of the CXCL12-CXCR4 axis in the development of deep rectal endometriosis. J Reprod Immunol. 2014;103:45–52. [DOI] [PubMed] [Google Scholar]
- 83.Ouyang Z, Sun JP, Tian XL, Chen MX, Zhai JJ. The expressions and the roles of SDF-1/CXCR-4 and SDF-1/CXCR-4 in human endometriosis. Zhonghua Yi Xue Za Zhi. 2018;98:1854–1858. [DOI] [PubMed] [Google Scholar]
- 84.Gazvani MR, Christmas S, Quenby S, Kirwan J, Johnson PM, Kingsland CR. Peritoneal fluid concentrations of interleukin-8 in women with endometriosis: relationship to stage of disease. Hum Reprod. 1998;13:1957–1961. [DOI] [PubMed] [Google Scholar]
- 85.Kalu E, Sumar N, Giannopoulos T, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–495. [DOI] [PubMed] [Google Scholar]
- 86.Jorgensen H, Hill AS, Beste MT, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. 2017;107:1191–1199.e2. [DOI] [PubMed] [Google Scholar]
- 87.Iwabe T, Harada T, Tsudo T, Tanikawa M, Onohara Y, Terakawa N. Pathogenetic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1998;69:924–930. [DOI] [PubMed] [Google Scholar]
- 88.Podgaec S, Abrao MS, Dias JA Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373–1379. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X, Xu H, Lin J, Qian Y, Deng L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG. 2005;112:1153–1155. [DOI] [PubMed] [Google Scholar]
- 90.Crist AM, Zhou X, Garai J, et al. Angiopoietin-2 inhibition rescues arteriovenous malformation in a Smad4 hereditary hemorrhagic telangiectasia mouse model. Circulation. 2019;139:2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alotaibi FT, Peng B, Klausen C, et al. Plasminogen activator inhibitor-1 (PAI-1 ) expression in endometriosis. PLoS ONE. 2019;14:e0219064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kubala MH, Punj V, Placencio-Hickok VR, et al. Plasminogen activator inhibitor-1 promotes the recruitment and polarization of macrophages in cancer. Cell Rep. 2018;25:2177–2191.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samarakoon R, Higgins SP, Higgins CE, Higgins PJ. TGF-beta1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-s rc)/EGFR(Y845) and Rho/ROCK signaling. J Mol Cell Cardiol. 2008;44:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Cao M, Cai Y, Li X, Zhao C, Cui R. Dissecting the role of the FGF19-FGFR4 signaling pathway in cancer development and progression. Front Cell Dev Biol. 2020;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen B, Liang Y, He Z, An Y, Zhao W, Wu J. Autocrine activity of BDNF induced by the STAT3 signaling pathway causes prolonged TrkB activation and promotes human non-small-cell lung cancer proliferation. Sci Rep. 2016;6:30404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saleh A, Shan L, Halayko AJ, Kung S, Gounni AS. Critical role for STAT3 in IL-1 7A-mediated CCL11 expression in human airway smooth muscle cells. J Immunol. 2009;182:3357–3365. [DOI] [PubMed] [Google Scholar]
- 97.Kras JV, Weisshaar CL, Quindlen J, Winkelstein BA. Brain-d erived neurotrophic factor is upregulated in the cervical dorsal root ganglia and spinal cord and contributes to the maintenance of pain from facet joint injury in the rat. J Neurosci Res. 2013;91:1312–1321. [DOI] [PubMed] [Google Scholar]
- 98.Simao AP, Mendonca VA, de Oliveira Almeida TM, et al. Involvement of BDNF in knee osteoarthritis: the relationship with inflammation and clinical parameters. Rheumatol Int. 2014;34:1153–1157. [DOI] [PubMed] [Google Scholar]
- 99.Laske C, Stransky E, Eschweiler GW, et al. Increased BDNF serum concentration in fibromyalgia with or without depression or antidepressants. J Psychiatr Res. 2007;41:600–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.